Abstract
Objective
Recent evidence suggests that younger- and middle-age adults who show greater cardiovascular reactivity (CVR) to acute mental stress demonstrate better reasoning and memory skills. The purpose of this study was to examine whether older adults would show a similar positive association between CVR and executive function, and whether regular engagement in mentally stimulating activities (MSA) would moderate this association.
Design
Secondary cross-sectional analysis.
Setting
Three general clinical research centers located in the West Coast, Midwest, and East Coast.
Participants
487 older adults participating in an on-going national survey.
Measurements
Heart rate (HR) and low (LF) and high frequency (HF) domains of heart rate variability (HRV) were measured at baseline and in response to standard mental stress tasks (Stroop color word task and mental arithmetic). Executive function was measured separately from the stress tasks using five neuropsychological tests. MSA was measured by self-report frequency of six common mentally stimulating activities.
Results
Higher HR reactivity was associated with better executive function after controlling for demographic and health variables and baseline HR activity and the interaction between HR reactivity and MSA was significant for executive function. Higher LF-HRV reactivity was also associated with executive function, but subsequent analyses indicated that frequency of MSA was the strongest predictor of executive function in models that included LF- or HF-HRV.
Conclusions
Higher HR reactivity to acute psychological stress is related to better executive function in older adults. For those with lower HR reactivity, engaging frequently in MSA showed significant compensatory benefits for executive function.
Keywords: cardiovascular reactivity, heart rate variability, acute stress, executive function, aging, mentally stimulating activities
Introduction
The notion that the mind (central nervous system) and body (peripheral systems) interact to contribute to both mental and physical health has become increasingly evident (1). For instance, both the sympathetic and parasympathetic branches of the autonomic nervous system regulate cardiovascular activity at rest and also in response to environmental challenges, measured as cardiovascular reactivity (CVR) from rest. Higher CVR to short-term (acute) stressors is implicated in cardiovascular health risk, such as increased incident coronary heart disease in patients with heart disease history, or elevated blood pressure (2, 3), but is also associated with positive health outcomes, including better self-perceived health, lower incident depression and obesity (4). More recent evidence suggests that both cognitive function and CVR to acute stressors are regulated by similar neural pathways, suggesting a new avenue to explore the neurobiological underpinnings of a health outcome that is critical for older adults – age-related cognitive decline (5).
A handful of recently published cross-sectional studies found that greater CVR to acute stress was associated with enhanced cognitive performance, in particular, attention, memory, and reasoning, in younger- or middle-age adults (6–8). Similarly, in a prospective cohort study in Scotland, lower CVR was a risk factor predicting future decline in reasoning and reaction time, and the relationship was stronger in old age relative to young and middle age groups (9). Reasoning, attention, and reaction time are components of executive function, which is a higher order cognitive system controlling multiple cognitive processes that regulate goal-directed behaviors and information organization (10). Executive function declines early in the aging trajectory (11). It is unclear whether the relationship between CVR and overall executive function remains at old ages. Further, the positive association between increased CVR to acute stress and better cognitive function, as well as the other positive health outcomes observed in prior studies, may reflect a more adaptive central nervous system (5). For instance, the prefrontal cortex (PFC), which regulates executive function, also regulates the autonomic nervous system during acute stress (4). Thus, examining the direct relationships between executive function and CVR may shed light on links between cognitive and autonomic regulation.
A potentially important contributor to individual differences in cognitive function is lifestyle behaviors (5, 12). Routine engagement in mentally stimulating activities (MSA), such as playing puzzles, Sudoku, or computer games, which rely on sustained attention and information processing, is a lifestyle behavior considered to protect against cognitive decline in the aging process; accumulated evidence support a positive causal relationship between routinely engaging in MSA and improving cognitive function or slowing cognitive decline (13, 14). Whether MSA relate to acute stress responses is unknown, but plausible. Active engagement in MSA appears to improve executive function by enhancing neuroplasticity in cortical networks (15), including networks of the PFC (16), which, as mentioned, also contributes to regulation of the autonomic nervous system and CVR (17, 18); thus, MSA may be related indirectly to CVR via their effects on central physiological stress regulation. This would suggest concurrent associations among MSA, CVR, and executive function. Further, MSA may serve to protect cognitive function in the context of neurobiological alterations that are typically associated with impaired cognitive performance. For example, mental activities compensated for high white matter lesions in protecting processing speed in aging process (19). Likewise, autonomic regulation may show less covariation with cognitive function, when cognitive function is being protected by MSA. As such, we examined whether regularly engaging in MSA would diminish the association between CVR and executive function in older adults.
In this cross-sectional study, we examined the association between CVR and cognitive function by measuring cardiac activity during acute stressors, i.e. stressful mental tasks, and executive function in older adults, as well as the possible moderating effect of regular engagement in MSA. Cardiac activity at rest and in response to acute stressors was indexed by both time and frequency domain indices derived from the electrocardiogram (ECG). The time domain index was heart rate (HR). Heart rate variability (HRV), a measure of the variation in the time interval between heart contractions, was derived from spectral analysis of the ECG signal to provide frequency domain indices. The high frequency domain of heart rate variability (HF-HRV, 0.15 – 0.5 Hz) indexed primarily parasympathetic (vagal) control of heart rate. Thus, this index provided information about whether heart rate increases (or decreases) were due primarily to withdrawal of (or increased input by) the parasympathetic nervous system. The low frequency domain of HRV (LF-HRV, 0.04 – 0.15 Hz) provided an index of both parasympathetic and sympathetic cardiac control (20). The acute stressors used in the study were a series of acute laboratory-based mental stress tasks including a mental arithmetic task (Math) and a Stroop word-color task (Stroop). Executive function was assessed by five cognitive tests sharing different executive components, and independent of the acute mental stress tasks, allowing for a more comprehensive assessment of executive function (9). We tested the following hypotheses: (1) greater CVR to the acute mental stress tasks is associated with better executive function; and (2) the association between CVR and executive function is moderated by the frequency of engagement in MSA.
Method
Participants
The Survey of Midlife Development in the United States (MIDUS), an on-going nationally representative longitudinal survey dataset was the primary dataset for this study. The baseline data (MIDUS I) collected between 1995 and 1996 focused on socio-demographic and psycho-behavioral assessments from over 7,000 non-institutionalized respondents. These assessments were repeated at a 10-year follow up as MIDUS II projects 1 (survey assessment). Four new categories of assessments were added in MIDUS II: daily diaries (project 2), cognitive function (project 3), biomarkers (project 4), and neuroscience (project 5). All participants who participated in Projects 2 to 5 must have completed Project 1 before the projects. The average lag of data collection was 24 months between Project 1 and Project 4, and 22 months between Project 3 and Project 4. More details describing information between MIDUS I and MIDUS II can be found elsewhere (21).
This study utilized data from MIDUS II projects 1, 3 and 4. There were a total of 1015 participants who participated in Projects 1, 3, and 4. We excluded those with flagged problems (i.e., problematic testing procedure due to test disruption, interview equipment failures, or other problems) in cognitive tests (n = 11), those did not attend the acute stress protocol (n = 33), as well as those aged 54 or younger (n = 486). The final sample for the current study included 487 participants aged 55 to 84 years (see the Appendix Figure).
Appendix Figure.
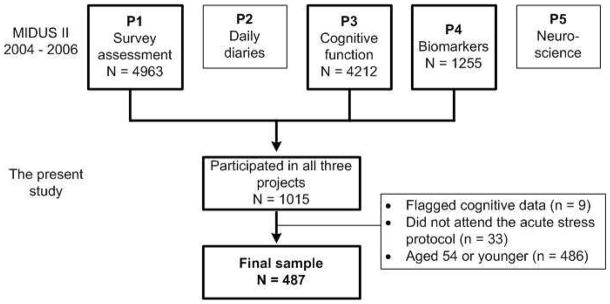
Flow Chart. Note: P = Project
Procedures
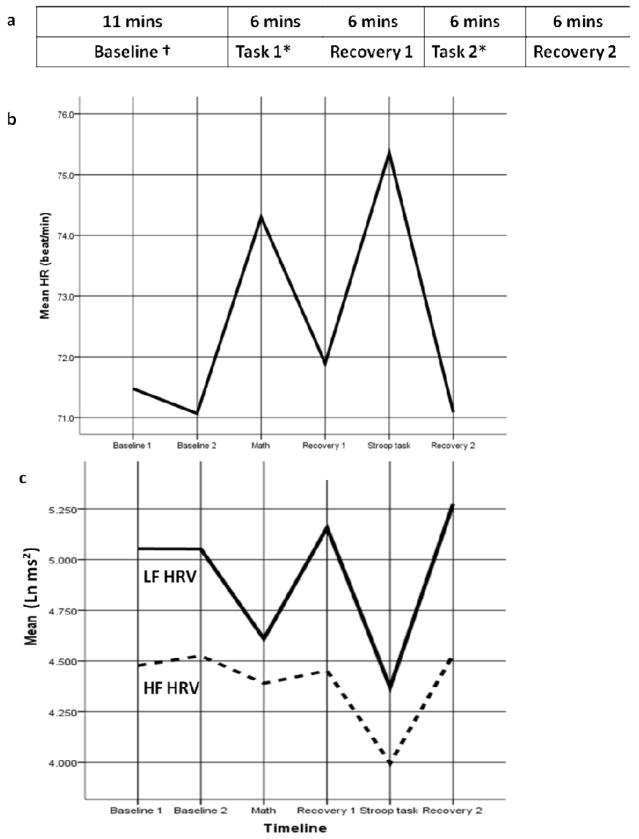
Project 1 was administered over telephone and by mail-in survey and included socio-demographic and psycho-behavioral assessments. Project 3, including a series of cognitive tests, was administered over the telephone. Project 4 included 2-day visits to one of three participating General Clinical Research Centers (GCRCs) located in the West Coast, Midwest, and East Coast. Institutional Review Board approval was obtained at each center, and informed written consent was obtained from all participants. More details on the protocol of Project 4 can be found elsewhere (21). Of relevance to the current study, on Day 1, participants completed a detailed medical history interview with GCRC clinicians as well as self-administrated questionnaires. On Day 2, medication use was assessed, and then participants engaged in the acute stress protocol while cardiovascular measures were recorded. The protocol order (see Figure 1a) was: resting status baseline (11 minutes, including two 5-minute epochs, Baseline 1 and Baseline 2), mental stress task 1 (randomly assigned to Math or Stroop task, 6 minutes), recovery 1 (6 minutes), mental stress task 2 (assigned to the other unfinished task: Math or Stroop task, 6 minutes), and recovery 2 (6 minutes). Participants were instructed to sit quietly during the baseline and recovery phases of the protocol. Among participants who attended the acute stress protocol, 61 participants had missing data for some epochs of interest (that is, missing heart rate data for one of the tasks, or for recovery, etc). A comparison of demographic information between this group and participants with complete data was conducted.
Figure 1.
Cardiac Measurement over the Psychophysiology Experimental Protocol
Note. † There were two sets of baseline variables (i.e., Baseline 1 and Baseline 2) within 11 minutes, each representing a 5 minutes epoch.
*Task 1 and Task 2 could be Math or Stroop task. The sequence of the two mental stress tasks was varied by participants. Regardless, there was a recovery period (6 minutes) between two tasks to avoid the influence of Task 1 on Task 2. In addition, there were 26 participants attending PASAT task instead of Math task (data was not shown). For the purpose of illustration, we recoded data and displayed the graphs (b and c) on HR, LF HRV, and HF HRV based on the type of mental stress task (Math or Stroop task) instead of the order of the task (Task 1 or 2).
Measures
Cardiac measures
HR (beats per minute) and HRV were derived from the ECG, which was collected at rest and during the acute mental tasks in Project 4. To collect ECG, standard ECG electrodes were placed using a standard lead-II electrode configuration (electrodes placed on the participant’s left and right shoulders, and in the left lower quadrant). ECG was continuously monitored during the acute stress protocol (Figure 1a). The beat-to-beat ECG waveforms, in particular, the series of intervals between consecutive R waves, were analyzed to calculate HR and frequency domain of HRV using proprietary event detection software (Gmark by Delano McFarlane). All data were analyzed with a 300-second epoch duration (for “resting status baseline” data, two 300-second epochs were analyzed). If any unscorable data (due to noisy signal) precluded a full 300-section segment, epoch duration was decreased by 60-section segments (i.e., 240 seconds or 180 seconds). The minimum epoch length analyzed was 180 seconds. There were 61 participants whose epoch length was less than 180 seconds, thus their ECG data were not analyzed. A comparison of their background characteristics with the rest sample was provided at the end of the “results” section. HR was determined as an average of all valid RR intervals for the specified length of time described and converted to beats per minute units. The frequency domain of HRV (i.e., LF and HF) was calculated by the spectra of RR interval series using an interval method for computing Fourier transforms (22). Because LF HRV and HF HRV were skewed, natural log transformation was applied prior to any analysis. More information about the standard procedure has been reported previously (23).
Baseline HR, LF- and HF-HRV was computed as the average HR or LF- or HF- HRV across Baseline 1 and 2. HR, LF- and HF- HRV during the Math and Stroop tasks were each calculated as the average measure across each task. HR, HF-HRV, and LF-HRV reactivity scores were calculated as the task average minus the baseline average. Preliminary analyses indicated that the Stroop task elicited greater cardiac reactivity across participants; as such, in analyses we used HR, LF- and HF-HRV reactivity during the Stroop task (see “results” section).
Executive Function
Two sets of neuropsychological tests were conducted over the phone at Project 3: the Brief Test of Adult Cognition by Telephone (BTACT) and the Stop and Go Switch Task (SGST) (24, 25). Details on the rationale for, and psychometric properties of, the BTACT and SGST and the composite scores have been published previously (24, 25). Of relevance to this report, we used a previously validated composite index of executive function derived from reported exploratory and confirmatory factor analyses of the subtests from the BTACT and SGST (12) and used in prior studies of MIDUS participants (26, 27). The executive function factor comprises five standard cognitive measures of multiple cognitive components regulated by executive function (10), including working memory (Digits Backward), verbal fluency (Category Fluency), inductive reasoning (Number Series), and processing speed (Backward Counting) from BTACT and attention switching and inhibitory control from SGST. An average of z-scores for all executive function measures was used in the data analysis, with higher scores indicating better executive function (12).
Engagement in MSA
Participants were asked about their current frequency of engagement of six mental activities as part of the Project 1, including reading, doing word games, playing cards, attending lectures, writing, and using a computer. Each participant indicated the frequency of engaging in these activities using a 6-point ordinal scale ranging from 1 (daily) to 6 (never). The mean score of all items was calculated with lower scores indicating more frequent mental activities. This scale was used in a previous study to examine the association with education and cognitive function (12) as well as cardiovascular risk factors and cognitive function (28). All participants completed Project 1 before participating in Projects 3 and 4, which ensured that their self report on the engagement in MSA occurred prior to the assessments of CVR and executive function.
Demographic and Health Information/Covariates
Demographic information, collected from Project 1, included age, sex, education (from “no education” to “doctoral degree”, grouped into three categories: “high school graduate, GED or less”, “some college”, and “college graduate”), and race (White vs. other racial/ethnic groups).
The following data were collected from Project 4. Depressive symptoms were measured using the Depressive Symptoms subscale from the Mood and Symptom Questionnaire (29). A total of 12 items of depressive symptoms was asked using a question “How much have you felt or experienced things this way during the past week?” Participants responded using a Likert scale from 1 “Not at all” to 5 “Extremely”. A sum score was computed with higher scores indicating more depressive symptoms. Internal consistency of the subscale was .90 in MIDUS II.
Perceived control was measured using a 19-item Self-Control Scale (30). Participants responded using a Likert scale from 1 “Strongly disagree” to 7 “Strongly agree”. A mean score was computed with higher scores indicating higher ability of self control. The internal consistency of the subscale was .71 in MIDUS II.
Data on smoking was collected using a single question, “Have you ever smoked regularly?” Data on alcohol intake was assessed using a single question that asked about the frequency of drinking. Active alcohol intake was defined as drinking 1 or more days/week. Hypertension was measured based on sitting systolic blood pressure ≥ 130 mmHg, or sitting diastolic blood pressure ≥ 85 mmHg, or currently taking antihypertensive treatment. Diabetes was defined as blood hemoglobin A1c ≥7 % or self-reported history in past 12 months (31). Data on heart attack was collected based on a single question of relevant health history. Use of beta-blockers was recorded using the information from the participant’s medication list. The interval (i.e., lag in months) between Project 3 and 4 was collected and controlled in the analysis.
Data Analysis
Analyses were conducted using IBM SPSS 19.0. All analyses were conducted separately for the three reactivity indices. The differences of each variable by the frequency of engagement in MSA were examined using independent t-test for continuous variables and χ2 for categorical variables. The cardiac activities in response to mental stress tasks or baseline status were examined using repeated measures ANOVA with pair-wise comparison using Bonferroni adjustment for multiple comparisons. The associations between CVR measures and executive function, and the moderating effect of engagement in MSA on this association were examined using multiple linear regression. Scores on HR, HF-HRV, and LF-HRV and engagement in MSA were centered. Models were adjusted for demographic and health characteristics along with the corresponding baseline value for HR or HRV; covariates were chosen based on previously reported associations between these variables and cardiovascular activity or cognitive function (6, 9, 32, 33). To further explore the subgroup differences in executive function if there were any interactions of CVR measure × MSA, multiple linear regression and ANCOVA were applied. Statistical significance for Bonferroni adjustment was set at α level of 0.018, while other analyses were using an overall α level of 0.05, two-sided.
Results
Sample Characteristics
Table 1 displays the sample characteristics. The average age of the sample was 65.06. Participants engaged in MSA on average “several times a month.”
Table 1.
Characteristics of Demographic Information and Health History
| Total sample (n = 487) | Engagement in MSA a, b
|
|||||
|---|---|---|---|---|---|---|
| High (n = 214) | Low (n = 266) | t or χ2 test | df | p | ||
| Age, mean (SD) | 65.06 (7.61) | 65.14 (7.31) | 64.95 (7.73) | 0.27 | 478 | .790 |
| Male, n (%) | 226 (46.4%) | 84 (39.3%) | 138 (51.9%) | 7.61 | 1 | .006 |
| White, n (%) | 449 (92.2%) | 200 (93.5%) | 244 (91.7%) | 0.51 | 1 | .475 |
| Education | 25.99 | 2 | < .001 | |||
| • High School graduate, GED or less | 138 (28.3%) | 38 (17.8%) | 99 (37.2%) | |||
| • Some college | 238 (48.9%) | 111 (51.9%) | 122 (45.9%) | |||
| • College graduate | 111 (22.8%) | 65 (30.4%) | 45 (16.9%) | |||
| Depressive symptoms, mean (SD) | 17.21 (5.35) | 17.01 (4.85) | 17.37 (5.78) | −0.72 | 477 | .467 |
| Perceived control, mean (SD) | 4.96 (0.53) | 4.95 (0.51) | 4.98 (0.55) | −0.62 | 478 | .532 |
| Regularly smoking, n (%) | 241 (49.5%) | 98 (45.8%) | 139 (52.3%) | 1.98 | 1 | .259 |
| Alcohol intake, n (%) | 204 (41.9%) | 98 (45.8%) | 103 (38.7%) | 2.44 | 1 | .118 |
| Use of beta blockers, n (%) | 108 (22.2%) | 43 (20.2%) | 64 (24.1%) | 1.02 | 1 | .312 |
| Hypertension, n (%) | 322 (66.1%) | 143 (66.8%) | 177 (66.5%) | 0.03 | 1 | .863 |
| Diabetes, n (%) | 68 (14.0%) | 32 (15.0%) | 34 (12.8%) | 0.47 | 1 | .492 |
| Heart attack, n (%) | 16 (3.3%) | 8 (3.7%) | 8 (3.0%) | 2.79 | 1 | .248 |
| MSA, mean (SD) c | 3.74 (0.86) | 2.97 (0.48) | 4.36 (0.53) | −30.02 | 472 | <.001 |
| Executive function, mean of Z scores (SD) | −0.09 (0.85) | 0.11 (0.79) | −0.26 (0.85) | 4.86 | 478 | <.001 |
Note.
7 cases were not available;
median score 3.50 as the cut-off score.
Lower scores indicated higher frequency.
Bold indicates a significant p value.
The median point 3.50 was used as the cutoff score to categorize the engagement in MSA into high vs. low frequency groups. Those participants engaging in more mental activities tended to be female, and have higher education and better executive function.
Baseline HR and HRV and Reactivity to Stressors
Figure 1a shows the protocol order of the psychophysiological experiment. Figure 1b and 1c displays the HR, LF-HRV, and HF-HRV throughout the protocol. Baseline HR, LF-HRV, and HF-HRV was calculated using relevant average scores of Baseline 1 and 2. HR and HRV measures were significantly different from baseline in response to the mental stress tasks (see Table 2, Repeated ANOVA, Math-Baseline and Stroop-Baseline, all p < .001): HR significantly increased in response to mental stress tasks (Baseline: M = 71.29, SD = 11.11; Math: M = 74.30, SD = 10.83; Stroop: M = 75.36, SD = 11.43; F[2,424] = 313.29) while LF-HRV (Baseline: M = 5.10, SD = 1.12; Math: M = 4.61, SD = 1.12; Stroop: M = 4.37, SD = 1.16; F[2,424] = 172.30) and HF-HRV (Baseline: M = 4.53, SD = 1.25; Math: M = 4.39, SD = 1.18; Stroop: M = 4.00, SD = 1.28; F[2,424] = 124.41) significantly decreased in response to the tasks. The Stroop task induced significantly larger increase in HR (Mean difference = −1.06, SE = 0.14; t[424] = 7.57) and larger decrease in LF-HRV (Mean difference = 0.22, SE = 0.04; t[424] = 5.50) and HF-HRV (Mean difference = 0.38, SE = 0.03; t[424] = 12.67) compared to the Math task (see Table 2, pairwise t test, Math-Stroop, all p < .001). Thus, HR, LF-HRV and HF-HRV reactivity during the Stroop task was used in all subsequent analyses of associations between HR and HRV and executive function.
Table 2.
Descriptive Data of Cardiac Measurement
| Descriptive (Mean and SD) | Baseline ‡ | Math task | Stroop task | Repeated ANOVA*
|
Reactivity§ | |||
|---|---|---|---|---|---|---|---|---|
| Bonferroni adjusted pairwise t test (df = 424) | F (2, 424) | |||||||
| Mean Difference (SE)
| ||||||||
| Math – Baseline | Stroop – Baseline | Math – Stroop | ||||||
| HR | 71.29 (11.11) | 74.30 (10.83) | 75.36 (11.43) | 2.94 (0.16) a | 4.00 (0.19) a | −1.06 (0.14) a | 313.29 a | 2.60 (0.27)† |
| LF HRV | 5.10 (1.12) | 4.61 (1.12) | 4.37 (1.16) | −0.50 (0.04) a | −0.72 (0.04) a | 0.22 (0.04) a | 172.30 a | −0.73 (0.87) |
| HF HRV | 4.53 (1.25) | 4.39 (1.18) | 4.00 (1.28) | −0.16 (0.03) a | −0.53 (0.04) a | 0.38 (0.03) a | 124.41 a | −0.52 (0.79) |
Note.
average score of baseline assessment at two time points;
61 cases were missing;
Reactivity = cardiac Stroop task activity cardiac baseline activity, given that Stroop task induced significantly greater reactivity than Math task did;
the distribution of raw scores was skewed and some scores were negative, thus natural log transformation was performed as ln (Stroop – Baseline + 10);
p < .001.
Table 3 displays the comparison of baseline cardiac measures and CVR by the frequency of engaging in MSA. Baseline LF-HRV (high MSA: M = 5.24, SD = 1.15; low MSA: M = 4.97, SD = 1.09; t[418] = 2.51, p = 0.012), LF-HRV reactivity (high MSA: M = −0.84, SD = 0.87; low MSA: M = −0.62, SD = 0.84; t[418] = −2.58, p = 0.010), and HF-HRV reactivity (high MSA: M = −0.61, SD = 0.80; low MSA: M = −0.44, SD = 0.77; t[418] = −2.24, p = 0.026) were significantly different by the frequency of engaging in MSA. That is, engaging in high MSA was significantly related to higher baseline LF-HRV and greater decrease in LF-HRV and HF-HRV reactivity.
Table 3.
Comparison of Cardiac Measurement by the Frequency of Engagement in MSA†
| Engagement in MSA | Baseline
|
Reactivity
|
||||
|---|---|---|---|---|---|---|
| HR | LF-HRV | HF-HRV | HR | LF-HRV | HF-HRV | |
|
|
|
|||||
| High, mean (SD) | 71.49 (10.76) | 5.24 (1.15) | 4.65 (1.28) | 2.61 (0.27) | −0.84 (0.87) | −0.61 (0.80) |
| Low, mean (SD) | 71.26 (11.40) | 4.97 (1.09) | 4.42 (1.21) | 2.59 (0.28) | −0.62 (0.84) | −0.44 (0.77) |
|
|
|
|||||
| t test (p value)‡ | 0.21 (.944) | 2.51 (.012) | 1.88 (.061) | 0.72 (.473) | −2.58 (.010) | −2.24 (.026) |
Note.
7 cases with missing MSA data and 61 cases were missing.
df = 418. Bold indicates a significant p value.
CVR, Engagement in MSA, and Executive Function
Table 4 displays the regression on executive function. Collinearity tolerance of predictors and covariates was all > 0.95 across all analyses, indicating there was not much redundant information between these predictors and covariates, thus, multicollinearity was not a concern in the analysis. Controlling for all covariates (i.e., age, gender, education, hypertension, diabetes, heart attack, regularly smoking, alcohol intake, perceived control, depressive symptoms, and interval between Projects 3 and 4), none of the baseline cardiac measures was associated with executive function. HR (B[SE] = 0.27 (0.14), t[406] = 2.00, p = 0.047) and LF-HRV reactivity (B[SE] = −0.09 (0.05), t[406] = −2.00, p = 0.046), but not HF-HRV reactivity (B[SE] = −0.05 (0.05), t[406] = −1.08, p = 0.281), were significant predictors of executive function (see Model 1s). HR and LF-HRV explained 1% of the variance in executive function, respectively. When considering the engagement in MSA in the model, MSA significantly predicted executive function in all models (HR: B[SE] = −0.32 (0.04), t[405] = −7.21, p < 0.001; LF-HRV: B[SE] = −0.31 (0.05), t[405] = −6.89, p < 0.001; HF-HRV: B[SE] = −0.31 (0.04), t[405] = −7.05, p < 0.001) (see Model 2s). MSA explained around 9% of the variance in executive function. HR reactivity had a significant interaction with the engagement in MSA in predicting executive function (B[SE] = 0.33 (0.14), t[404] = 2.33, p = 0.020) (see Model 3s). Such interaction explained another 1% of the variance in executive function.
Table 4.
Regression Models of Cardiac Measurement and Engagement in MSA on Executive Function (N = 419)†
| Model 1
|
Model 2
|
Model 3
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B (SE) | t(406) | p | B (SE) | t(405) | p | B(SE) | t(404) | p | |
|
|
|
|
|||||||
| Baseline cardiac activity | 0.002 (0.01) | 0.46 | .646 | 0.003 (0.01) | 0.74 | .391 | 0.003 (0.01) | 0.90 | .369 |
| HR | 0.27 (0.14) | 2.00 | .047 | 0.29 (0.13) | 2.24 | .026 | 0.29 (0.13) | 2.23 | .026 |
| MSA | −0.32 (0.04) | −7.21 | <.001 | −0.32 (0.04) | −7.19 | <.001 | |||
| MSA × HR | 0.33 (0.14) | 2.33 | .020 | ||||||
| R2 = 0.23, F(13, 406) = 9.40, p < .001 | R2 = 0.32, F(14, 405) = 13.53, p < .001 | R2 = 0.33, F(15, 404) = 13.13, p < .001 | |||||||
| Model 1
|
Model 2
|
Model 3
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B(SE) | t(406) | p | B(SE) | t(405) | p | B(SE) | t(404) | p | |
|
|
|
|
|||||||
| Baseline cardiac activity | −0.002 (0.04) | −0.04 | .965 | −0.01 (0.04) | −0.36 | .717 | −0.01 (0.04) | −0.36 | .717 |
| LF-HRV | −0.09 (0.05) | −2.00 | .046 | −0.06 (0.04) | −1.37 | .173 | −0.06 (0.04) | −1.35 | .177 |
| MSA | −0.31 (0.05) | −6.89 | <.001 | −0.31 (0.05) | −6.88 | <.001 | |||
| MSA × LF-HRV | 0.004 (0.09) | 0.10 | .926 | ||||||
| R2 = 0.23, F(13, 406) = 5.35, p < .001 | R2 = 0.31, F(14, 405) = 6.69, p < .001 | R2 = 0.31, F(15, 404) = 6.25, p < .001 | |||||||
| Model 1
|
Model 2
|
Model 3
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| B(SE) | t(406) | p | B(SE) | t(405) | p | B(SE) | t(404) | p | |
|
|
|
|
|||||||
| Baseline cardiac activity | −0.02 (0.03) | −0.59 | .555 | −0.02 (0.03) | −0.72 | .469 | −0.02 (0.03) | −0.74 | .457 |
| HF-HRV | −0.05 (0.05) | −1.08 | .281 | −0.03 (0.05) | −0.73 | .463 | −0.04 (0.05) | −0.78 | .438 |
| MSA | −0.31 (0.04) | −7.05 | <.001 | −0.32 (0.05) | −7.15 | <.001 | |||
| MSA × HF-HRV | −0.07 (0.05) | −1.32 | .187 | ||||||
| R2 = 0.23, F(13, 406) = 5.21, p < .001 | R2 = 0.31, F(14, 405) = 6.66, p < .001 | R2 = 0.31, F(15, 404) = 6.27, p < .001 | |||||||
Note. Controlled for age, gender, education, hypertension, diabetes, heart attack, regularly smoking, alcohol intake, perceived control, depressive symptoms, and interval between Projects 3 and 4.
7 cases with missing MSA data and 61 cases with missing cardiac data were excluded from the analysis.
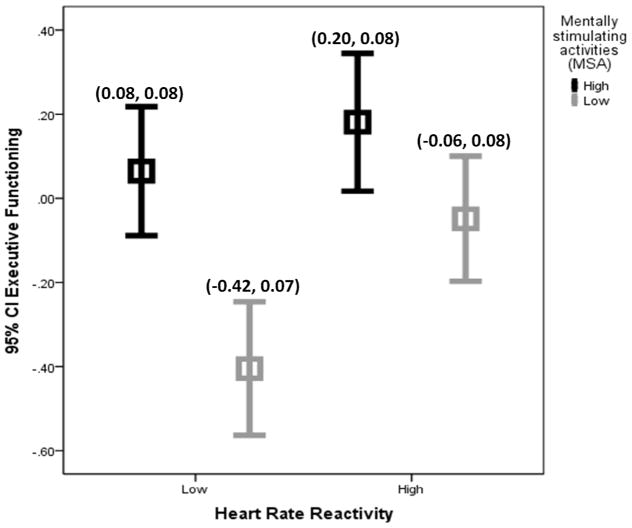
Given the interaction between HR reactivity and the engagement in MSA in predicting executive function, the association of HR reactivity and executive function was further examined by two subgroups of MSA. After controlling for all covariates, the significant relationship between HR reactivity and executive function remained for the low (B[SE] = 0.43 (0.19), t[406] = 2.25, p = 0.030), but not high (B[SE] = 0.06 (0.18), t[406] = 0.33, p = 0.750), MSA group. Figure 2 further illustrates the interaction. HR reactivity was categorized by the median score (M = 2.60) to high vs. low HR reactivity. Among the four subgroups (high MSA/high HR reactivity, high MSA/low HR reactivity, low MSA/high HR reactivity, and low MSA/low HR reactivity), the high MSA/high HR reactivity subgroup had highest adjusted mean for executive function, while the low MSA/low HR reactivity had lowest adjusted mean for executive function.
Figure 2.
Illustration of Interaction between HR Reactivity and the Engagement in MSA in Predicting Executive Function. Note. For the purpose of illustration, HR reactivity was categorized by the median score (M = 2.60) to high vs. low HR reactivity. Adjusted mean and standard error of executive function was reported for each subgroup controlling for age, gender, education, hypertension, diabetes, heart attack, regularly smoking, alcohol intake, perceived control, depressive symptoms, interval between Projects 3 and 4, and baseline HR.
Analysis of Missing Data
Although all participants included in the analysis attended the acute stress protocol, the data on cardiac activities in 61 participants was not recordable. This subset of participants were significantly older than those completed the Stroop task (Mage = 68.21 vs. 64.61, t[60] = −3.50, p = .001). Other demographic and health characteristics and executive function were similar.
Discussion
The present study examined cross-sectional associations between CVR to acute stress and executive function in older adults, and the role of engagement in MSA in these associations. Similar to prior studies of younger- and middle-aged adults, we found that higher HR reactivity was associated with better executive function after controlling for demographic and health variables and baseline HR. The direction of the relationship between HR reactivity and cognitive function in the current study is consistent with two recently published cross-sectional or prospective studies showing greater HR reactivity associated with better memory (6) and reasoning (9). The present study extends these findings to executive function, a broad cognitive construct consisting of a set of complex, partially overlapping cognitive abilities including reasoning, but also extending to working memory, attention, processing speed, response inhibition and problem-solving that, all together, guide self-regulation or goal-oriented behaviors. One strength of this study is that we derived a composite score from a number of comprehensive executive tasks tapping these abilities rather than relying on one dimension exclusively, and these tasks were independent from the acute mental stress task (Stroop) that also relies on some aspects of executive function (e.g., attention and inhibition). To our knowledge, this is the first study to comprehensively examine executive function, and identify significant associations between HR reactivity and executive function. Our data are cross-sectional and therefore cannot clarify causality, but they support investment in future work to identify directional links between executive function and CVR. The top-down role of PFC in regulating both the CVR and executive function is well recognized, which may somewhat explain the link between CVR and executive function (34). However, a bottom-up mechanism is also plausible. Greater CVR may reflect more adaptive endothelial function and metabolic balance, and may promote adequate blood pressure, which are all important for maintaining healthy cerebrovascular function, especially in the frontal lobe (2, 35). The contribution of the cardiovascular system to brain function, and, ultimately, executive function, is less recognized and worthwhile of further exploration.
Notably, higher LF-HRV reactivity was also associated with executive function, although including frequency of MSA in the model rendered this association non-significant. We did not observe an association between HF-HRV reactivity and executive function. LF-HRV is mediated by both sympathetic and vagal mechanisms, whereas, HF-HRV is almost exclusively vagally mediated. In light of our discrepant LF- and HF-HRV findings, the association between LF-HRV changes and executive function may reflect associations between cognitive performance and HRV that was primarily due to sympathetic nervous system control of the heart. Previous studies found a reduced influence of the parasympathetic/vagal system in the regulation of attentional resources as individuals age (34). Further research is needed to characterize the components of autonomic regulation of CVR that may be linked to cognitive function.
An important caveat to the observed relationship between HR reactivity and executive function is the moderating effect of MSA. Specifically, among older adults who more frequently engaged in MSA, the association between lower HR reactivity and poorer executive function was attenuated. On the contrary, among infrequent users of MSA, lower HR reactivity was associated with lower executive function. One intriguing possibility suggested by these findings is that MSA may preserve executive function even when other central regulatory processes, such as those involved in stress adaptation, are compromised. MSA in this study included activities of learning, computer use, playing puzzles, etc. that may create an enriched environment for older adults. Previous studies found that these mental activities compensated for low education (12) or high white matter lesions (19), protecting episodic memory or processing speed. Importantly, in this study, MSA explained a much larger amount of variance in executive function, compared to HRV, suggesting that MSA engagement may be a potentially efficacious intervention to modify the central nervous system and relevant self-regulation or goal-oriented behaviors. As a cross-sectional study, although data on MSA was obtained before executive function and CVR, the lag between the projects in MIDUS was not extensive enough to determine MSA’s causal role in modifying the CVR-executive function association. Future studies should examine the moderating effect of MSA in a prospective design, and address whether frequent engagement in MSA also compensates for changes in central networks, especially PFC, that regulate CVR and cognitive function.
In addition to the moderating effects of MSA on the relationship between HR reactivity and executive function, we observed associations between MSA and HRV indices. Participants who engaged in more MSA tended to have higher baseline LF HRV, and greater decreases in LF HRV and HF HRV reactivity in response to mental stress tasks. The notion that MSA may influence systemic cardiac function through central stress regulation (36) may shed light on the role of neuroplasticity in stress adaptation. Cognitive interventions can improve the structure and function of PFC and relevant cortical networks in groups at risk for cognitive impairment (37, 38). Future cognitive intervention studies may further explore whether such brain changes would alter cardiovascular reactivity to stressors.
In this study, baseline cardiac activity was not associated with executive function. Some investigators report similar non-significant results (6), while others have observed a significant association between higher HRV at rest and better cognitive function (32, 39–41). The differences in results may be explained by the type of participants (e.g., older adults vs. young athletes or sailors), length of epoch time recorded (e.g., 11 minutes vs. 2 to 24 hours), or types or presence of covariates.
Limitations of the present study should be noted. First, we did not account for the potential influence of respiration rate and tidal volume on HF HRV. It is unclear whether respiration rate could contribute to the explanation of the non-significant association between HF HRV and executive function in this study, but future studies should include these variables. Second, another index of frequency domain of HRV, very low frequency (VLF), was not collected as part of the MIDUSII study. VLF HRV at rest has been related to cognitive function (32) and this should also be considered when designing future studies on mental stress and CR. Finally, given the cross-sectional design of the study, the causal relationships between CVR and executive function cannot be determined. Although a previous prospective study supported such a causal relationship (9), there is still a possibility that baseline executive function shapes physiological adaptation to environmental challenges given that CVR to psychological challenge is assumed to be regulated by the central nervous system (20, 42).
Our findings contribute to the growing literature investigating the relationship between physiological stress responses and executive function. Importantly, increasing the frequency of mental activities may not only hold promise for modifying risks of cognitive decline, but may also modify stress regulation in older adults.
Acknowledgments
Funding/Sources of Support:
MIDUS II was supported by from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS (Midlife in the U.S.) investigation. The authors reported no financial or other relationships relevant to the subject of the study.
Note
- CVR
cardiovascular reactivity
- HRV
heart rate variability
- MSA
mentally stimulating activities
- HR
heart rate
- LF-HRV
low frequency heart rate variability
- HF-HRV
high frequency heart rate variability
- PFC
prefrontal cortex
- VLF-HRV
very low frequency heart rate variability
Footnotes
No Disclosures to Report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Taylor AG, Goehler LE, Galper DI, et al. Top-down and bottom-up mechanisms in mind-body medicine: development of an integrative framework for psychophysiological research. Explore (NY) 2012;6:29–41. doi: 10.1016/j.explore.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treiber FA, Kamarck T, Schneiderman N, et al. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychol Bull. 1984;96:435–464. [PubMed] [Google Scholar]
- 4.Carroll D, Lovallo WR, Phillips AC. Are Large Physiological Reactions to Acute Psychological Stress Always Bad for Health? Social and Personality Psychology Compass. 2009;3:725–743. [Google Scholar]
- 5.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2012;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginty AT, Phillips AC, Roseboom TJ, et al. Cardiovascular and cortisol reactions to acute psychological stress and cognitive ability in the Dutch Famine Birth Cohort Study. Psychophysiology. 2012;49:391–400. doi: 10.1111/j.1469-8986.2011.01316.x. [DOI] [PubMed] [Google Scholar]
- 7.Ell SW, Cosley B, McCoy SK. When bad stress goes good: increased threat reactivity predicts improved category learning performance. Psychon Bull Rev. 2011;18:96–102. doi: 10.3758/s13423-010-0018-0. [DOI] [PubMed] [Google Scholar]
- 8.Duschek S, Muckenthaler M, Werner N, et al. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol Psychol. 2009;81:110–117. doi: 10.1016/j.biopsycho.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Ginty AT, Phillips AC, Der G, et al. Heart rate reactivity is associated with future cognitive ability and cognitive change in a large community sample. Int J Psychophysiol. 2011;82:167–174. doi: 10.1016/j.ijpsycho.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Drag LL, Bieliauskas LA. Contemporary review 2009: cognitive aging. J Geriatr Psychiatry Neurol. 2010;23:75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- 11.Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- 12.Lachman ME, Agrigoroaei S, Murphy C, et al. Frequent cognitive activity compensates for education differences in episodic memory. Am J Geriatr Psychiatry. 2011;18:4–10. doi: 10.1097/JGP.0b013e3181ab8b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes TF. Promotion of cognitive health through cognitive activity in the aging population. Aging health. 2011;6:111–121. doi: 10.2217/ahe.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73:854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- 15.Mesulam M. Representation, inference, and transcendent encoding in neurocognitive networks of the human brain. Ann Neurol. 2008;64:367–378. doi: 10.1002/ana.21534. [DOI] [PubMed] [Google Scholar]
- 16.Valenzuela MJ, Matthews FE, Brayne C, et al. Multiple biological pathways link cognitive lifestyle to protection from dementia. Biol Psychiatry. 2012;71:783–791. doi: 10.1016/j.biopsych.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Thayer JF, Ahs F, Fredrikson M, et al. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 19.Saczynski JS, Jonsdottir MK, Sigurdsson S, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63:848–854. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamarck TW, Lovallo WR. Cardiovascular reactivity to psychological challenge: conceptual and measurement considerations. Psychosom Med. 2003;65:9–21. doi: 10.1097/01.psy.0000030390.34416.3e. [DOI] [PubMed] [Google Scholar]
- 21.Dienberg Love G, Seeman TE, Weinstein M, et al. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2011;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events particularly for heart rate variability data. IEEE Trans Biomed Eng. 1984;31:384–387. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- 23.Shcheslavskaya OV, Burg MM, McKinley PS, et al. Heart rate recovery after cognitive challenge is preserved with age. Psychosom Med. 2010;72:128–133. doi: 10.1097/PSY.0b013e3181c94ca0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the Brief Test of Adult Cognition by Telephone. Age Ageing. 2006;35:629–632. doi: 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- 25.Tun PA, Lachman ME. Age differences in reaction time and attention in a national telephone sample of adults: education, sex, and task complexity matter. Dev Psychol. 2008;44:1421–1429. doi: 10.1037/a0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeman TE, Miller-Martinez DM, Stein Merkin S, et al. Histories of social engagement and adult cognition: midlife in the U.S. study. J Gerontol B Psychol Sci Soc Sci. 2010;66 (Suppl 1):i141–152. doi: 10.1093/geronb/gbq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin F, Friedman E, Quinn J, et al. Effect of leisure activities on inflammation and cognitive function in an aging sample. Arch Gerontol Geriatr. 2012;54:e398–404. doi: 10.1016/j.archger.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin F, Friedman E, Quinn J, et al. Effect of Leisure Activities on Inflammation and Cognitive Function in an Aging Sample. Archives of Gerontology and Geriatrics. 2012 doi: 10.1016/j.archger.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 30.Singelis TM. The measurement of independent and interdependent self-construals. Personality and Social Psychology Bulletin. 1994;20:580–591. [Google Scholar]
- 31.Adults EPoDEToHBCi. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 32.Shah AJ, Su S, Veledar E, et al. Is heart rate variability related to memory performance in middle-aged men? Psychosom Med. 2011;73:475–482. doi: 10.1097/PSY.0b013e3182227d6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roepke SK, Grant I. Toward a more complete understanding of the effects of personal mastery on cardiometabolic health. Health Psychol. 2011;30:615–632. doi: 10.1037/a0023480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thayer JF, Hansen AL, Saus-Rose E, et al. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 35.Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66:153–164. doi: 10.1097/01.psy.0000116719.95524.e2. [DOI] [PubMed] [Google Scholar]
- 36.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 37.Carlson MC, Erickson KI, Kramer AF, et al. Evidence for neurocognitive plasticity in at-risk older adults: the experience corps program. J Gerontol A Biol Sci Med Sci. 2009;64:1275–1282. doi: 10.1093/gerona/glp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belleville S, Clement F, Mellah S, et al. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain. 2011;134:1623–1634. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- 39.Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48:263–274. doi: 10.1016/s0167-8760(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 40.Luft CD, Takase E, Darby D. Heart rate variability and cognitive function: effects of physical effort. Biol Psychol. 2009;82:164–168. doi: 10.1016/j.biopsycho.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Kim DH, Lipsitz LA, Ferrucci L, et al. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: Women’s Health and Aging Study I. J Am Geriatr Soc. 2006;54:1751–1757. doi: 10.1111/j.1532-5415.2006.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Ann Behav Med. 2009;37:126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]