Abstract
Background
Both insufficiency and resistance to the actions of the adipocyte-derived hormone leptin promote hunger, increased food intake, and greater body weight. Some studies suggest adults reporting binge eating have increased serum leptin compared to those without binge eating, even after adjusting for the greater adiposity that characterizes binge eaters. Pediatric binge or loss of control (LOC) eating are prospective risk factors for excessive weight gain and may predict development of metabolic abnormalities, but whether LOC eating is associated with higher leptin among children is unknown. We therefore examined leptin and LOC eating in a pediatric cohort.
Methods
A convenience sample of 506 lean and obese youth (7–18y) was recruited from Washington, DC and its suburbs. Serum leptin was collected after an overnight fast. Adiposity was measured by dual-energy x-ray absorptiometry or air displacement plethysmography. LOC eating was assessed by interview methodology.
Results
Leptin was strongly associated with fat mass (r=.79, p<.001). However, even after adjusting for adiposity and other relevant covariates, youth with LOC eating had higher serum leptin compared to those without LOC episodes (15.42±1.05 vs. 12.36±1.04 ng/mL, p<.001). Neither reported amount of food consumed during a recent LOC episode nor number of LOC episodes in the previous month accounted for differences in leptin (ps>.05). The relationship between LOC eating and leptin appeared to be significant for females only (p=0.002).
Conclusions
Reports of LOC eating were associated with higher fasting leptin in youth, beyond the contributions of body weight. Prospective studies are required to elucidate if LOC eating promotes greater leptin or if greater leptin resistance may promote LOC eating.
Keywords: Binge Eating, Leptin, Loss of Control Eating, Hormone Resistance, Adiposity
INTRODUCTION
Leptin is an adipose tissue-derived hormone implicated in body weight regulation.1 Among its manifold functions, leptin acts as a regulatory signal reflecting adipose tissue stores for the central nervous system, and influences energy homeostasis, eating behaviors,2 appetite regulation,3 and energy expenditure.4 Among weight-stable normal weight and obese adults, leptin is strongly positively correlated with body fat mass.5 Following acute energy restriction, sharp declines in serum leptin concentrations are observed.6 Conversely, overeating leads to an increase in serum leptin that precedes any changes in weight.7
States of leptin insufficiency, or resistance to its action, promote hunger, food intake, and body weight gain.8 High serum leptin concentrations have also been found prospectively to predict weight gain and body fat gain among children at high risk for adult obesity, independent of baseline body fat.9 High circulating leptin may be an indicator of increased leptin resistance10 and may act by down-regulating the cellular response to leptin.8
Since leptin influences food intake,5 it may also play a role in the development of disordered eating behaviors. In patients with anorexia nervosa, plasma leptin concentrations are sharply decreased due to low body weight and reduced fat stores.11 These decreases have also been observed among normal weight individuals with bulimia nervosa,12 suggesting that factors other than body fat stores may play a role in leptin concentrations. Among individuals with binge eating disorder (BED), reduced leptin might explain increased sensations of hunger, which could drive such individuals to binge eat6, 13 or to consume more comfort foods.14 However, data are mixed with regard to the relationship between leptin and BED. Some research indicates increased serum leptin among overweight adults with BED compared to weight-matched controls.6, 13, 15 Among healthy, normal weight women, consumption of a large amount of food in one sitting, similar to the overeating involved in a binge episode, resulted in acute alteration of the diurnal pattern of leptin secretion, indicating that eating behaviors may alter leptin dynamics, at least on a momentary basis.16 D'amore and colleagues15 hypothesize that the relationship between severe binge eating and leptin concentration is mediated by lower dietary restraint and greater fat intake, suggesting that leptin may be a sensitive marker of metabolic changes occurring during binge eating episodes. An impaired sensitivity to leptin's action could also be pathogenic and maintain binge eating behavior. Some data, however, suggest that leptin is unrelated to BED.17, 18
While full-syndrome BED is rarely observed in childhood, loss of control (LOC) eating episodes are often reported by youth.19 LOC eating is characterized by eating episodes wherein individuals report an experience of lack of control over their eating, irrespective of the amount of food consumed. LOC eating can be comprised of classic, objective binge episodes (consumption of an objectively large amount of food accompanied by a sense of LOC) or subjective binge episodes (consumption of an amount of food that is not necessarily large, with a sense of LOC). The term `LOC eating' thus refers to both objectively large and subjectively large episodes of binge eating.20 LOC eating is effectively assessed by interview in children as young as 8 years and adolescents.21, 22 Compared with children who do not report LOC, those with LOC have greater adiposity and report increased symptoms of depression and anxiety, higher dietary restraint, more disordered eating attitudes, and lower self-esteem.23 Prospective studies suggest children and adolescents who endorse even infrequent LOC are at risk for gaining excess weight24, 25 and for developing partial or full-syndrome BED.25, 26 LOC with overeating in youth may also predict worsening in some components of the metabolic syndrome.20
No study has examined the association between leptin and LOC eating in youth. In general, the pediatric literature has focused on the relationship between leptin and anorexia nervosa27 or bulimia nervosa.28 The results of a preliminary longitudinal study of overweight pre-adolescent girls (n=20) indicated a significant negative correlation between cognitive restraint measured with the Three-Factor Eating Questionnaire29 and leptin at 6 months, even when controlling for fat mass30 such that girls with lower dietary restraint scores had significantly higher leptin 6 months later. Although dietary restraint is associated with LOC,21, 22 prospective models including both constructs suggest that LOC, but not restraint, may be the more salient variable in the prediction of excess weight gain24 and BED.22
The aim of the present study was to explore the relationships among leptin, adiposity, and LOC eating in a sample of children and adolescents. Based on past findings that leptin tends to be higher among adults with BED compared to weight-matched controls,6, 13, 15 we hypothesized that youth who report LOC would exhibit higher concentrations of serum leptin compared to youth without LOC, after accounting for adiposity. If youth reporting LOC eating also experience dysregulation in appetitive hormones, targeting LOC eating may serve as an important potential point of intervention. As an exploratory aim, we also anticipated that among youth who endorse LOC, those who report objective binge episodes would exhibit higher leptin compared to those who report subjective binge episodes, given increased amounts of fat stores as well as frequent bouts of positive energy balance presumably observed among youth who consume objectively large quantities of food during an LOC episode. Objective binge episodes may be reflective of youth being over-sated more frequently than youth with subjective binge episodes only. Additionally, obese adult females with BED consume significantly more energy and a greater percentage of energy from fat during a laboratory binge meal compared to those who do not report objectively large binge episodes.31 Such comparisons have not been fully examined among youth with objective compared to subjective binge episodes.
METHODS AND MATERIALS
Participants
A convenience sample was assembled from participants in several studies of eating behavior and obesity conducted at the National Institute of Child Health and Human Development (NICHD) and the Uniformed Services University of the Health Sciences (USUHS). Youth between the ages of 7 and 18y who were interviewed using the Eating Disorder Examination and had measures of serum leptin were included. Participants were either non-treatment seeking boys and girls serving as healthy volunteers (ClinicalTrials.gov ID: NCT00320177 (n=198), NCT00631644 (n=71), NCT00001195 (n=10) & NCT00001522 (n=10)), girls participating in an excess weight-gain prevention pilot study with a BMI between the 75th and 97th percentiles (NCT00263536, n=20), girls participating in an excess weight-gain prevention study with a BMI between the 75th and 97th percentiles who also reported at least one episode of LOC eating in the previous month (NCT00680979, n=64), or treatment-seeking Non-Hispanic Black and White adolescents with a BMI at or above the 95th percentile with at least one obesity-related comorbidity such as hypertension or sleep apnea (NCT00001723, n=134). Youth were excluded for major medical issues (other than an obesity-related comorbidity for the treatment-seeking study), major psychiatric conditions, pregnancy, or medications known to affect weight. Individuals who had lost more than 5% of their body weight in the 3 months prior to assessment or who were currently involved in weight loss treatment programs were also excluded.
Participants were recruited through the NIH clinical trials website, local area community flyer postings, posters at physician offices, and direct mailing to homes within a 50-mile radius of Bethesda, Maryland. All studies were approved by the NICHD institutional review board (IRB), and were performed in accordance with ethical guidelines and regulations. NCT00680979 and NCT00631644 were also approved by the USUHS IRB. Written consent and assent were provided by parents and children, respectively.
Procedures
For NCT00320177, NCT00631644, NCT00001195, NCT00263536, NCT00001522, and NCT00001723 potential participants and a parent or guardian were seen at the NIH Hatfield Clinical Research Center. For NCT00680979, interested families participated in two screening assessments at USUHS and the NIH on separate days. All data were collected at screening or baseline visits, prior to the initiation of any treatment. Data for participants were included only if leptin was collected within 6 months of the Eating Disorder Examination.
Measures
Loss of Control (LOC) Eating
The Eating Disorder Examination version 12OD/C.2 (EDE)32 or the EDE adapted for children (children age<14 years)33 was administered by trained interviewers to determine the presence of four types of eating episodes in the month prior to assessment. Participants were categorized as those who: i) endorsed at least one episode of unambiguous overeating with a sense of lack of control over their eating (objective binge episode,); ii) reported LOC, but the amount consumed was ambiguously large, but viewed as excessive by the interviewee (subjective binge episode); iii) reported overeating without LOC (objective overeating); or iv) endorsed no episodes of overeating or LOC (no episodes). For the purposes of the current study, LOC eating was defined by the presence of one or more objective binge episodes and/or subjective binge episodes in the previous month. The EDE for youth ≥ 14y has good inter-rater reliability for all episode types.21 Additionally, the child EDE has demonstrated good inter-rater reliability as well as discriminant validity in eating disorder samples and matched controls ages 6–14y.34 The EDE also generates a restraint subscale that assesses behavioral and cognitive restraint. As described previously,22 the variables generating the restraint subscale are independent of those identifying eating episodes.
Leptin
Serum leptin concentrations were collected during a fasting blood draw between 0800 and 1000 and determined by commercially available assays (Linco, St. Louis, MO or Mayo medical laboratories, New England, USA) that were found not to differ significantly in the relationship between measured value and body fat mass.9 The functional sensitivity for the leptin assay was 0.4–0.5 ng/mL; intra-assay and inter-assay variabilities were <8% and <18%, respectively.
Body Composition
Fasting weight and height were measured, as described previously,35 using calibrated electronic instruments. Height was measured to the nearest 1 mm with a stadiometer that was calibrated against a standard height before each use. Weight was measured to the nearest 0.01 kg with an electronic scale that was calibrated against a standard weight before each measurement. BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2). BMI standard deviation scores (BMI-z) for age and sex were calculated according to the Centers for Disease Control and Prevention 2000 growth charts.36 Body fat mass (kg) was measured either by dual-energy x-ray absorptiometry (DXA) using Hologic QDR-2000 or Hologic QDR 4500A equipment (Hologic, Bedford, MA, USA) or air displacement plethysmography (Bod Pod; Life Measurement Inc., Concord, CA, USA). As in prior studies,37 we adjusted measurements of adiposity to account for known differences between the different assessment techniques.
Pubertal Status
A physical examination performed by an endocrinologist or trained nurse practitioner was used to determine pubertal status. Testicular volume for males and Tanner breast staging for females (by palpation) were used to categorize youth as those in pre-puberty (males: testes, <4 mL; females: Tanner stage 1), early to mid-puberty (males: testes = 4–12 mL; females: Tanner stages 2–3), or late puberty (males: testes, >12 mL; females: Tanner stages 4–5). In cases in which the stage was discordant between the right and left testes/breast, the higher stage was assigned. Testicular volume (mL) was measured by using a set of orchidometer beads as standards,38 and breast development was assigned according to the 5 stages of Tanner.39, 40
Statistical Analysis
All analyses were performed with SPSS 20.0. Procedures advocated by Behrens41 were used to examine study variables to determine whether the assumptions of univariate and multivariate analyses were met. Leptin values were log-transformed to approximate a normal distribution. All other data were screened for problems of outliers, skew, and kurtosis. Outliers (<5% of all data points) were Winsorized to fall 1.5 times the interquartile range below or above the 25th or 75th percentile. This strategy was used because it minimizes the influence of outliers on the characteristics of the distribution, minimally changes the distribution overall, and avoids potential bias associated with the elimination of outliers altogether. This correction did not significantly alter the direction or magnitude of any result.
As we were interested in seeing if the presence of LOC (subjective binge episodes and/or objective binge episodes) compared to no LOC eating (no episode, objective overeating) would contribute to differences in leptin, analysis of covariance (ANCOVA) was used to compare differences in serum leptin. We were also interested in examining whether LOC episode size (objective binge episodes vs. subjective binge episodes) contributes to leptin. Therefore, ANCOVA was used to investigate leptin among the four eating episode groups (objective binge episodes, subjective binge episodes, objective overeating, and no episode). Two-tailed, least squares difference tests were used to follow-up on significant a priori planned comparisons between groups. Covariates in both models included age, race (coded as non-Hispanic white or non-white), sex, socioeconomic status (SES) assessed with the Hollingshead Index,42 fat mass (kg), height (cm), dietary restraint, and treatment-seeking status. As leptin concentrations are altered by puberty (e.g., leptin binding activity increases during pre-puberty and then falls during puberty),43 we also controlled for pubertal status in all analyses. We also considered the sex by LOC interaction, but it did not contribute to the model and was therefore removed. Differences between groups were considered significant when p values were <.05. All tests were two-tailed.
RESULTS
Participants were 338 girls and 168 boys between the ages of 7 and 18y (M±SD 13.77±2.4y). Based on participants' responses to the EDE, 39% (n=196) endorsed at least one episode of LOC eating in the month prior to assessment. Sample demographics based on presence or absence of reported LOC eating are presented in Table 1, and zero-order correlations stratified by LOC status are presented in Table 2 and 3. Among youth with LOC, the majority (50%, n=98) reported subjective binge episodes, 34% (n=67) reported objective binge episodes, and 16% (n=31) reported both subjective binge episodes and objective binge episodes in the past month. The number of reported LOC episodes ranged from 1 to 39 (M±SD 3.7±5.1). The average time between the EDE administration and the fasting blood draw was 21.9 days (SD= 45 days).
Table 1.
Baseline demographic and anthropometric characteristics of youth with and without loss of control eating
| Variable | Loss of Control Eating1 | No Loss of Control Eating | T or χ2 | P-Value |
|---|---|---|---|---|
| n | 195 | 311 | ||
| Age (y)2 | 14.1±2.5 | 13.6±2.5 | 2.53 | 0.01 |
| Sex | 82% female (n=160) | 58% female (n=179) | 31.08 | <0.001 |
| 18% male (n=36) | 42% male (n=132) | |||
| Race (% Non-Hispanic White) | 50% Non-Hispanic White (n=97) | 44% Non-Hispanic White (n=136) | 1.61 | 0.21 |
| 50% Non-White (n=99) | 56% Non-White (n=175) | |||
| SES (median) | 2 | 3 | 6.48 | 0.26 |
| BMI(kg/m2)2,3 | 29.1±8.9 | 29.8±11.9 | 0.78 | 0.43 |
| BMI-z2,4 | 1.58±0.84 | 1.48±1.22 | 1.01 | 0.31 |
| Fat Mass (kg)2,5 | 31.46±18 | 31.10±23 | 0.19 | 0.85 |
LOC= Loss of Control Eating, defined as the presence of at least 1 episode, irrespective of size, in the month prior to assessment on the Eating Disorder Examination.
M ± SD
BMI= Body Mass Index
BMI-z= Body mass index standard deviation score for age and sex, calculated according to the Centers for Disease Control and Prevention 2000 growth charts36.
Fat Mass, as measured by either by dual-energy x-ray absorptiometry (DXA) or air displacement plethysmography (Bod Pod). Measures of adiposity were adjusted to account for known differences between the different assessments37.
Table 2.
Bivariate correlations among leptin and all covariates, for youth reporting LOC eating in the previous month (n=196)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Fasting Leptin | -- | .31** | .66** | .72** | .71** | .28** | .31** | .23** |
| 2. Age | -- | .28** | .07 | .29** | .61** | .15* | .71** | |
| 3. BMI | -- | .85** | .96** | .31** | .19** | .22** | ||
| 4. BMI-z | -- | .80** | .23** | .26** | .10 | |||
| 5. Fat Mass | -- | .38** | .21** | .21** | ||||
| 6. Height | -- | .16* | .59** | |||||
| 7. Dietary Restraint | -- | .16* | ||||||
| 8. Tanner Stage | -- |
Note: Correlations marked with an asterisk (*) were significant at p < .05. Correlations marked with two asterisks (**) were significant at p < .01.
Table 3.
Bivariate correlations among leptin and all covariates, for youth reporting no LOC eating in the previous month (n=311)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1. Fasting Leptin | -- | .34** | .82** | .81** | .83** | .26** | .38** | .29** |
| 2. Age | -- | .42** | .22** | .36** | .76** | .12* | .71** | |
| 3. BMI | -- | .88** | .97** | .38** | .39** | .36** | ||
| 4. BMI-z | -- | .84** | .30** | .39** | .24** | |||
| 5. Fat Mass | -- | .38** | .37** | .29** | ||||
| 6. Height | -- | .06 | .61** | |||||
| 7. Dietary Restraint | -- | .05 | ||||||
| 8. Tanner Stage | -- |
Note: Correlations marked with an asterisk (*) were significant at p < .05. Correlations marked with two asterisks (**) were significant at p < .01.
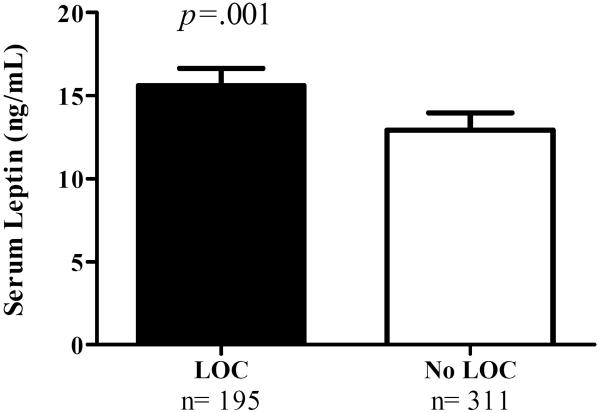
Serum leptin was positively correlated with fat mass (r=0.79, p<0.001). After accounting for adiposity, sex, pubertal status, and all other covariates, there was a main effect of LOC status, such that those reporting at least one episode of LOC in the past month (subjective binge episodes and/or objective binge episodes) had significantly higher leptin (M±SE 15.42±1.05 ng/mL) compared to those reporting no LOC episodes (12.36±1.04 ng/mL, F(1, 440)=13.24, p<0.001, Figure 1).
Figure 1.
Youth with loss of control (LOC) eating had higher fasting serum leptin than youth without LOC eating. Data from ANCOVA adjusted for age, race, sex, SES, fat mass (kg), height (cm), restraint score, pubertal status, and treatment-seeking status (p<0.001). The adjusted mean + standard error of the mean is shown for children with LOC eating (filled bar) and without LOC eating (open bar).
When comparing youth endorsing no episodes, objective overeating, subjective binge episodes only, objective binge episodes only, and both subjective binge episodes/ objective binge episodes, there was a significant main effect of group for serum leptin (F(4, 436)= 5.39, p<0.001). Youth reporting both subjective binge episodes/ objective binge episodes (M±SE=16.67±1.22 ng/mL) as well as youth reporting subjective binge episodes only (16.75±1.07 ng/mL) had significantly higher leptin than youth reporting no episodes (12.74±1.04 ng/mL) or objective overeating (11.30±1.07 ng/mL), adjusting for covariates (ps<0.05). Leptin concentrations of youth reporting objective binge episodes only (13.31±1.08) were less than among youth reporting subjective binge episodes only (p=0.02), but were not significantly different from youth reporting both subjective binge episodes/ objective binge episodes (p=0.10). Neither the number of reported LOC episodes in the previous month (p=0.12) nor the total number of aberrant eating episodes in the previous month (p=0.30) contributed significantly to leptin concentrations.
We conducted an exploratory follow-up analysis to examine whether dietary restraint contributed to differences in serum leptin after accounting for LOC. Accounting for all covariates, both LOC presence and dietary restraint appeared to be positively related to leptin (p=0.001), such that greater dietary restraint was also associated with higher leptin. In this model, dietary restraint contributed 0.57% of the variance of leptin, whereas LOC presence accounted for 1.11% of the variance of leptin. When comparing youth on dietary restraint, there was a significant main effect of group (F(4, 470)= 12.07, p<0.001). Youth reporting both subjective binge episodes/objective binge episodes (M±SE=1.17±0.16) as well as youth reporting objective binge episodes only (.84±0.11) and subjective binge episodes only (1.19±0.09) had significantly higher dietary restraint than youth reporting no episodes (.51±0.06) or objective overeating (.57±0.09), adjusting for covariates (ps<0.05). However, when comparing youth with subjective binge episodes to those with objective binge episodes, dietary restraint did not differ significantly (p=0.10). We also examined whether lower dietary restraint mediated the relationship between LOC eating and leptin. Using the Sobel test for mediation, we found that increased (rather than decreased) dietary restraint was a partial mediator of the relationship between LOC and leptin.
We also conducted an exploratory analysis to examine sex differences in the relationship between LOC and fasting leptin. Females displayed significantly higher fasting leptin (M±SE= 16.63±1.03 ng/mL) than males (9.77±1.05 ng/mL, p<0.001). Among males (n=155), after controlling for all relevant covariates, there were no differences in leptin between those with (M±SE= 8.57±1.09) and without LOC (8.22±1.05, F(1,145)=0.166, p=0.68). By contrast, among females (n=295), youth with LOC (19.50±1.05) had significantly higher leptin concentrations compared to those without LOC (15.49±1.05, F(1,285)=9.59, p=0.002). As there were two different techniques used to assess fat mass (n=403 were measured with DEXA and n= 104 were measured with Bod Pod), we conducted analyses where we co-varied for fat mass technique in our analyses examining the main effect of LOC status on leptin. The results remained significant (F(1,471)=8.12, p=0.005).
DISCUSSION
In a sample of children and adolescents of various ages and weight strata, we found LOC eating to be associated with higher fasting serum leptin, after accounting for the contribution of body composition, pubertal status, and demographic variables. The reported number of LOC episodes in the previous month did not significantly account for the observed differences in leptin concentrations.
Findings from the present study are in accord with three adult studies reporting higher leptin in overweight adults with BED.6, 13, 15 One explanation, as put forth by several investigators,6, 16 is that higher serum concentrations of leptin among individuals with LOC may reflect a transient positive energy balance experienced during an LOC eating episode. As Monteleone and colleagues13 suggest, persistent compensatory hyperleptinemia may occur for individuals with LOC as part of the counter-regulatory response to the excessive energy intake of an LOC eating episode. Alternatively, the association between LOC and leptin in youth may indicate resistance to leptin's actions. This explanation would be consistent with the presence of defects in proximal leptin signaling that could potentially be a cause for LOC eating.
Theoretical models suggest that LOC eating may be a means of coping with negative affective states.44 LOC eating is maintained and reinforced by stimulating the release of opioids, as well as dopamine, which increase the reward value of food.45 While leptin is primarily responsible for modulating energy homeostasis, it has also been implicated in the regulation of food-related reward mechanisms.46 Insulin and leptin can decrease food reward behaviors and modulate the function of neurotransmitter systems and neural circuitry that mediate food reward. Figlewicz and Benoit50 suggest that insulin and leptin affect the reward circuitry bimodally, such that low concentrations of both sustain dopaminergic activity and contribute to the rewarding value of food, intermediate levels suppress reward function, and elevated levels lead to impaired dopaminergic function. Therefore, affective theories might offer an explanation of links between obesity and eating disorders.
Interestingly, the reported size and frequency of LOC episodes in the previous month did not account for the relationship between LOC and leptin. In fact, children reporting subjective binge episodes had higher serum leptin than those reporting objectively large binge episodes. This finding calls into question the hypothesis that frequent bouts of positive energy balance account for differences in leptin concentrations. For youth, the size of an LOC episode may not be as critical as its macronutrient content, for the prediction of leptin concentrations. Studies suggest few differences between youth who describe LOC episodes characterized by the consumption of an “unambiguously large” amount of food versus an amount of food not considered unambiguously large, with respect to weight concerns, depressive symptoms, and disordered eating attitudes.47, 48 Additionally, some studies find that youth who report LOC do not necessarily consume more calories during a test meal than youth who do not report LOC, nor do they self-report consuming more calories during an interview.49 Rather, youth with reported LOC may consume meals characterized by consumption of a greater proportion of calories from carbohydrates, including snacks and desserts, and less from protein.49 The consumption of more sweets and snacks, as well as foods higher in fat content, could potentially contribute to increased leptin secretion.
Our finding of a sex dichotomy in leptin, even after controlling for fat mass, is consistent with the adult and child literature, and may be suggestive of differences in body composition between men and women. Serum leptin has been found to be strongly correlated with the degree of obesity and female sex, and it is hypothesized that testosterone may possess an inhibitory effect on adipocyte ob gene transcription.50, 51 Among children and adolescents, obese girls have significantly higher leptin concentrations than boys at the same degree of adiposity.52
Low levels of reported dietary restraint have previously been hypothesized as one mechanism to explain the relationship between LOC and leptin.15, 30 Additionally, restraint theory53 proposes that binge and LOC eating occur in response to excessive dietary restraint. In contrast to prior research,15, 30 we found higher levels of dietary restraint were associated with serum leptin, after accounting for LOC status and adiposity. In fact, higher levels of dietary restraint appeared to partially mediate the relationship between LOC and leptin. Unlike prior studies using self-report questionnaires which primarily assess dietary restraint,15, 30 the current study employed the EDE restraint subscale, which assesses both cognitive and behavioral restraint. High EDE restraint scores identify youth who are trying to restrict their intake whether or not they succeed. Thus those with high EDE restraint may be youth with disinhibited eating as opposed to successful restrictors. While dietary restraint is associated with LOC in youth,23,22 prospective models including both constructs suggest that LOC, but not restraint, may be the more salient variable in the prediction of excess weight gain24 and development of BED.54
Strengths of the current study include the large sample size, use of a well-validated, interview measure of LOC eating and direct estimation of fat mass using criterion methods as opposed to relying on BMI as the metric for adiposity. However, the sample was not recruited in an unbiased fashion; therefore results may not be generalizable to all children. Although we controlled for differences across the pooled studies (for example, modeling treatment-seeking status in the analysis), there are limitations associated with combining diverse cohorts. The cross-sectional relationship between LOC and adiposity (BMI, BMI-z, fat mass) differs based upon the sample being studied. Typically, in treatment seeking samples, no difference is observed among those with and without LOC.21, 55 By contrast, among non-treatment seeking samples, children with LOC tend to be heavier than those without LOC.22,56 The present sample includes a mix of treatment-seeking youth, non-treatment seekers, and prevention seeking youth (an understudied group). Therefore, it was unclear whether or not children with LOC would be heavier than those without LOC eating. However, all available longitudinal studies have demonstrated that LOC eating predicts excess weight gain.24,25, 57, 58
Another limitation is that the study's cross-sectional design does not provide information regarding the directionality of the relationship between leptin and LOC eating. A further limitation is that we used a single fasting blood measure of leptin as an indicator of tonic levels of leptin. However, leptin patterns are variable and change acutely in response to factors such as meals and fat stores.59 Additionally, we did not examine leptin-binding protein, which may be important to obtain a more accurate reflection of circulating free leptin concentrations.60 nor did we collect post-LOC episode leptin data, which might provide important insight into the relationship between leptin and binge eating behaviors.
We conclude that reported LOC eating may be uniquely related to fasting serum leptin in youth, beyond the contributions of body adipose tissue and dietary restraint. Future prospective studies that examine alterations in food regulation pathways among youth who report LOC are needed to fully understand these relationships in youth.
ACKNOWLEDGEMENTS
This research was supported by National Research Service Award 1F32HD056762 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (to L.B.S.), Uniformed Services University of the Health Sciences grant R072IC (to M.T.K.), National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant 5R01DK080906-04 (to M.T.K.) and Intramural Research Program Grant 1ZIAHD000641 from the NICHD, with supplemental funding from the Division of Nutrition Research Coordination, the National Institute of Minority Health and Health Disparities, and the Office of Behavioral and Social Sciences Research (to J.A.Y.). J.A.Y. is a Commissioned Officer in the U.S. Public Health Service, Department of Health and Human Services. The funding organizations played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of data; or the preparation or review of the manuscript. Drs. Yanovski and Tanofsky-Kraff take full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
National Institutes of Health ClinicalTrials.gov IDs: NCT00001522, NCT00001195, NCT00001723, NCT00320177, NCT00631644, NCT00263536, NCT00680979
Research Support: National Research Service Award 1F32HD056762 (NICHD) (to L.B.S.), Uniformed Services University of the Health Sciences grant R072IC (to M.T.K.), National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) grant R01DK080906-04 (to M.T.K.) and Intramural Research Program Grant 1ZIAHD000641 from the NICHD
Footnotes
CONFLICT OF INTEREST For all authors, no potential biomedical conflicts of interest, financial or otherwise, relevant to this article were reported (R.M., M.T.K., L.B.S., S.E.F., L.H., S.R., M.M., N.S., S.M.B., T.C., J.C.R., S.Z.Y., and J.A.Y.).
REFERENCES
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Wang Q, Bing C, Al-Barazanji K, Mossakowaska DE, Wang XM, McBay DL, et al. Interactions between leptin and hypothalamic neuropeptide Y neurons in the control of food intake and energy homeostasis in the rat. Diabetes. 1997;46(3):335–41. doi: 10.2337/diab.46.3.335. [DOI] [PubMed] [Google Scholar]
- 3.McDuffie JR, Riggs PA, Calis KA, Freedman RJ, Oral EA, DePaoli AM, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. The Journal of clinical endocrinology and metabolism. 2004;89(9):4258–63. doi: 10.1210/jc.2003-031868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–89. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr Rev. 2002;60(10 Pt 2):S1–14. doi: 10.1301/002966402320634878. discussion S68–84, 85–7. [DOI] [PubMed] [Google Scholar]
- 6.Adami GF, Campostano A, Cella F, Scopinaro N. Serum leptin concentration in obese patients with binge eating disorder. Int J Obes Relat Metab Disord. 2002;26(8):1125–8. doi: 10.1038/sj.ijo.0802010. [DOI] [PubMed] [Google Scholar]
- 7.Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. The Journal of clinical endocrinology and metabolism. 1996;81(11):4162–5. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- 8.Knight ZA, Hannan KS, Greenberg ML, Friedman JM. Hyperleptinemia is required for the development of leptin resistance. PloS one. 2010;5(6):e11376. doi: 10.1371/journal.pone.0011376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleisch AF, Agarwal N, Roberts MD, Han JC, Theim KR, Vexler A, et al. Influence of serum leptin on weight and body fat growth in children at high risk for adult obesity. The Journal of clinical endocrinology and metabolism. 2007;92(3):948–54. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuillan PP, Ng D, Han JC, Sapp JC, Wetsch K, Spaulding E, et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. The Journal of clinical endocrinology and metabolism. 2011;96(3):E528–35. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp W, Blum WF, Ziegler A, Mathiak K, Lubbert H, Herpertz S, et al. Serum leptin and body weight in females with anorexia and bulimia nervosa. Horm Metab Res. 1998;30(5):272–5. doi: 10.1055/s-2007-978882. [DOI] [PubMed] [Google Scholar]
- 12.Jimerson DC, Wolfe BE, Carroll DP, Keel PK. Psychobiology of purging disorder: reduction in circulating leptin levels in purging disorder in comparison with controls. The International journal of eating disorders. 2010;43(7):584–8. doi: 10.1002/eat.20738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monteleone P, Di Lieto A, Tortorella A, Longobardi N, Maj M. Circulating leptin in patients with anorexia nervosa, bulimia nervosa or binge-eating disorder: relationship to body weight, eating patterns, psychopathology and endocrine changes. Psychiatry research. 2000;94(2):121–9. doi: 10.1016/s0165-1781(00)00144-x. [DOI] [PubMed] [Google Scholar]
- 14.Tomiyama AJ, Schamarek I, Lustig RH, Kirschbaum C, Puterman E, Havel PJ, et al. Leptin concentrations in response to acute stress predict subsequent intake of comfort foods. Physiology & behavior. 2012;107(1):34–9. doi: 10.1016/j.physbeh.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d'Amore A, Massignan C, Montera P, Moles A, De Lorenzo A, Scucchi S. Relationship between dietary restraint, binge eating, and leptin in obese women. Int J Obes Relat Metab Disord. 2001;25(3):373–7. doi: 10.1038/sj.ijo.0801565. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AE, Hubbard J, Anderson EJ. Impact of binge eating on metabolic and leptin dynamics in normal young women. The Journal of clinical endocrinology and metabolism. 1999;84(2):428–34. doi: 10.1210/jcem.84.2.5502. [DOI] [PubMed] [Google Scholar]
- 17.Geliebter A, Yahav EK, Gluck ME, Hashim SA. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiology & behavior. 2004;81(5):735–40. doi: 10.1016/j.physbeh.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Karhunen L, Haffner S, Lappalainen R, Turpeinen A, Miettinen H, Uusitupa M. Serum leptin and short-term regulation of eating in obese women. Clin Sci (Lond) 1997;92(6):573–8. doi: 10.1042/cs0920573. [DOI] [PubMed] [Google Scholar]
- 19.Tanofsky-Kraff M, Marcus MD, Yanovski SZ, Yanovski JA. Loss of control eating disorder in children age 12 years and younger: proposed research criteria. Eat Behav. 2008;9(3):360–5. doi: 10.1016/j.eatbeh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanofsky-Kraff M, Shomaker LB, Stern EA, Miller R, Sebring N, Dellavalle D, et al. Children's binge eating and development of metabolic syndrome. International journal of obesity. 2012;36(7):956–62. doi: 10.1038/ijo.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasofer DR, Tanofsky-Kraff M, Eddy KT, Yanovski SZ, Theim KR, Mirch MC, et al. Binge eating in overweight treatment-seeking adolescents. Journal of pediatric psychology. 2007;32(1):95–105. doi: 10.1093/jpepsy/jsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanofsky-Kraff M, Yanovski SZ, Wilfley DE, Marmarosh C, Morgan CM, Yanovski JA. Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. Journal of consulting and clinical psychology. 2004;72(1):53–61. doi: 10.1037/0022-006X.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanofsky-Kraff M, Faden D, Yanovski SZ, Wilfley DE, Yanovski JA. The perceived onset of dieting and loss of control eating behaviors in overweight children. Int J Eat Disord. 2005;38(2):112–22. doi: 10.1002/eat.20158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. Int J Eat Disord. 2009;42(1):26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field AE, Austin SB, Taylor CB, Malspeis S, Rosner B, Rockett HR, et al. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics. 2003;112(4):900–6. doi: 10.1542/peds.112.4.900. [DOI] [PubMed] [Google Scholar]
- 26.Tanofsky-Kraff M, Cohen, Yanovski SZ, Cox C, Theim KR, Keil M, et al. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117(4):1203–9. doi: 10.1542/peds.2005-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munoz MT, Argente J. Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances. Eur J Endocrinol. 2002;147(3):275–86. doi: 10.1530/eje.0.1470275. [DOI] [PubMed] [Google Scholar]
- 28.Jimerson DC, Mantzoros C, Wolfe BE, Metzger ED. Decreased serum leptin in bulimia nervosa. The Journal of clinical endocrinology and metabolism. 2000;85(12):4511–4. doi: 10.1210/jcem.85.12.7051. [DOI] [PubMed] [Google Scholar]
- 29.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 30.Laessle RG, Wurmser H, Pirke KM. Restrained eating and leptin levels in overweight preadolescent girls. Physiology & behavior. 2000;70(1–2):45–7. doi: 10.1016/s0031-9384(00)00243-2. [DOI] [PubMed] [Google Scholar]
- 31.Yanovski SZ, Leet M, Yanovski JA, Flood M, Gold PW, Kissileff HR, et al. Food selection and intake of obese women with binge-eating disorder. Am J Clin Nutr. 1992;56(6):975–80. doi: 10.1093/ajcn/56.6.975. [DOI] [PubMed] [Google Scholar]
- 32.Fairburn CG, Cooper Z. The Eating Disorders Examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, & Treatment. 12th edn Guilford; New York, NY: 1993. pp. 317–360. [Google Scholar]
- 33.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: a pilot study. Int J Eat Disord. 1996;19(4):391–7. doi: 10.1002/(SICI)1098-108X(199605)19:4<391::AID-EAT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 34.Christie D, Watkins B, Lask BD. Assessment. In: Lask B, Bryant-Waugh R, editors. Anorexia nervosa and related eating disorders in childhood and adolescence. 2nd edn Psychology Press; Hove, East Sussex, UK: 2000. pp. 105–125. [Google Scholar]
- 35.Nicholson JC, McDuffie JR, Bonat SH, Russell DL, Boyce KA, McCann S, et al. Estimation of body fatness by air displacement plethysmography in African American and white children. Pediatric research. 2001;50(4):467–73. doi: 10.1203/00006450-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Advance data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 37.Robotham DR, Schoeller DA, Mercado AB, Mirch MC, Theim KR, Reynolds JC, et al. Estimates of body fat in children by Hologic QDR-2000 and QDR-4500A dual-energy X-ray absorptiometers compared with deuterium dilution. Journal of pediatric gastroenterology and nutrition. 2006;42(3):331–5. doi: 10.1097/01.mpg.0000189373.31697.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanner JM. Growth and maturation during adolescence. Nutrition reviews. 1981;39(2):43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 39.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of disease in childhood. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of disease in childhood. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Behrens JT. Principles and procedures of exploratory data analysis. Psychological Methods. 1997;2(2):131–160. [Google Scholar]
- 42.Hollingshead AB. Hollingshead two factor index of social position (1957) In: Miller DC, editor. Handbook of research design and social measurement. 5th edn Sage Publications; Newbury Park, CA: 1991. pp. 351–359. [Google Scholar]
- 43.Quinton ND, Smith RF, Clayton PE, Gill MS, Shalet S, Justice SK, et al. Leptin binding activity changes with age: the link between leptin and puberty. The Journal of clinical endocrinology and metabolism. 1999;84(7):2336–41. doi: 10.1210/jcem.84.7.5834. [DOI] [PubMed] [Google Scholar]
- 44.Arnow B, Kenardy J, Agras WS. Binge eating among the obese: a descriptive study. J Behav Med. 1992;15(2):155–70. doi: 10.1007/BF00848323. [DOI] [PubMed] [Google Scholar]
- 45.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Figlewicz DP, Benoit SC. Insulin, leptin, and food reward: update 2008. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;296(1):R9–R19. doi: 10.1152/ajpregu.90725.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldschmidt AB, Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Yanovski SZ, et al. Subtyping children and adolescents with loss of control eating by negative affect and dietary restraint. Behav Res Ther. 2008;46(7):777–87. doi: 10.1016/j.brat.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shomaker LB, Tanofsky-Kraff M, Elliott C, Wolkoff LE, Columbo KM, Ranzenhofer LM, et al. Salience of loss of control for pediatric binge episodes: does size really matter? Int J Eat Disord. 2010;43(8):707–16. doi: 10.1002/eat.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, et al. Laboratory assessment of the food intake of children and adolescents with loss of control eating. The American journal of clinical nutrition. 2009;89(3):738–45. doi: 10.3945/ajcn.2008.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hickey MS, Israel RG, Gardiner SN, Considine RV, McCammon MR, Tyndall GL, et al. Gender differences in serum leptin levels in humans. Biochemical and molecular medicine. 1996;59(1):1–6. doi: 10.1006/bmme.1996.0056. [DOI] [PubMed] [Google Scholar]
- 51.Vettor R, De Pergola G, Pagano C, Englaro P, Laudadio E, Giorgino F, et al. Gender differences in serum leptin in obese people: relationships with testosterone, body fat distribution and insulin sensitivity. European journal of clinical investigation. 1997;27(12):1016–24. doi: 10.1046/j.1365-2362.1997.2270773.x. [DOI] [PubMed] [Google Scholar]
- 52.Wabitsch M, Blum WF, Muche R, Braun M, Hube F, Rascher W, et al. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. The Journal of clinical investigation. 1997;100(4):808–13. doi: 10.1172/JCI119595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herman CP, Polivy J. Anxiety, restraint, and eating behavior. J Abnorm Psychol. 1975;84(6):66–72. [PubMed] [Google Scholar]
- 54.Tanofsky-Kraff M, Shomaker LB, Olsen C, Roza CA, Wolkoff LE, Columbo KM, et al. A prospective study of pediatric loss of control eating and psychological outcomes. J Abnorm Psychol. 2011;120(1):108–18. doi: 10.1037/a0021406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Decaluwé V, Braet C. Prevalence of binge-eating disorder in obese children and adolescents seeking weight-loss treatment. International Journal of Obesity. 2003;27(3):404–409. doi: 10.1038/sj.ijo.0802233. [DOI] [PubMed] [Google Scholar]
- 56.Croll J, Neumark-Sztainer D, Story M, Ireland M. Prevalence and risk and protective factors related to disordered eating behaviors among adolescents: relationship to gender and ethnicity. The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 2002;31(2):166–75. doi: 10.1016/s1054-139x(02)00368-3. [DOI] [PubMed] [Google Scholar]
- 57.Stice E, Cameron RP, Killen JD, Hayward C, Taylor CB. Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. J Consult Clin Psychol. 1999;67(6):967–74. doi: 10.1037//0022-006x.67.6.967. [DOI] [PubMed] [Google Scholar]
- 58.Field AE, Javaras KM, Aneja P, Kitos N, Camargo CA, Jr., Taylor CB, et al. Family, peer, and media predictors of becoming eating disordered. Archives of pediatrics & adolescent medicine. 2008;162(6):574–9. doi: 10.1001/archpedi.162.6.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chapelot D, Aubert R, Marmonier C, Chabert M, Louis-Sylvestre J. An endocrine and metabolic definition of the intermeal interval in humans: evidence for a role of leptin on the prandial pattern through fatty acid disposal. Am J Clin Nutr. 2000;72(2):421–31. doi: 10.1093/ajcn/72.2.421. [DOI] [PubMed] [Google Scholar]
- 60.Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem. 2001;276(9):6343–9. doi: 10.1074/jbc.M009795200. [DOI] [PubMed] [Google Scholar]