Abstract
Most of our knowledge on cell kinetics stems from in vitro studies of continuously dividing cells. In this study, we determine in vivo cell-cycle parameters of pancreatic β-cells, a largely quiescent population, using drugs that mimic or prevent glucose-induced replication of β-cells in mice. Quiescent β-cells exposed to a mitogenic glucose stimulation require 8 h to enter the G1 phase of the cell cycle, and this time is prolonged in older age. The duration of G1, S, and G2/M is ∼5, 8, and 6 h, respectively. We further provide the first in vivo demonstration of the restriction point at the G0-G1 transition, discovered by Arthur Pardee 40 years ago. The findings may have pharmacodynamic implications in the design of regenerative therapies aimed at increasing β-cell replication and mass in patients with diabetes.
Introduction
Most of our knowledge about the mammalian cell-division cycle is based on studies of cultured cells. Whether basic cell-cycle concepts and quantitative parameters of the cell cycle are conserved between cells growing on plastic in artificial medium and cells in their natural niche is hard to determine. One reason is the difficulty in applying and withdrawing specific, direct mitogens in vivo.
Insulin-producing β-cells reside in the islets of Langerhans and are essential for maintaining normal glucose levels. Insufficient mass of β-cells is a central factor in human diabetes, and the identification of methods to expand β-cell mass is a prime challenge for regenerative biology. Similarly to most differentiated cell types, β-cells in the adult organism are largely quiescent. However, β-cells do divide rarely, and their duplication is key for the maintenance of β-cell mass homeostasis during healthy adult life (1–5) as well as after a diabetogenic injury (6). We have recently shown that the key physiological trigger for β-cell proliferation is glucose (7). Mitogenic signaling is transduced by glucokinase, catalyzing the first step of glycolysis, followed by closure of ATP-dependent potassium channels, leading to membrane depolarization. Indeed, small-molecule glucokinase activators (GKAs), being developed to augment insulin secretion in diabetes (8), double the fraction of replicating β-cells when administered to mice (7). Coadministration of diazoxide, a drug preventing membrane depolarization, cancels the mitogenic effect of GKA (7).
In this study, we use these drugs to probe β-cell kinetics in vivo by taking advantage of the ability to time the administration of a direct mitogen. This enabled the timing of the transition from quiescence to G1 phase of the cell cycle, the duration of G1, S, and G2/M, as well the duration of continued mitogen activity that is required for β-cells to commit to the cell cycle.
Research Design and Methods
Mice and Drugs
GKA was dissolved in 79% saline, 20% DMSO (Sigma-Aldrich), 1% polysorbate–Tween 80, and injected intraperitoneally at 20 or 50 mg/kg. Control mice received the same volume of DMSO (20% of total volume). The injection of DMSO did not affect replication rates of β-cells when compared with mice that were injected with saline. Diazoxide was dissolved similarly to GKA and injected intraperitoneally at 40 mg/kg. BrdU, 5-chloro-2′-deoxyuridine (CldU; MD-Biomedical), and iododeoxyuridine (IdU; Sigma-Aldrich) were dissolved in PBS (10 mg/mL) and injected intraperitoneally at 10 mg/kg.
We used ICR male mice aged 5 weeks or 6 months. Injections of drug or vehicle were typically performed at 4 p.m., and the animals were killed the next morning at 9 a.m. For the G0-G1 experiments, mice were injected with GKA at 8 a.m. and killed at different time points. At sacrifice, the pancreas was fixed in formalin and embedded in paraffin, and 4-μm thick sections were immunostained.
Immunostaining and Analysis
Images were captured on a Nikon C1 confocal microscope (Nikon). For each mouse, >2,000 β-cells (defined as Insulin+Pdx1+ cells) were counted from multiple islets in nonadjacent sections. For each data point, we used three to five mice. Primary antibodies were: guinea pig anti-insulin (1:200; DakoCytomation), rabbit anti-Ki67 (1:200; NeoMarkers), mouse anti-BrdU (Cell Proliferation Kit; 1:300; Amersham Biosciences), rabbit anti–phosphorylated histone H3 (PH3) Ser10 (1:100; Cell Signaling Technology), mouse anti-Cdc47 (1:100; Thermo Fisher Scientific), goat anti-Pdx1 (1:250; a generous gift from Dr. Christopher Wright, Vanderbilt University), rat anti-CldU (1:200; AbD Serotec), and mouse anti-IdU (1:100; BD Biosciences). Secondary antibodies were from all from Jackson ImmunoResearch Laboratories.
RT-PCR
Total RNA was prepared using Qiagen RNeasy microkit (Qiagen) according to the manufacturer's protocol. Total RNA (50 ng) was used for first-strand cDNA synthesis using random primers (Roche) and reverse transcriptase (ImProm-II; Promega). Quantitative real-time PCR was performed with SYBR Green PCR master mix (Applied Biosystems) in 96-well plates using the 7900HT instrument (Applied Biosystems). All reactions were performed in triplicates. The relative amount of mRNA was calculated using the comparative threshold cycle method after normalization to β-actin. The following primers were used: β-actin, 5′-CACAGCTTCTTTGCAGCTCCT-3′ and 5′-GTCATCCATGGCGAACTGG-3′; Ki67, 5′-TTGACCGCTCCTTTAGGTATGAA-3′ and 5′-TTCCAAGGGACTTTCCTGGA-3′; Top2A, 5′-AGCAGATTAGCTTCGTCAACAGC-3′ and 5′-ACATGTCTGCCGCCCTTAGA-3′; and CcnA2, 5′-CAAGACTCGACGGGTTGCTC-3′ and 5′-GAAGGACCAGCAGTGACATGC-3′.
Statistical Analyses
Statistical analyses were performed using a two-tailed Student t test. In all graphs: *P < 0.05, **P < 0.01, ***P < 0.005, and P > 0.05 is not significant. Results are reported as means ± SE.
Results
Time From Mitogenic Stimulus to Cell‐Cycle Entry of β-Cells In Vivo
Most β-cells reside in a quiescent state in vivo. A small fraction of these cells is engaged in the cell cycle under baseline conditions, and an additional fraction can respond to mitogenic stimuli and enter the cell-division cycle. Cycling β-cells can be identified by staining for cell-cycle markers such as Ki67 (marking all stages in the cell cycle, but not G0 quiescent cells), PH3 (marking cells in late G2 and mitosis), Cdc47 (marking cycling cells starting in late G1), and the incorporation of thymidine analogs such as BrdU, CldU, and IdU (occurring during S phase and maintained in the DNA permanently). By timing the administration of a β-cell mitogen to adult mice, one can assess the time it takes a quiescent β-cell, residing in G0, to reach the G1 phase of the cell cycle. This can be defined at the first point after injection of a mitogen when a significant increase in the proportion of replicating β-cells is observed. In other words, this will reflect the time it took competent quiescent β-cells to enter the cell cycle (assuming that the G0 state is homogenous with regard to the distance from G1).
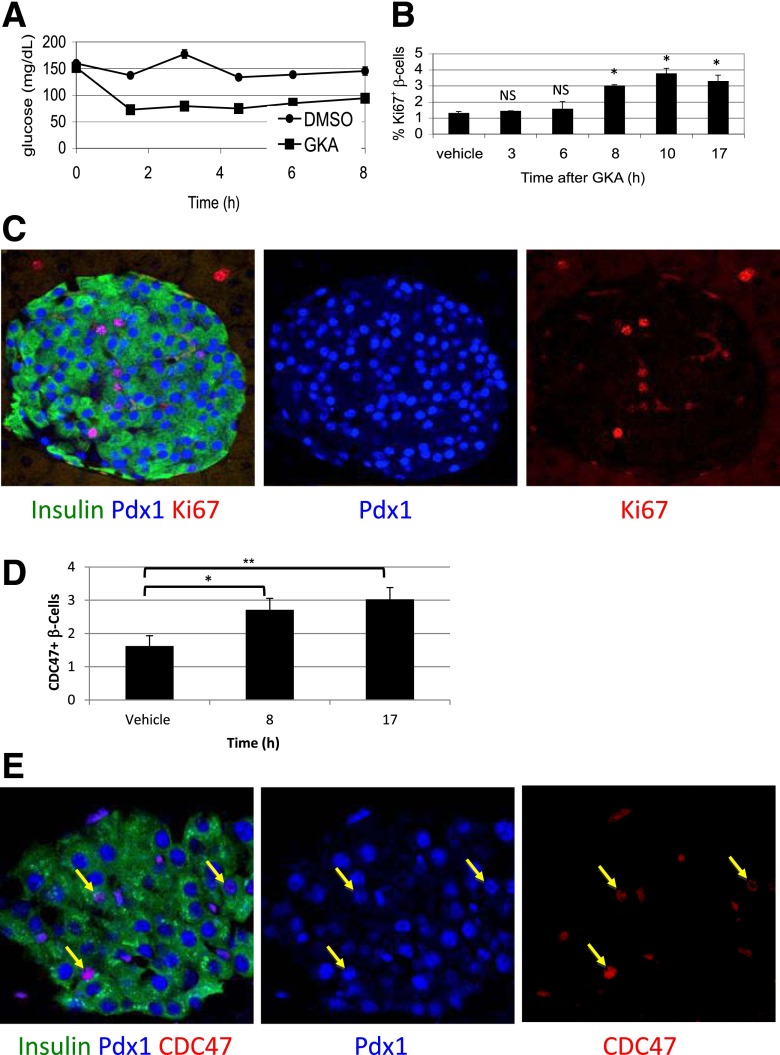
As previously shown, injection of GKA causes rapid onset of hypoglycemia due to an increase in β-cell glycolysis and insulin secretion (Fig. 1A). A significant increase in the fraction of β-cells engaged in the cell cycle (expressing Ki67) occurs 8 h after injection of GKA, but not earlier (Fig. 1B and C). This suggests that the transition of β-cells from G0 to G1 in vivo takes ∼8 h. Staining for Cdc47 (Fig. 1D and E) and quantitative RT-PCR for cyclin A2, Top2A, and Ki67 (Supplementary Fig. 1) provided results consistent with this conclusion.
Figure 1.
Using GKA to define the time from quiescence to G1 in pancreatic β-cells in vivo. A: Blood glucose levels drop upon treatment of mice with a GKA, reflecting increased glycolysis-stimulated insulin secretion in β-cells. B: GKA increases the fraction of replicating β-cells 8 h after administration. C: Representative immunofluorescence image documenting replication of β-cells in vivo using the cell-cycle marker Ki67, 17 h after administration of GKA. D: The fraction of Cdc47+ β-cells increases 8 h after administration of GKA. E: Representative immunofluorescence image of Cdc47 staining in islets, 17 h after administration of GKA. Arrows point to Insulin+Pdx1+Cdc47+ cells. *P < 0.05, **P < 0.01. NS, not significant.
Duration of G1
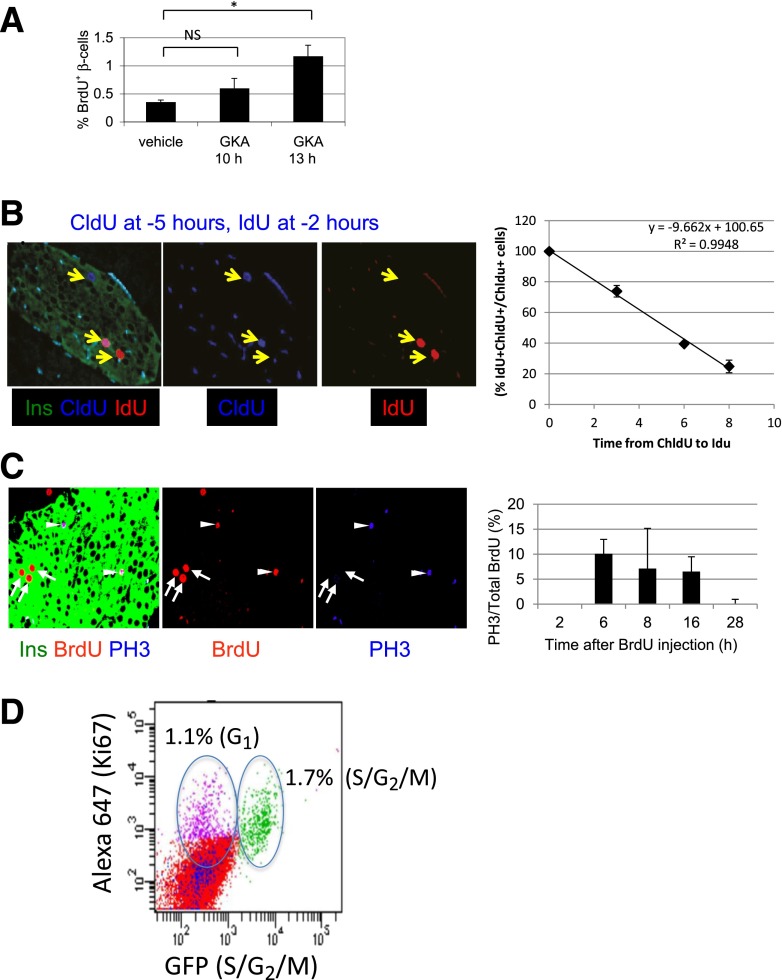
Following a similar reasoning, the duration of G1 can be calculated from the time after injection of a mitogen when a significant increase in the proportion of β-cells that incorporate the thymidine analog BrdU is observed. This reflects the time required by competent β-cells to move from G0 to the DNA synthesis phase. The time of G1 can therefore be defined as (time from G0 to S) − (time from G0 to G1). Mice were administered with GKA and killed at different time points after pulsing with BrdU for 2 h. Thirteen hours (but not 10 h) after injection of GKA, the proportion of β-cells incorporating the thymidine analog BrdU is increased. This suggests that the duration of G1 in β-cells in vivo is ∼5 h (13 h from G0 to S − 8 h from G0 to G1) (Fig. 2A).
Figure 2.
Defining the duration of G1, S, and G2/M in β-cells in vivo. A: GKA increases BrdU incorporation in β-cells 13 h after administration. B: Double labeling with thymidine analogs to estimate duration of S phase. Left panels: Representative image from a mouse killed 2 h after IdU injection. CldU was injected 3 h prior to IdU. Arrows point to a CldU+ β-cell (left), CldU+ IdU+ double positive β-cell (middle), and IdU+ β-cell (right). Right panel: Graph depicting the percent of CldU+ β-cells that costain for IdU, when IdU is injected at different times after CldU. C: BrdU-PH3 staining to estimate duration of G2 phase. Left panels: Representative immunofluorescence image. BrdU was injected 6 h prior to sacrifice. Arrowheads point to BrdU+ PH3+ mitotic β-cells. Arrows point to BrdU+ PH3− β-cells. Right panel: Percent of BrdU+ β-cells that stain for PH3, at different time points after injection of BrdU, reflecting the duration of G2 as described in text. D: FACS analysis of dissociated islet cells from 3-week-old CyclinB1-GFP mice (n = 4 mice, pooled), costained for GFP and Ki67. A total of 2.8% of islet cells are Ki67+, 1.7% of islet cells costain for Ki67 and GFP, marking cells in S-G2 phases of the cell cycle, and 1.1% are Ki67+GFP−, representing G1 cells. *P < 0.05. NS, not significant.
Duration of S and G2
To calculate the duration of S phase in vivo, mice were pulsed with two distinct thymidine analogs, CldU and IdU, which can be identified using specific antibodies (2,9). We reasoned that if the two analogs are injected consecutively, the same cell will be labeled by both only if its S phase spanned between the two pulses. The shortest gap between CldU and IdU injection that does not result in double-labeled cells should therefore represent the duration of S phase. Control experiments confirmed the specificity of each antibody to its designated thymidine analog (Fig. 2B and Supplementary Fig. 2). When the two analogs were coinjected, all labeled β-cells stained for both antibodies (Supplementary Fig. 2). We then injected CldU followed by IdU in increasing intervals and killed mice 2 h after the IdU injection. As the gap between injections of the analogs increased, the fraction of CldU-labeled β-cells that were also stained for IdU has gradually declined and estimated to reach zero at ≥10.4 h (Fig. 2B). Given that thymidine analogs are available to cells in vivo at least 2 h after injection (9,10), we therefore conclude that the duration of S phase in β-cells in vivo is ∼8 h.
To assess the duration of G2, we measured the minimal time it takes β-cells to travel from S phase to mitosis. We injected mice with BrdU to label cells in S phase, killed at different time points later, and costained for BrdU and the mitotic marker PH3. The first time after BrdU injection at which BrdU+ β-cells stain for PH3 should represent the duration of G2. In addition, the last time after BrdU injection at which BrdU+ β-cells stain for PH3 should represent the combined duration of S and G2 (capturing cells that were at the beginning of S phase when BrdU was injected and at M phase when mice were killed). BrdU+ β-cells stained for PH3 starting at 6 h after BrdU injection, suggesting that the duration of G2 in β-cells in vivo is 6 h (Fig. 2C). PH3+ β-cells that were labeled with BrdU could be observed up to 16 h after BrdU injection (Fig. 2C). Assuming that BrdU is available for 2 h after injection, this suggests that the duration of S + G2 phases in β-cells in vivo is ∼14 h, consistent with S phase = 8 h and G2 phase = 6 h.
We then wished to independently validate the conclusions about the duration of cell-cycle stages. We took advantage of a transgenic mouse that we have recently developed, in which replicating cells at the S-G2/M phases express GFP (11). We dissociated islets from these mice at 3.5 weeks of age, stained with antibodies against GFP and Ki67, and used FACS to determine the fraction of replicating islet cells in any stage of the cell cycle (Ki67+) and the fraction of islet cells at S/G2/M (GFP+). As expected, all GFP+ cells were also Ki67+ (Fig. 2D and Supplementary Fig. 3). At this young age, 2.8% of islet cells were Ki67+, representing the population at G1, S, G2, and M phases, plus the cells that finished mitosis and have not yet degraded Ki67. Among the Ki67+ cells, 60% were also GFP+ (Fig. 2D). This result suggests that the duration of S/G2/M is 60% of the combined duration of G1, S, G2, M, and the half-life of Ki67 protein postmitosis [estimated to be 1.5 h (12)]. The experiments above suggested that S + G2 = 14 h and that G1 + S + G2 = 19 h. Assuming that Ki67 is detected for 2 h after mitosis in two daughter cells, the FACS results (S+ G2 + M/G1 + S + G2 + M + post-M = 0.6) are thus consistent with the results from Figs. 1Aand 2A–C (G1 + S + G2 + M + post-M = 23 h; S + G2 + M = 14 h).
Old Age Prolongs the Time From Mitogenic Stimulus to Cell-Cycle Entry
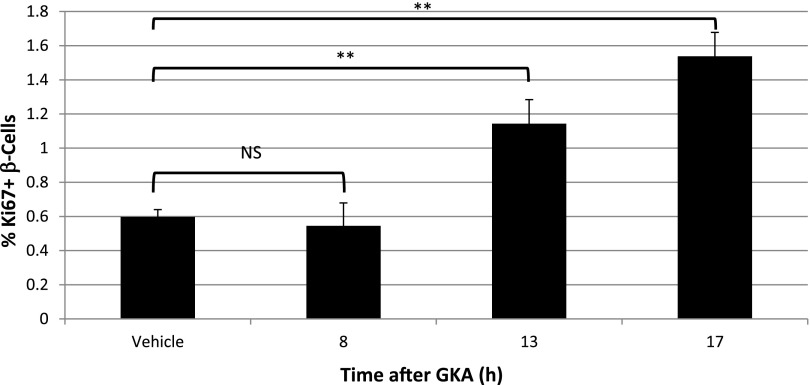
All of the experiments described above were performed using 5-week-old mice. While it is established that the fraction of replicating β-cells declines dramatically with age (13–17), it is not known if the duration of different cell-cycle phases is age-dependent. To address this interesting question, we treated 6-month-old mice with GKA and examined if and when the fraction of replicating β-cells increases. As shown in Fig. 3 and consistent with published results, the baseline replication of β-cells in these middle-aged mice was lower (∼0.6%). GKA increased the fraction of replicating β-cells (stained for Ki67) as in young mice, but with an apparent delay: at 8 h postinjection, no effect was seen, while 13 and 17 h after injection, the fraction of replicating β-cells has doubled. These results suggest that in old age, quiescent β-cells that do respond to mitogenic signals require more time to do so. More work is required to understand the mechanistic basis of this phenomenon and to identify the specific stages of the cell cycle that are affected by age.
Figure 3.
Delayed cell-cycle entry of β-cells in old age. Six-month-old mice were injected with GKA, killed at different time points later, and the fraction of Ki67+ β-cells was determined. There is no increase in the fraction of replicating β-cells 8 h after drug administration, a time point showing a robust increase in Ki67 staining in 5-week-old mice. **P < 0.01. NS, not significant.
Restriction Point in β-Cells In Vivo
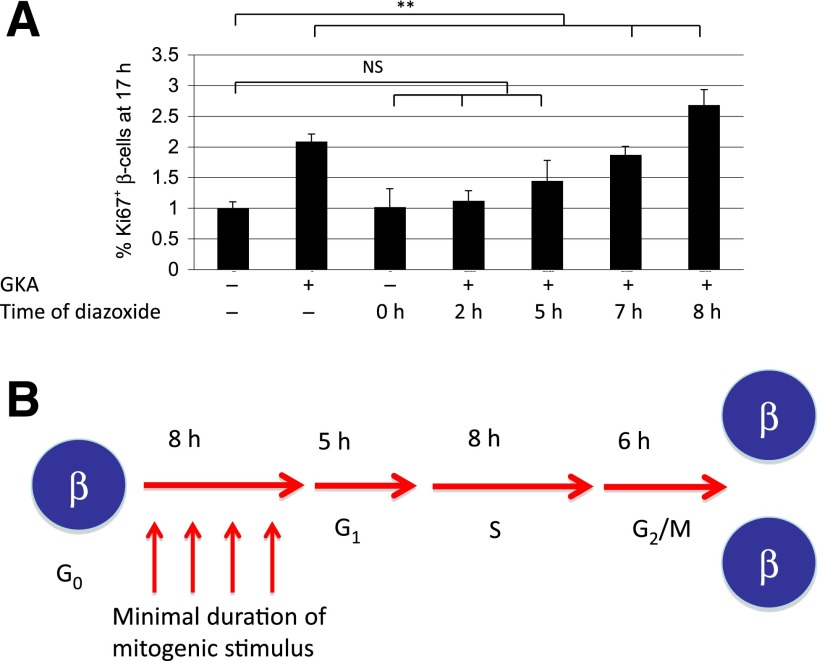
Finally, we attempted to characterize in vivo the restriction point as defined by Arthur Pardee: the point in the cell cycle when replication becomes independent of further mitogenic stimulus (18–20). In this study, we took advantage of our previous finding that the mitogenic signal of GKA is transduced via membrane depolarization and can be cancelled by pharmacologic or genetic prevention of membrane depolarization (7). Mice were injected with GKA, followed by diazoxide to cancel the mitogenic effect, and the fraction of Ki67+ β-cells was determined at sacrifice, 17 h after GKA administration. Diazoxide prevented the mitogenic effect of GKA when given up until 7 to 8 h after GKA (Fig. 4A). This suggests that increased glycolysis and membrane depolarization must continue for 7 to 8 h to ensure commitment of β-cells to the cell cycle. Beyond this time, cell-cycle progression is independent of GKA activity. Thus, quiescent β-cells cross a restriction point 7 to 8 h after mitogen exposure, a time coinciding with G1 entry.
Figure 4.
Demonstration of the restriction point in β-cells triggered to divide in vivo. A: Timed application of a mitogen (GKA) and a downstream inhibitor of its mitogenic activity (diazoxide) identifies the restriction point in β-cells in vivo after 7 to 8 h of mitogen activity. B: Model summarizing findings on β-cell kinetics in vivo. **P < 0.01. NS, not significant.
Discussion
We show in this study that adult quiescent β-cells responding to a mitogen require ∼8 h to enter G1. Mitogenic signaling has to continue throughout this time for the cells to commit to the cell cycle. The duration of G1 is ∼5 h, DNA replication takes ∼8 h, and G2/M phases take ∼6 h (Fig. 4B). These values are in agreement with cell kinetics data measured in cultured (non-β) cells (21,22). The universal conservation of the G0-G1 restriction point in vivo is particularly interesting. Why mammalian cells require specifically this time to commit to replication remains unknown.
According to our results, the total duration of the cell cycle of adult β-cells in vivo, from mitogenic stimulation to cell division, is 27 h. Previous estimates of cell cycle duration of β-cells, based on in vitro studies, varied from 14.9 (23) to 40 h (24). More relevant to our study, glucose infusion to adult mice was reported to increase β-cell replication (BrdU incorporation) after 2 days, but not after 1 day (25). However, the resolution of this study was in days and not hours, which could have led to skipping an early wave of glucose-induced β-cell replication.
Importantly, our results reflect averaged values for cell-cycle progression of β-cells. While it was previously shown that all β-cells are equivalent in their replicative potential (2,3), it is possible that different β-cells progress differently in the cell cycle. Testing this idea may require time lapse studies on individual replicating β-cells, preferably in vivo, which is beyond reach at present. We note, however, that cell-cycle entry of β-cells in response to GKA appears in a sharp step rather than gradually (Fig. 1), suggesting synchronization of quiescent, G0 cells.
Our results also suggest that organismal age causes not only a decline in the total number of β-cells engaged in the cell cycle (16,17,26,27), but also prolongs the time from mitogenic stimulus to cell-cycle entry, an effect on cell kinetics not previously described. It will be interesting to study the molecular nature of this phenomenon and to ask if other cell-cycle parameters such as the restriction point and the duration of other cell-cycle phases is also altered. Future studies can use the methodology described in this study to ask how factors such as stress, regenerative signals (6,28), specific mitogens (29,30), and genetic components (27,31,32) affect β-cell kinetics in vivo.
Our results have interesting implications for the design of drugs aimed at increasing β-cell replication for the cure of diabetes (assuming that these results translate to human islets). Such drugs must remain active for 7 to 8 h, or, alternatively, administration has to be repeated. Furthermore, drug exposure beyond this duration is not necessary and maybe undesirable based on evidence from a single patient with a severe gain-of-function glucokinase mutation (33) and our unpublished observations. Finally, our data may explain why meals do not trigger β-cell replication in healthy individuals: the surge in blood glucose is normally cleared after 1 to 2 h, before β-cells commit to division. Compensatory replication of β-cells in insulin resistant people may result from prolonged hyperglycemia, persisting beyond the restriction point.
Supplementary Material
Article Information
Acknowledgments. The authors thank Richard Kulka (Hebrew University), Ran Kafri (Harvard University), and Shalev Itzkovitz (Weizmann Institute) for critical reading of the manuscript and discussions and Norma Kidess-Bassir (Hebrew University) for excellent technical assistance.
Funding. This work was supported by grants from the Juvenile Diabetes Research Foundation, National Institutes of Health (Beta Cell Biology Consortium), Israel Cancer Research Fund (Barbara Goodman PC-RCDA), European Union (European Research Council and the Seventh Framework Programme under grant 241883), the Leona M. and Harry B. Helmsley Charitable Trust, the Dutch Friends of Hebrew University, and the I-CORE Program of the Planning and Budgeting Committee and The Israel Science Foundation #41.11 (to Y.D.). A.H. was supported by a generous fellowship from Alan Harper.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.H. and S.S. designed and performed the experiments. A.K. performed the experiments. J.G. designed the experiments. M.B., B.G., and Y.D. designed the experiments and wrote the manuscript. Y.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-1035/-/DC1.
References
- 1.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 2.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 3.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol 2007;5:e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georgia S, Bhushan A. Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 2004;114:963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier JJ, Butler AE, Saisho Y, et al. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 2008;57:1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003;301:370–373 [DOI] [PubMed] [Google Scholar]
- 9.Bauer S, Patterson PH. The cell cycle-apoptosis connection revisited in the adult brain. J Cell Biol 2005;171:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi T, Nowakowski RS, Caviness VS., Jr BUdR as an S-phase marker for quantitative studies of cytokinetic behaviour in the murine cerebral ventricular zone. J Neurocytol 1992;21:185–197 [DOI] [PubMed] [Google Scholar]
- 11.Klochendler A, Weinberg-Corem N, Moran M, et al. A transgenic mouse marking live replicating cells reveals in vivo transcriptional program of proliferation. Dev Cell 2012;23:681–690 [DOI] [PubMed] [Google Scholar]
- 12.Heidebrecht HJ, Buck F, Haas K, Wacker HH, Parwaresch R. Monoclonal antibodies Ki-S3 and Ki-S5 yield new data on the ‘Ki-67’ proteins. Cell Prolif 1996;29:413–425 [DOI] [PubMed] [Google Scholar]
- 13.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA. Very slow turnover of beta-cells in aged adult mice. Diabetes 2005;54:2557–2567 [DOI] [PubMed] [Google Scholar]
- 14.Stolovich-Rain M, Hija A, Grimsby J, Glaser B, Dor Y. Pancreatic beta cells in very old mice retain capacity for compensatory proliferation. J Biol Chem 2012;287:27407–27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong ES, Le Guezennec X, Demidov ON, et al. p38MAPK controls expression of multiple cell cycle inhibitors and islet proliferation with advancing age. Dev Cell 2009;17:142–149 [DOI] [PubMed] [Google Scholar]
- 16.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes 2009;58:1312–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 2009;58:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Planas-Silva MD, Weinberg RA. The restriction point and control of cell proliferation. Curr Opin Cell Biol 1997;9:768–772 [DOI] [PubMed] [Google Scholar]
- 19.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA 1974;71:1286–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zetterberg A, Larsson O, Wiman KG. What is the restriction point? Curr Opin Cell Biol 1995;7:835–842 [DOI] [PubMed] [Google Scholar]
- 21.Zwang Y, Sas-Chen A, Drier Y, et al. Two phases of mitogenic signaling unveil roles for p53 and EGR1 in elimination of inconsistent growth signals. Mol Cell 2011;42:524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA 1985;82:5365–5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swenne I. The role of glucose in the in vitro regulation of cell cycle kinetics and proliferation of fetal pancreatic B-cells. Diabetes 1982;31:754–760 [DOI] [PubMed] [Google Scholar]
- 24.Saisho Y, Manesso E, Gurlo T, et al. Development of factors to convert frequency to rate for beta-cell replication and apoptosis quantified by time-lapse video microscopy and immunohistochemistry. Am J Physiol Endocrinol Metab 2009;296:E89–E96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development 2010;137:3205–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Gu X, Liu Y, et al. PDGF signalling controls age-dependent proliferation in pancreatic β-cells. Nature 2011;478:349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab 2010;21:151–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson O, Adams BA, Yoo D, et al. Adenosine signaling promotes regeneration of pancreatic β cells in vivo. Cell Metab 2012;15:885–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Annes JP, Ryu JH, Lam K, et al. Adenosine kinase inhibition selectively promotes rodent and porcine islet β-cell replication. Proc Natl Acad Sci USA 2012;109:3915–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Gu X, Su IH, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev 2009;23:975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhawan S, Tschen SI, Bhushan A. Bmi-1 regulates the Ink4a/Arf locus to control pancreatic beta-cell proliferation. Genes Dev 2009;23:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kassem S, Bhandari S, Rodríguez-Bada P, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med 2010;362:1348–1350 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.