Abstract
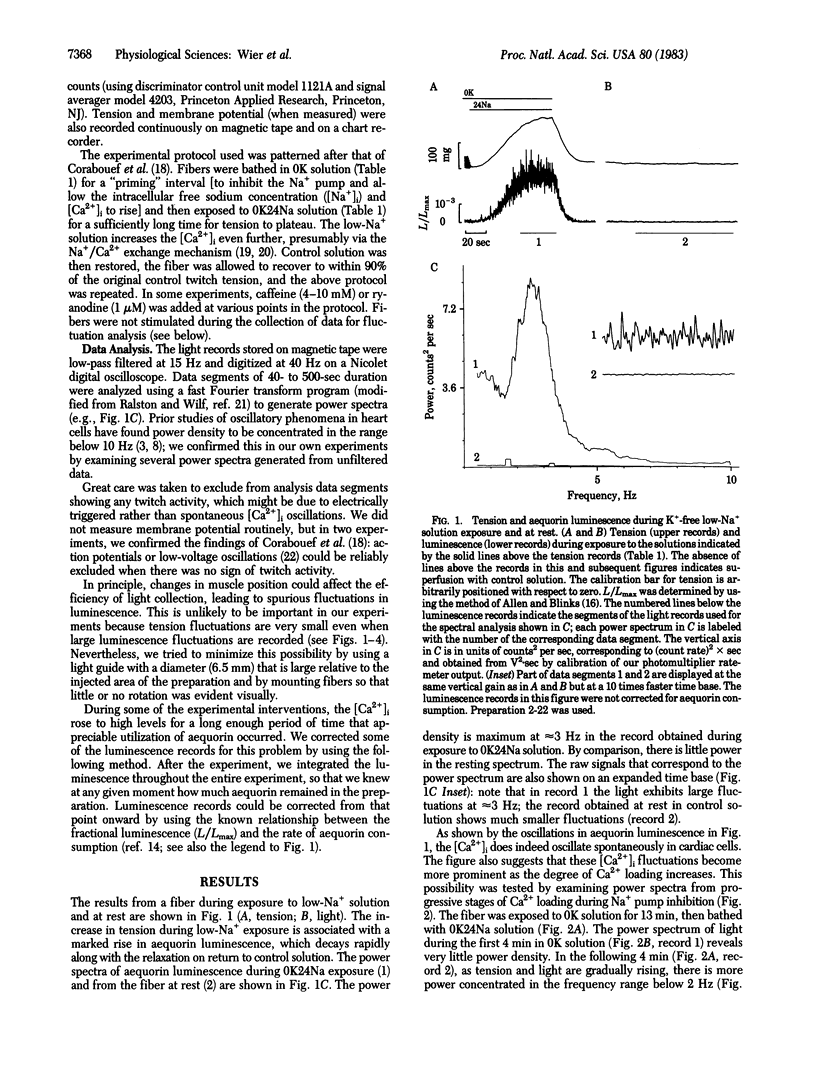
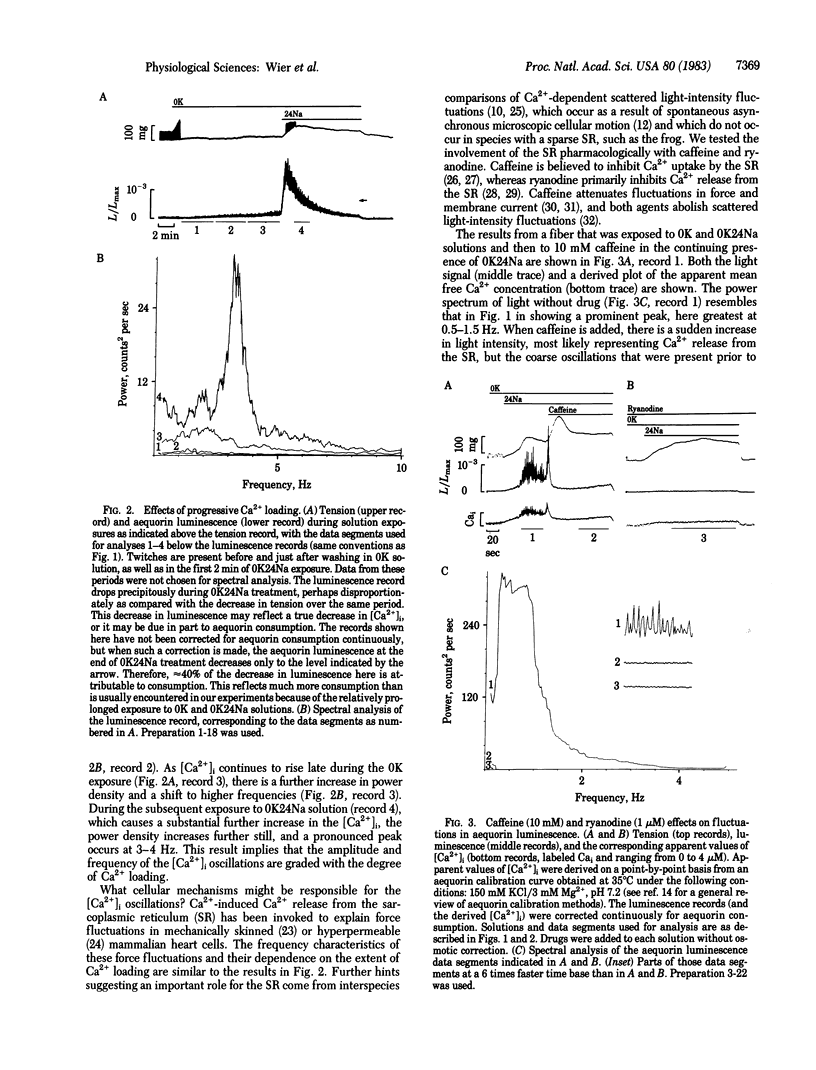
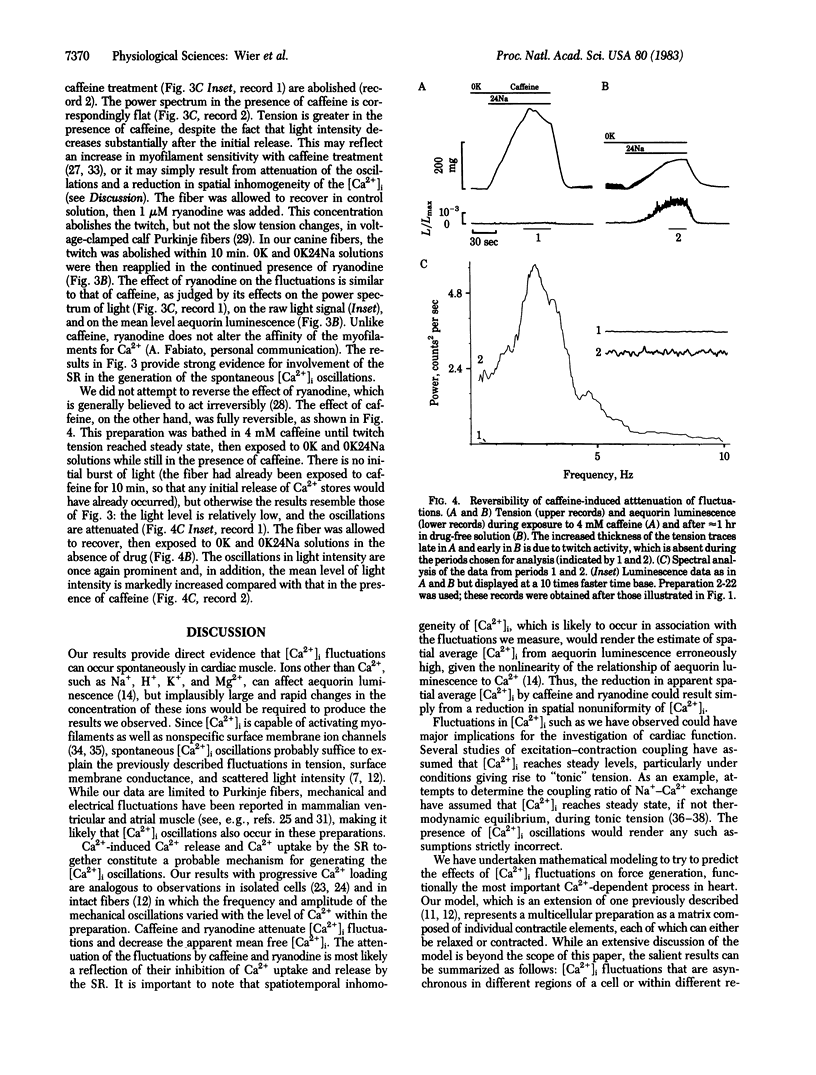
Indirect evidence suggests that fluctuations, or oscillations, in the intracellular free calcium concentration [( Ca2+]i) can occur spontaneously in intact cardiac preparations, but such [Ca2+]i fluctuations have never been demonstrated directly. We used the bioluminescent Ca2+-sensitive protein aequorin to detect fluctuations in the [Ca2+]i in canine cardiac Purkinje fibers. Noise analysis of the aequorin luminescence reveals prominent peaks of power density at frequencies of 1-4 Hz; these peaks become larger and shift to higher frequencies as the [Ca2+]i increases. Caffeine and ryanodine abolish the [Ca2+]i fluctuations, suggesting that Ca2+ release and uptake by the sarcoplasmic reticulum generate these events. When [Ca2+]i fluctuations are present, less tension is produced at any given level of mean aequorin luminescence. Thus, [Ca2+]i fluctuations will undermine attempts to relate [Ca2+]i and force in intact myocardium.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassingthwaighte J. B., Fry C. H., McGuigan J. A. Relationship between internal calcium and outward current in mammalian ventricular muscle; a mechanism for the control of the action potential duration? J Physiol. 1976 Oct;262(1):15–37. doi: 10.1113/jphysiol.1976.sp011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Olson C. B., Jewell B. R., Bravený P. Influence of caffeine and other methylxanthines on mechanical properties of isolated mammalian heart muscle. Evidence for a dual mechanism of action. Circ Res. 1972 Apr;30(4):367–392. doi: 10.1161/01.res.30.4.367. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Croaboeuf E., Gautier P., Giuraudou P. Potential and tension changes induced by sodium removal in dog Purkinje fibres: role of an electrogenic sodium-calcium exchange. J Physiol. 1981 Feb;311:605–622. doi: 10.1113/jphysiol.1981.sp013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani A. M., Cittadini A., Inesi G. Calcium transport and contractile activity in dissociated mammalian heart cells. Am J Physiol. 1979 Sep;237(3):C147–C155. doi: 10.1152/ajpcell.1979.237.3.C147. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The control of tonic tension by membrane potential and intracellular sodium activity in the sheep cardiac Purkinje fibre. J Physiol. 1983 Feb;335:723–743. doi: 10.1113/jphysiol.1983.sp014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Activation of skinned cardiac cells. Subcellular effects of cardioactive drugs. Eur J Cardiol. 1973 Dec;1(2):143–155. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Excitation-contraction coupling of isolated cardiac fibers with disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic contractions. Circ Res. 1972 Sep;31(3):293–307. doi: 10.1161/01.res.31.3.293. [DOI] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. The mechanism of oscillatory activity at low membrane potentials in cardiac Purkinje fibres. J Physiol. 1969 Jan;200(1):255–265. doi: 10.1113/jphysiol.1969.sp008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagueuzian H. S., Katzung B. G. Voltage-clamp studies of transient inward current and mechanical oscillations induced by ouabain in ferret papillary muscle. J Physiol. 1982 Jun;327:255–271. doi: 10.1113/jphysiol.1982.sp014230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982 Jun;38(3):259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H. The informational role of calcium in the cytosol. Adv Cyclic Nucleotide Res. 1979;11:1–26. [PubMed] [Google Scholar]
- Lakatta E. G., Lappé D. L. Diastolic scattered light fluctuation, resting force and twitch force in mammalian cardiac muscle. J Physiol. 1981 Jun;315:369–394. doi: 10.1113/jphysiol.1981.sp013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappé D. L., Lakatta E. G. Intensity fluctuation spectroscopy monitors contractile activation in "resting" cardiac muscle. Science. 1980 Mar 21;207(4437):1369–1371. doi: 10.1126/science.7355295. [DOI] [PubMed] [Google Scholar]
- Marban E., Rink T. J., Tsien R. W., Tsien R. Y. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980 Aug 28;286(5776):845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- Matsuda H., Noma A., Kurachi Y., Irisawa H. Transient depolarization and spontaneous voltage fluctuations in isolated single cells from guinea pig ventricles. Calcium-mediated membrane potential fluctuations. Circ Res. 1982 Aug;51(2):142–151. doi: 10.1161/01.res.51.2.142. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D., Kort A. A., Bhatnagar G. M., Lakatta E. G. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J Gen Physiol. 1983 Jul;82(1):119–153. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Willerson J. T., Templeton G. H., Jones L. R., Besch H. R., Jr Ryanodine: its alterations of cat papillary muscle contractile state and responsiveness to inotropic interventions and a suggested mechanism of action. J Pharmacol Exp Ther. 1979 Apr;209(1):37–47. [PubMed] [Google Scholar]
- Tsien R. W., Kass R. S., Weingart R. Cellular and subcellular mechanisms of cardiac pacemaker oscillations. J Exp Biol. 1979 Aug;81:205–215. doi: 10.1242/jeb.81.1.205. [DOI] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]


