Abstract
Acute administration of glucagon-like peptide 1 (GLP-1) and its agonists slows gastric emptying, which represents the major mechanism underlying their attenuation of postprandial glycemic excursions. However, this effect may diminish during prolonged use. We compared the effects of prolonged and intermittent stimulation of the GLP-1 receptor on gastric emptying and glycemia. Ten healthy men received intravenous saline (placebo) or GLP-1 (0.8 pmol/kg ⋅ min), as a continuous 24-h infusion (“prolonged”), two 4.5-h infusions separated by 20 h (“intermittent”), and a 4.5-h infusion (“acute”) in a randomized, double-blind, crossover fashion. Gastric emptying of a radiolabeled mashed potato meal was measured using scintigraphy. Acute GLP-1 markedly slowed gastric emptying. The magnitude of the slowing was attenuated with prolonged but maintained with intermittent infusions. GLP-1 potently diminished postprandial glycemia during acute and intermittent regimens. These observations suggest that short-acting GLP-1 agonists may be superior to long-acting agonists when aiming specifically to reduce postprandial glycemic excursions in the treatment of type 2 diabetes.
Introduction
Acute administration of glucagon-like peptide 1 (GLP-1) to healthy humans, and patients with type 2 diabetes, lowers blood glucose concentrations by stimulating insulin, suppressing glucagon secretion, and slowing gastric emptying (1). GLP-1 agonists have been incorporated into standard algorithms to treat hyperglycemia in patients with type 2 diabetes, and while the objective of these treatment regimens is to reduce glycemia safely (2), the importance of specifically targeting postprandial glycemia is increasingly being recognized (3). The capacity for GLP-1, and its agonists, to slow gastric emptying represents the dominant mechanism by which they reduce postprandial glycemic excursions (4,5).
Long-acting GLP-1 agonists are attractive, since fewer injections are required (6,7). However, there is preliminary evidence that the slowing of gastric emptying by long-acting agonists becomes attenuated over time (6,8–10), although only one study has hitherto examined directly whether sustained GLP-1 receptor activation induces tachyphylaxis for the effects of GLP-1 on gastric emptying (11). In this study, the delay in gastric emptying of a liquid meal was reported to be diminished after administration of intravenous GLP-1 for 270 min compared with 30 min (11), but methodological limitations included the use of a suboptimal dye dilution technique to quantify gastric emptying and the provision of a second meal only 4 h after the first, with potential for incomplete emptying of the first meal or ongoing nutrient stimulation of the small intestine to influence the disposition of the second meal. Furthermore, this previous study did not evaluate the effect of intermittent GLP-1 receptor stimulation, which is of substantial clinical relevance.
We undertook the current study to determine accurately whether tachyphylaxis to the effect of GLP-1 on gastric emptying occurs rapidly and affects postprandial glycemia. The primary hypothesis was that intermittent administration of GLP-1 would slow gastric emptying more than prolonged continuous administration. Secondary hypotheses were that 1) prolonged infusion of GLP-1 would still slow gastric emptying compared with placebo, 2) the effects of intermittent and acute GLP-1 infusions on gastric emptying would be similar, and 3) postprandial glycemic excursions would be related to the rate of gastric emptying.
Research Design and Methods
Healthy men aged 18–35 years were eligible, and those with diabetes (HbA1c >6.0% or 42.1 mmol/mol), impaired renal function or anemia, currently smoking, consuming >20 g/day alcohol, receiving medication known to affect gastrointestinal motility or glycemia, or with a history of gastric or small intestinal surgery were excluded.
Each subject attended the hospital after an overnight fast on two occasions separated by at least 4 days to be studied under regimens A and B in a randomized, double-blind fashion. Randomization was carried out by the Royal Adelaide Hospital Pharmacy using a Web-based program. Allocation concealment was maintained throughout. An intravenous catheter was inserted into each arm for drug delivery and blood sampling. Gastric emptying was measured at approximately the same time of day in each subject. During study visits, energy intake was standardized and subjects remained sitting or lying, unless toileting. GLP-1 (7–36)amide (Bachem, Germany) was infused at a rate of 0.8 pmol/kg/min, which is known to result in receptor stimulation representative of pharmacological agents (11). Placebo was 0.9% sodium chloride, and all study drugs were infused at 1 mL/min.
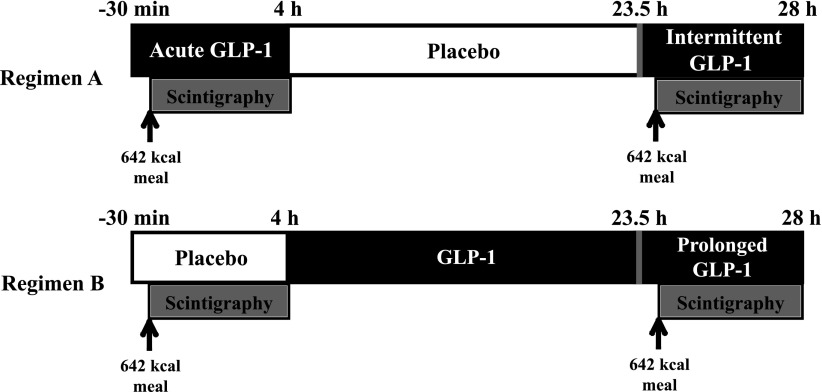
Regimen A
Subjects received an intravenous infusion of GLP-1 for 4.5 h followed by 19.5 h of placebo and, finally, 4.5 h of GLP-1. The response to “intermittent” GLP-1 was assessed during the second GLP-1 infusion (Fig. 1).
Figure 1.
Study protocol. Scintigraphic measurements of gastric emptying were performed over 4 h both from T = 0 h to T = 4 h and T = 24 h to T = 28 h. Infusion of study drug was commenced 30 min prior to ingestion of meal to allow for plasma concentrations to reach steady-state.
Regimen B
Subjects received a 4.5-h intravenous infusion of placebo followed by 24 h of GLP-1. The effect of “prolonged” GLP-1 exposure was assessed after 20 h of GLP-1 infusion (Fig. 1). The study protocol was approved by the Royal Adelaide Hospital Research Ethics Committee and registered as a clinical trial. Written informed consent was obtained from the subjects.
Gastric Emptying
Radioisotopic data were acquired with the subjects seated with their back against a γ camera (GE Healthcare). On four occasions (Fig. 1), subjects ingested a test meal comprising 65 g powdered mashed potato (Deb Instant; Continental, Sydney, Australia), 45 g margarine (Flora Original; Unilever, Sydney, Australia), 20 g glucose, and 200 mL water, labeled with 20 MBq 99mTc-calcium-phytate colloid. The meal contained 2,687 kJ (642 kcal), with 72.3 g carbohydrate, 35.5 g fat, and 8.1 g protein. Scintigraphic images were acquired every minute for the first hour and then at 3-min intervals for a further 3 h. A left lateral image of the stomach was acquired to correct for γ-ray attenuation (12). Data were also corrected for radioactive decay and subject movement. A region of interest was drawn around the total stomach, and percent retention was determined at 0, 30, 60, 90, 120, 150, 210, and 240 min. The time taken for the stomach to empty 50% (T50) was also calculated (12).
Blood Glucose and Insulin
Blood glucose concentrations were measured using a blood gas analyzer (ABL800 Flex; Radiometer Medical, Brønshøj, Denmark), and serum insulin was measured by ELISA (Mercodia) (13). Blood was sampled at −30, 0, 30, 60, 90, 120, and 180 min.
Statistical Analysis
Data are presented as mean (95% CI). Area under the blood glucose curve (AUC) was derived using the trapezoidal rule. The sample size was calculated using SD of 25% around the mean gastric emptying of a similar meal (14), with an anticipated reduction in gastric slowing during prolonged GLP-1 administration of 50% (11). Allowing for 10% greater margins, at least nine subjects were required (β = 0.8, α = 0.05); therefore, we studied 10 subjects.
A mixed-effects maximum likelihood model, with infusion as a fixed effect, was used to determine differences between infusions. If an effect was present, post hoc pairwise analysis was conducted between infusions, with Bonferonni corrections for repeated testing. P values <0.05 were considered significant.
Because after a similar meal we have previously reported 1) strong associations between glycemic excursions during the initial 60 min and the rate of gastric emptying (14) and 2) serum insulin concentrations markedly elevated for 180 min, glycemia was calculated as AUC60 and insulinemia as AUC180. The relationships between gastric emptying, glycemia, and insulinemia were determined using univariate analysis, with correction for within-subject correlations (15).
Results
Ten healthy male subjects (age 24 [95% CI 23–25] years, BMI 25 [22–27] kg/m2) completed the study without any adverse effects.
Gastric Emptying
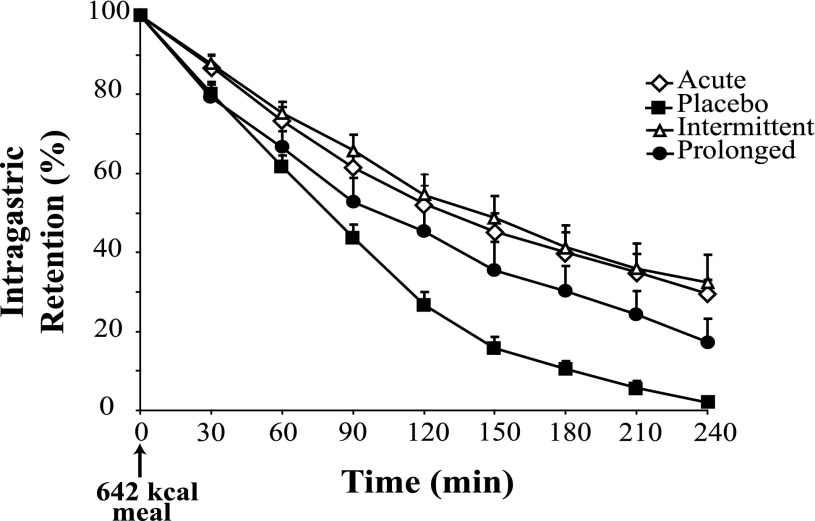
All GLP-1 infusions slowed gastric emptying compared with placebo (P < 0.001) (Fig. 2). Acute GLP-1 compared with placebo increased intragastric retention (P = 0.001), as did intermittent compared with prolonged infusion (P = 0.04) (Fig. 2). While prolonged infusion increased retention in comparison with placebo (P = 0.003), acute and intermittent infusions had similar effects (P = 1.0) (Fig. 2). T50 was affected by the study infusion (P = 0.01), with acute infusion (143 min [104–183] min) delaying the T50 compared with placebo (79 min [65–94]); P = 0.02) and a trend for intermittent (168 min [109–225]) to delay compared with prolonged (122 min [75–170]; P = 0.09). However, there was no significant difference in T50 between prolonged and placebo (P = 0.26) or acute and intermittent (P = 0.69) infusions.
Figure 2.
The effect of different GLP-1 regimens on gastric emptying. There was an effect between the various infusions (P < 0.001). Acute GLP-1 compared with placebo increased intragastric retention (P = 0.001), as did intermittent compared with prolonged infusion (P = 0.04). While prolonged infusion increased retention in comparison with placebo (P = 0.003), acute and intermittent infusions had similar effects (P = 1.0). Data are mean ± SEM. A mixed-effects maximum likelihood model was used to determine differences with post hoc testing adjusted for multiple comparisons.
Blood Glucose and Serum Insulin
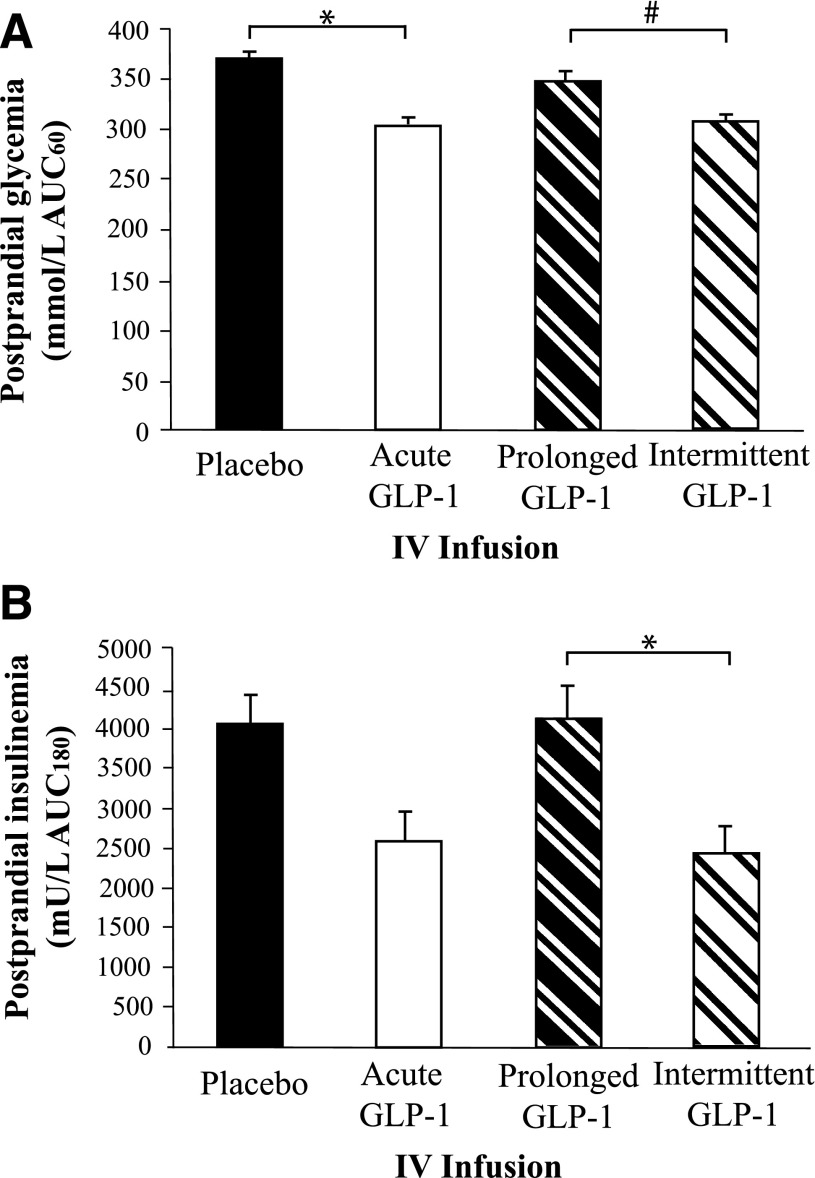
There was a small, but statistically significant, difference in fasting blood glucose concentrations prior to saline or GLP-1 infusions (T = −30: placebo 5.5 mmol/L [5.2–5.7] vs. acute 5.0 mmol/L [4.7–5.3]; P < 0.001). Postprandial glycemia (AUC60) was affected by the infusion (P < 0.001) (Supplementary Fig. 1). Acute GLP-1 reduced postprandial glycemia (AUC60) compared with placebo (P = 0.001), as did intermittent compared with prolonged (P = 0.007), but there was no difference between prolonged and placebo (P = 0.21) or acute and intermittent (P = 1.0) regimens (Fig. 3A). The peak blood glucose concentration was affected by the infusion (P < 0.001), being greater during placebo 7.1 mmol/L (6.4–7.7) than either acute (5.8 mmol/L [5.4–6.3]), intermittent (6.0 mmol/L [5.5–6.4]), or prolonged (6.6 mmol/L [6.1–7.2]) GLP-1 infusions.
Figure 3.
The effect of different GLP-1 regimens on postprandial glycemia and insulinemia. A: There was an effect between infusions (P < 0.001). Acute GLP-1 reduced postprandial glycemia (AUC0–60) compared with placebo (*P = 0.001), as did intermittent compared with prolonged (#P = 0.007), but there was no difference between prolonged and placebo (P = 0.21) or acute and intermittent (P = 1.0) regimens. B: There was an effect between the various infusions (P < 0.001). Postprandial insulin concentrations were reduced during intermittent compared with prolonged infusion (*P = 0.003); comparing acute infusion with placebo, the difference was not statistically significant (P = 0.07). Postprandial insulin concentrations were comparable between acute and intermittent infusions (P = 1.0) and between prolonged infusion and placebo (P = 0.21). Data are mean ± SEM. A mixed-effects maximum likelihood model was used to determine differences with post hoc testing adjusted for multiple comparisons. IV, intravenous.
Fasting insulin concentrations were similar [T = −30: placebo (5 mU/L [4–6]) vs. acute (5 mU/L [3–6]; P = 0.15)]. Overall insulin secretion was affected by the study infusion (P < 0.001) (Supplementary Fig. 2). Postprandial insulin concentrations were reduced during intermittent compared with prolonged infusion (P = 0.003), but there was no statistically significant difference between acute GLP-1 and placebo (P = 0.07) (Fig. 3B). Postprandial insulin concentrations were comparable between acute and intermittent infusions (P = 1.0) and between prolonged infusion and placebo (P = 0.85) (Fig. 3B).
Peak insulin concentrations were also affected (P = 0.003), with secretion less during acute (25.1 mU/L [16.4–33.8]) than placebo (39.8 mU/L [31.9–47.6]), P = 0.03, and less during intermittent (21.0 mU/L [14.6–27.4 when compared with prolonged (43.0 mU/L [28.0–58.0]), P = 0.03. However, peak insulin concentrations were similar between prolonged and placebo and between acute and intermittent (P = 1.0 for both). The insulin-to-glucose ratios (AUC180) were similarly affected. Due to slower emptying, the ratios were less with acute than placebo (P = 0.035) and intermittent compared with prolonged (P = 0.002) (Supplementary Fig. 3).
Relationship Between Gastric Emptying, Postprandial Glycemia, and Insulinemia
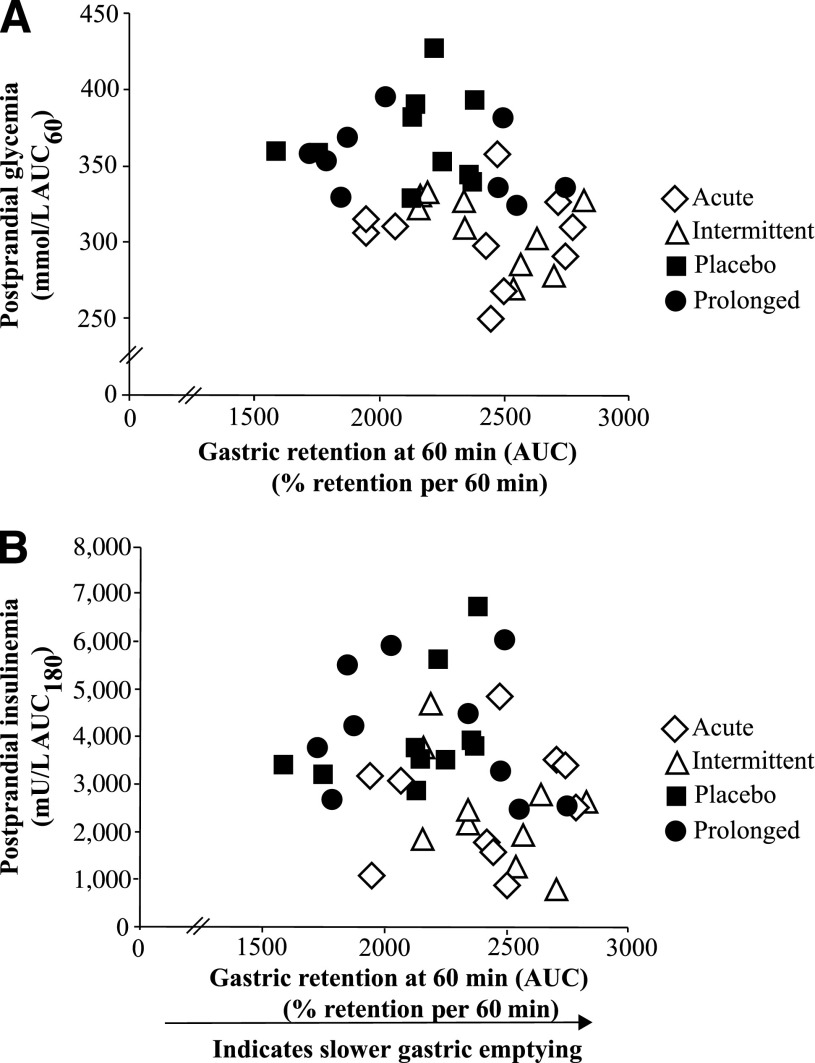
There were strong associations between gastric emptying and postprandial glycemia (AUC60: r = −0.49; P = 0.005) (Fig. 4A) and between gastric emptying and insulinemia (AUC240: r = −0.41; P = 0.02 [Fig. 4B] and % gastric retention AUC60 vs. insulin peak: r = −0.46; P = 0.01), so that when gastric emptying was faster, glycemic and insulin excursions were greater.
Figure 4.
Correlation between gastric emptying, glycemia, and insulinemia. The rate of gastric emptying (% gastric retention AUC60) was closely associated with postprandial glycemia (AUC60: r = −0.49; P = 0.005) (A) and postprandial insulinemia (AUC180: r = −0.41; P = 0.02) (B). Data are correlated within subject (15).
Discussion
This study establishes that while acute, intermittent, and prolonged infusions of exogenous GLP-1 all slow gastric emptying substantially in health, the magnitude of this effect is attenuated during prolonged stimulation, which reduces the effect of GLP-1 on postprandial glycemic excursions.
These observations were anticipated and are consistent with the notion that short-acting agonists appear to have a substantial, and sustained, effect to slow gastric emptying, whereas the acute effects of long-acting agonists on gastric emptying diminish with ongoing use (6,9,10,16). Indeed, while prolonged stimulation with exenatide once a week lowers postprandial glycemia, the magnitude of lowering is greater when exenatide twice daily is administered (6).
While a similar effect on gastric emptying was suggested by both Nauck et al. (11) and Näslund et al. (8) there were limitations with both studies. In both studies, the methods used to measuring measure gastric emptying were less than optimal and, in the former study, were also confounded by potential order effects, while in the latter study intermittent stimulation was achieved using suprapharmacological bolus dosing. However, building on these previous observations (8,11), our data establish unequivocally that tachyphylaxis to the gastric motor effects of GLP-1 occurs. Moreover, our study design allowed us not only to compare the effects of continuous and intermittent GLP-1 receptor stimulation at plasma concentrations that are similar to plasma concentrations achieved during standard dosing with commercially available short and long-acting GLP-1 agonists (6,11,16,17) but also to evaluate its impact on postprandial glycemia, which indicates that this phenomenon is clinically relevant.
It should be recognized that with prolonged (~24 h) GLP-1 receptor stimulation, the slowing of gastric emptying compared with placebo was attenuated but not abolished. This is important given that even modest changes in gastric emptying have the potential to affect postprandial glycemia substantially in patients with diabetes (18). It remains to be determined whether effects on gastric emptying would be further attenuated with receptor stimulation beyond 24 h.
It remains uncertain why there is tachyphylaxis to the effect of GLP-1 to slow gastric emptying and not to the islet cell effects, but there is limited evidence to suggest a role for vagal pathways (11). It should be recognized that the test meal had a substantial carbohydrate and calorie content and that potentially the magnitude of attenuation of the slowing of gastric emptying by exogenous GLP-1 may be influenced by meal composition (14,19). Other potential limitations are that we chose to study healthy volunteers in order to minimize potential confounding by marked hyperglycemia (which is itself known to slow gastric emptying) (20), autonomic neuropathy (which may influence the effects of GLP-1 on the proximal stomach) (21), and the potential for abnormally slow gastric emptying at baseline (22,23). The latter variable may be particularly important, as the capacity for GLP-1 to slow gastric emptying is dependent on the underlying rate of emptying (5). Aside from these factors, it is anticipated that patients with type 2 diabetes would respond similarly to healthy subjects, but this is not assured (2,6,7,24).
The clinical relevance of our study will require confirmatory studies comparing GLP-1 agonists in type 2 patients that have problematic postprandial glycemic excursions, particularly in those with normal, or rapid, gastric emptying. While long-acting agonists may have greater potency to reduce HbA1c in unselected patients with type 2 diabetes (6,25), the use of an isotope breath test to measure gastric emptying has the potential to determine whether short-acting agonists may be more appropriate for a particular patient (16). Such an approach would, however, need to evaluate postprandial effects against any potential worsening of preprandial glycemia (26).
In conclusion, there is a marked attenuation of the magnitude of slowing of gastric emptying with prolonged compared with intermittent stimulation of the GLP-1 receptor in health, supporting the concept of rapid tachyphylaxis. Moreover, the reduction in postprandial glycemia induced by GLP-1 is dependent on the magnitude of slowing of emptying. These data suggest that the choice of GLP-1 agonist for a given patient should be based on the relative importance of targeting postprandial glycemia for an individual patient.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge the assistance of Kylie Lange (University of Adelaide), biostatistician, who supervised all of the statistical analyses performed and Raj Sardana (University of Adelaide) for technical support in performing the gastric-emptying measurements.
Funding. The study was supported by project grant 1025648 from the National Health and Medical Research Council of Australia.
Duality of Interest. M.A.N. is employed by Diabeteszentrum Bad Lauterberg, Germany, and has received research grants (to his institution, Diabeteszentrum Bad Lauterberg) from Berlin-Chemie AG/Menarini; Eli Lilly & Co.; Merck Sharp & Dohme; Novartis Pharma; AstraZeneca; Boehringer Ingelheim; GlaxoSmithKline; Lilly Deutschland GmbH; MetaCure, Inc.; Roche Pharma AG; Novo Nordisk Pharma GmbH; and Tolerx, Inc. for participation in multicentric clinical trials. M.A.N. has also received consulting fees or/and honoraria for membership in advisory boards or/and honoraria for speaking from Amylin Pharmaceuticals; AstraZeneca; Berlin-Chemie AG/Menarini; Boehringer Ingelheim; Bristol-Myers Squibb; Diartis Pharmaceuticals; Eli Lilly & Co.; F. Hoffmann-La Roche Ltd.; GlaxoSmithKline; Intarcia Therapeutics, Inc.; Lilly Deutschland GmbH; MannKind Corp.; Merck Sharp & Dohme GmbH; Merck Sharp & Dohme Corp.; Novartis Pharma AG; Novo Nordisk A/S; Novo Nordisk Pharma GmbH; Sanofi Pharma; Takeda; and Wyeth Research, including reimbursement for travel expenses. M.H. has participated in advisory boards and/or symposia for Novo Nordisk, Sanofi, Novartis, Eli Lily, Boehringer Ingelheim, AstraZeneca, Satlogen, and Meyer Nutraceuticals. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.M.U. was responsible for acquisition of data, statistical analysis, and drafting the manuscript. M.Y.L. contributed to the acquisition of data. K.L.J. was responsible for analysis and interpretation of the scintigraphic data and contributed to the study design and critical revision of the manuscript for important intellectual content. C.E.A., C.E.C., and L.G.T. contributed to the acquisition of data. C.K.R., M.J.C., M.A.N., and M.H. contributed to the study design and critical revision of the manuscript for important intellectual content. A.M.D. was responsible for the study conception and design, obtaining funding, acquisition of data, interpretation, manuscript review, and important intellectual content. A.M.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-0893/-/DC1.
See accompanying commentary, p. 407.
References
- 1.Hare KJ, Vilsbøll T, Asmar M, Deacon CF, Knop FK, Holst JJ. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 2010;59:1765–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract 2007;77:280–285 [DOI] [PubMed] [Google Scholar]
- 4.Meier JJ, Kemmeries G, Holst JJ, Nauck MA. Erythromycin antagonizes the deceleration of gastric emptying by glucagon-like peptide 1 and unmasks its insulinotropic effect in healthy subjects. Diabetes 2005;54:2212–2218 [DOI] [PubMed] [Google Scholar]
- 5.Deane AM, Chapman MJ, Fraser RJ, et al. Effects of exogenous glucagon-like peptide-1 on gastric emptying and glucose absorption in the critically ill: relationship to glycemia. Crit Care Med 2010;38:1261–1269 [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ, Buse JB, Taylor K, et al. DURATION-1 Study Group Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
- 7.Deacon CF, Mannucci E, Ahrén B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes Metab 2012;14:762–767 [DOI] [PubMed] [Google Scholar]
- 8.Näslund E, King N, Mansten S, et al. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr 2004;91:439–446 [DOI] [PubMed] [Google Scholar]
- 9.Flint A, Kapitza C, Hindsberger C, Zdravkovic M. The once-daily human glucagon-like peptide-1 (GLP-1) analog liraglutide improves postprandial glucose levels in type 2 diabetes patients. Adv Ther 2011;28:213–226 [DOI] [PubMed] [Google Scholar]
- 10.Jelsing J, Vrang N, Hansen G, Raun K, Tang-Christensen M, Knudsen LB. Liraglutide: short-lived effect on gastric emptying — long lasting effects on body weight. Diabetes Obes Metab 2012;14:531–538 [DOI] [PubMed] [Google Scholar]
- 11.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011;60:1561–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins PJ, Horowitz M, Cook DJ, Harding PE, Shearman DJ. Gastric emptying in normal subjects—a reproducible technique using a single scintillation camera and computer system. Gut 1983;24:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MY, Fraser JD, Chapman MJ, et al. The effect of exogenous glucose-dependent insulinotropic polypeptide in combination with glucagon-like peptide-1 on glycemia in the critically ill. Diabetes Care 2013;36:3333–3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deane AM, Nguyen NQ, Stevens JE, et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J Clin Endocrinol Metab 2010;95:215–221 [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Calculating correlation coefficients with repeated observations: part 1—correlation within subjects. BMJ 1995;310:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin-Méry A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab 2013;15:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calara F, Taylor K, Han J, et al. A randomized, open-label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic exendin-4). Clin Ther 2005;27:210–215 [DOI] [PubMed] [Google Scholar]
- 18.Jones KL, Horowitz M, Wishart MJ, Maddox AF, Harding PE, Chatterton BE. Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. J Nucl Med 1995;36:2220–2228 [PubMed] [Google Scholar]
- 19.Nicolaus M, Brödl J, Linke R, Woerle HJ, Göke B, Schirra J. Endogenous GLP-1 regulates postprandial glycemia in humans: relative contributions of insulin, glucagon, and gastric emptying. J Clin Endocrinol Metab 2011;96:229–236 [DOI] [PubMed] [Google Scholar]
- 20.Schvarcz E, Palmér M, Aman J, Horowitz M, Stridsberg M, Berne C. Physiological hyperglycemia slows gastric emptying in normal subjects and patients with insulin-dependent diabetes mellitus. Gastroenterology 1997;113:60–66 [DOI] [PubMed] [Google Scholar]
- 21.Delgado-Aros S, Vella A, Camilleri M, et al. Effects of glucagon-like peptide-1 and feeding on gastric volumes in diabetes mellitus with cardio-vagal dysfunction. Neurogastroenterol Motil 2003;15:435–443 [DOI] [PubMed] [Google Scholar]
- 22.Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med 1996;37:1643–1648 [PubMed] [Google Scholar]
- 23.Camilleri M, Bharucha AE, Farrugia G. Epidemiology, mechanisms, and management of diabetic gastroparesis. Clin Gastroenterol Hepatol 2011;9:5–12 [DOI] [PMC free article] [PubMed]
- 24.Christensen M, Knop FK, Vilsbøll T, Holst JJ. Lixisenatide for type 2 diabetes mellitus. Expert Opin Investig Drugs 2011;20:549–557 [DOI] [PubMed] [Google Scholar]
- 25.Buse JB, Rosenstock J, Sesti G, et al. LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39–47 [DOI] [PubMed] [Google Scholar]
- 26.Sathananthan A, Vella A. Personalized pharmacotherapy for type 2 diabetes mellitus. Per Med 2009;6:417–422 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.