Abstract
Posttranslational modifications (PTMs) of protein embedded arginines are increasingly being recognized as playing an important role in both prokaryotic and eukaryotic biology, and it is now clear that these PTMs modulate a number of cellular processes including DNA binding, gene transcription, protein-protein interactions, immune system activation, and proteolysis. There are currently four known enzymatic PTMs of arginine ( i.e., citrullination, methylation, phosphorylation, ADP-ribosylation), and two non-enzymatic PTMs (i.e., carbonylation, advanced glycation end-products (AGEs)). Enzymatic modification of arginine is tightly controlled during normal cellular function, and can be drastically altered in response to various second messengers and in different disease states. Non-enzymatic arginine modifications are associated with a loss of metabolite regulation during normal human aging. This abnormally large number of modifications to a single amino acid creates a diverse set of structural perturbations that can lead to altered biological responses. While the biological role of methylation has been the most extensively characterized of the arginine PTMs, recent advances have shown that the once obscure modification known as citrullination is involved in the onset and progression of inflammatory diseases and cancer. This review will highlight the reported arginine PTMs and their methods of detection, with a focus on new chemical methods to detect protein citrullination.
Introduction
The state of a cell is determined by external and internal signals that allow adaptations to complex environments. These stresses help to regulate normal cellular processes through the induction of PTMs, which induce or inhibit cell signaling pathways that ultimately determine the fate of the cell. Among the more than 200 known PTMs, arginine modifications (Figure 1) have emerged as important PTMs that impact multiple cellular processes including cell growth, division, gene transcription, kinase signaling, proteolysis, and DNA/RNA binding. The fact that arginine modifications can effect so many different cellular processes is unsurprising because this residue is structurally unique in that the guanidinium is both positively charged and can form extended hydrogen bonding networks with both proteins and nucleic acids.
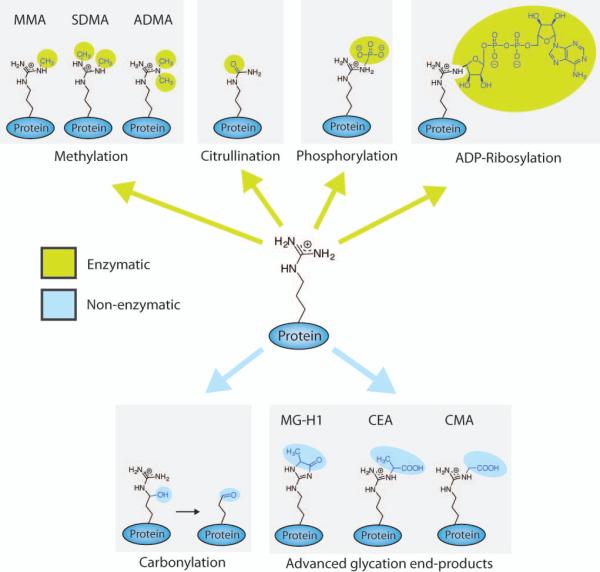
Figure 1.
Posttranslational modifications (PTMs) of arginine that occur within proteins and have been detected in vivo. MMA = Monomethylarginine, SDMA = Symmetric dimethylarginine, ADMA = Asymmetric dimethylarginine, MG-H1 = 5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine, CEA = Carboxyethylarginine, CMA = Carboxymethylarginine.
Of the known arginine PTMs, four occur enzymatically (i.e., methylation, citrullination, phosphorylation, and ADP-ribosylation) and two occur non-enzymatically (i.e., carbonylation and advanced glycation end-products). While most specific arginine residues in proteins have only been shown to be modified by one PTM, histones show multiple examples where the same arginine residue is subject to both methylation and citrullination, and it is known that these two modifications antagonize each other, leading to alterations in gene transcription.1-4 This type of crosstalk is likely to exist for all of the enzymatic and non-enzymatic PTMs, and given the importance of arginine, it should be clear how dysregulation of one of these pathways could contribute to the onset and progression of human disease.5
Given that our understanding of arginine PTMs has been hindered by a lack of robust and selective detection methods to study their role in human health and disease, below we highlight several recently described chemical probes that can be used to characterize arginine PTMs, focusing in particular on protein citrullination. We also describe methods to detect the other enzymatic and non-enzymatic PTMs, with the hope that the successful development of citrulline specific probes will inspire the development of new classes of tools focused on identifying and elucidating the role of the other arginine modifications.
Biological role of arginine citrullination
Citrullination, which is also termed deimination, is a reaction that converts the guanidinium group of arginine to a ureido group, resulting in the loss of both positive charge and two potential hydrogen bond donors (Figure 1). This reaction, which is catalyzed by the protein arginine deiminases (PADs) (i.e., PAD1, 2, 3, 4, 6),5 is an irreversible reaction (there is no known ‘decitrullinase’) that can antagonize methylation of the same arginine residue. Methyl-arginines are poor PAD substrates, with rates that are 150- to 1,000-fold slower than for an unmodified arginine; thus, unmodified arginines are the physiologically relevant substrates of the PADs.1,2,4,6,7
The PADs have gained significant interest over the past decade due to their role in eukaryotic gene regulation and involvement in human disease, particularly inflammatory diseases and cancer.5,8 Interest in the PADs is likely to accelerate, especially with the recent demonstration that the PAD inhibitor Cl-amidine, developed by the Thompson lab, as well as the closely related compounds 2-chloroacetamidine and YW3-56, show efficacy in multiple animal models of human disease, including rheumatoid arthritis,9 ulcerative colitis and Crohn’s disease,10 spinal cord injury,11 cancer,12-14 and multiple sclerosis.15
Detection of peptidyl-citrulline
Though aberrant PAD activity and protein citrullination have been linked to many human diseases, elucidating the exact role of this PTM is human cell signaling remains a challenge, especially since it has been difficult to identify novel PAD substrates. For example, unlike other PTMs, the ureido group does not provide a chemoselective handle that can be used to isolate and enrich for citrullinated proteins, as is the case with phosphorylated proteins. Furthermore, the small 1 Dalton mass increase that occurs upon citrullination is difficult to disambiguate from other hydrolytic PTMs (e.g. glutamine hydrolysis), which makes the detection of citrullinated proteins by MS and MS/MS difficult. Moreover, the fact that citrullination is a hydrolytic reaction prevents the use of alternative substrates that contain bioorthogonal handles (e.g., azides, alkynes, and fluorophores) that can be used in place of the normal substrate to detect and isolate posttranslationally modified proteins. An excellent example of the latter approach is the demonstration that the AMPylator VopS can use alkyne or fluorescein derivatized ATP analogues in place of ATP to facilitate the detection/isolation of AMPylated proteins.16,17
To overcome these challenges, we have focused on creating chemical tools to detect citrullination that are sensitive and allow the addition of functional handles for analysis. We will describe these probes, as well as several other chemical biology methods that have been developed for peptidyl-citrulline detection.
Detection of citrulline by the COLDER assay
Classically, the color development reagent (COLDER) assay is the most common method used for detecting citrulline levels, and we and others have used this assay extensively to study the citrullination of small molecule arginine mimetics, peptides, and even proteins.18-20,21,22 For this assay, citrulline levels are quantified by reacting the modified substrate with 2,3-butanedione monoxime and thiosemicarbazide (TSC) in the presence of NH4Fe(SO4)2 under strongly acidic conditions.4,19 The ureido group reacts with 2,3-butanedione monoxime to form an imidazolone that is stabilized by TSC, while Fe3+ facilitates rapid color development at 95° C. While this assay is reasonably fast and cost effective, it is an endpoint assay and the relatively high limit of detection (0.6 nM) limits its utility for quantifying the citrullination of less concentrated protein substrates. Additionally, this method cannot be use to visualize protein citrullination. Regardless of its limitations, this assay remains important for studying in vitro PAD activity.
Antibody based detection of chemically modified citrulline
A critical advance in the PAD field was the development of an antibody-based approach to detect chemically modified citrullines.23 Described by Senshu and colleagues, this method involves the transfer of citrullinated proteins to a nitrocellusose or PVDF membrane. The blot is then treated with 2,3-butanedione and antipyrine under highly acidic conditions (6M H2SO4 and 3M H3PO4) in the presence of FeCl3. The blots are then washed and incubated with a polyclonal antibody that recognizes chemically modified citrulline residues. The antibody was raised by injecting citrullinated histones, chemically modified by the above reagents, into rabbits, and the antibodies were purified using a modified citrulline column. This method has been successfully used for western blotting, as well as immunohistochemistry to study the tissue specific distribution of citrullinated proteins.24,25 Millipore ultimately commercialized this approach for detecting protein citrullination under the name Anti-citrulline (modifed) (ACM) detection kit. Using the ACM kit, this methodology has been used to detect and identify (by MS) a number of citrullinated proteins in RA synovium; however, the citrullinated residues were not identified.26,27,28 Fast and colleagues also combined this approach with protein microarray technology to identify a number of PAD4 substrates, including 40S ribosomal protein S2 (RPS2) and ING4.29,30 Although the ACM kit is effective, it suffers from a number of limitations including sensitivity, the need for long incubation times, and the adducts generated are not readily detected by most MS methods.
Proost and colleagues recently developed an alternative immunological detection method by raising antibodies against three peptides containing citrulline residues that were also chemically modified by 2,3-butanedione and antipyrine under acidic conditions (YAGCit*LLTK-NH2, PIECit*TKLY-NH2 and PIECit*TYLK-NH2).21 The antibody reacts with all three peptides in which citrulline is chemically modified, but not unmodified control peptides. The authors then demonstrated that this antibody could be used to detect protein citrullination in 96 well microtiter plates in either a Sandwich ELISA or ELISA based mode (Figure 2). In the Sandwich ELISA method (Figure 2B), modified citrullinated proteins are incubated in 96-well plates coated with an antibody that targets the protein of interest. Subsequently, the anti-modified citrulline antibody is added and its presence quantified with a peroxidase coupled secondary antibody. In the ELISA based format, which can be used to directly measure PAD activity, peptide coated microtiter plates are first incubated with a PAD, and then citrulline residues generated by the enzyme are derivatized in place, the plates washed, and citrullinated peptides detected with the anti-modified citrulline antibody and a peroxidase coupled secondary antibody. Bozdag et al. have shown that this assay can be used to detect changes in PAD activity as a function of added inhibitor.31
Figure 2.
Proost and colleagues developed an assay that uses antipyrine modification of citrulline and detection by ELISA. A) Peptidyl-citrulline is chemically modified by 2,3-butanedione and antipyrine under acidic conditions. B) Modified citrulline is detected with an antibody raised against this moiety, and subsequent ELISAs can be performed to detect protein citrullination.
Antibodies that directly bind citrullinated proteins
A number of antibodies that detect unmodified citrulline have also been developed, including the F95 monoclonal antibody and several commercially available antibodies from Abcam. The F95 monoclonal antibody was developed by injecting mice with a deca-citrulline peptide with a C-terminal Gly-Gly-Cys linker covalently fused to KLH by maleimide chemistry.32 The Abcam antibodies were generated by coupling citrulline directly to KLH. In both cases, the goal was to generate antibodies that recognize citrullinated proteins in a context independent manner. A number of sequence dependent antibodies have also been developed specifically for detecting citrulline in the N-terminal tail of histone H3 (e.g., citrulline 2, 8, and 17, ab77164 from Abcam), and histone H4 (e.g., citrulline 3, ab81797 from Abcam). These antibodies were developed by inoculation with peptides specific for the histone sequence where arginines were replaced with citrullines. Although all of these antibodies have shown great utility, they lack complete specificity for citrulline, thereby limiting their overall utility.
Phenylglyoxal based probes to detect protein citrullination
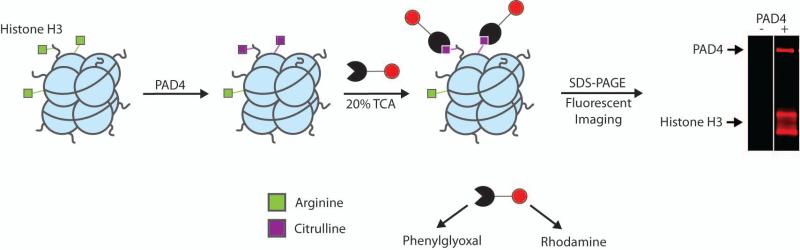
Fleckenstein and colleagues made a major contribution towards the detection of citrullinated proteins and furthered our understanding of the chemistry behind the chemical modification of citrulline by isolating and characterizing (by NMR and MS) the adducts formed upon reacting citrullinated peptides with 2,3-butanedione and antipyrine under acidic pH.18 As an extension of these studies, Tutturen et al. showed that phenylglyoxal also modified citrulline under acidic conditions, and then they synthesized a chemically cleavable phenylglyoxal derivative on PL-DMA resin and demonstrated that it could be used to isolate citrullinated proteins.33 While this method represented a huge improvement over existing antibody based methods, it cannot be used to visualize proteins. To overcome these limitations, we synthesized a rhodamine tagged phenylglyoxal derivative, termed Rh-Pg, that can be used to directly visualize protein citrullination (Figure 3).8 For the resin bound approach, Tutturen et al. conjugated phenolglyoxal to the resin via a para hydroxy group. In our hands, however, derivatization at this position was not possible, likely due to the poor nucleophilicity of the para hydroxy group, which results from conjugation to the glyoxal. To overcome this synthetic challenge, we first derivatized 3-aminoacetophenone with 2-bromopropionyl bromide. Displacement of the bromo group with sodium azide generated a click chemistry compatible compound that survives the harsh oxidizing conditions used to form the glyoxal; alkynes are unstable under these conditions. The fluorophore was then installed via the copper catalyzed azide-alkyne cycloaddition reaction using alkyne tagged rhodamine as the second substrate.
Figure 3.
Rh-PG detection and quantitation of protein citrullination assay developed by Thompson and colleagues.
Once synthesized, we demonstrated that Rh-Pg rapidly labels histone H3 and autodeiminated PAD4 under relatively mild conditions (20% TCA for 30 minutes at 37 °C). This assay provides a simple and straightforward solution to detect femtomole amounts of any citrullinated protein. The method is also quantitative and can readily be used to determine the steady state kinetic parameters for a variety of protein substrates. An additional advantage of Rh-Pg is that labeling and analysis is complete in less than 3 h versus >24 h for the antibody based methods.
In addition to its utility for identifying the numbers and types of citrullinated proteins, we envisioned that our probes would be useful for assessing target engagement; that is, inhibition of PAD activity in animal models of diseases. As an extension of this idea, we also hypothesized that these compounds would facilitate the discovery of novel citrullinated disease biomarkers that could be used for clinical diagnosis and monitoring the efficacy of various drug regimens. We further hypothesize that this approach will be superior to traditional biomarkers (the classic examples being cholesterol and PSA levels) because we will not be detecting gross changes in the level of a particular protein or metabolite, but instead a posttranslationally modified protein. To provide support for this idea, we used Rh-Pg to analyze citrulline levels in archived serum samples from a mouse model of DSS induced ulcerative colitis that had either been treated with the PAD inhibitor Cl-amidine or vehicle control.34,10,35 Impressively, we observed statistically significant reductions in total protein citrullination in the mice treated with Cl-amidine compared to the vehicle control. Moreover, this analysis identified 5 distinct proteins that showed a marked difference in citrullination levels, as a function of disease severity. Notably, for four of these proteins, higher citrullination levels strongly correlated with a number of clinical metrics of disease severity, including colon length and inflammation score.8
Work to generate biotin tagged versions of Rh-Pg is currently ongoing in our lab. These compounds will facilitate the isolation and identification of the citrullinated proteins present in whole proteomes, as well as the specific residues that are modified by the PADs in response to a variety of stimuli. Although the full scope and significance of the Rh-PG and other related probes is unclear, we predict that they will be powerful chemical probes that will enable the detection of citrullinated protein biomarkers for other diseases where the PADs play a role.
Fluorescent sensors to detect PAD activity
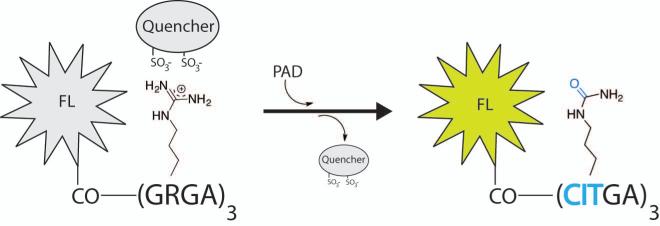
Lawrence and colleagues recently described a fluorescent reporter assay that monitors citrullination in a continuous fashion.36 The assay utilizes a fluorophore that is attached to an arginine rich peptide backbone, and a fluorescence quencher that electrostatically interacts with the positively charged arginines (Figure 4). Upon citrullination by a PAD, the loss of charge releases the fluorescence quencher, which in turn activates the fluorophore, resulting in the production of a colorimetric readout at specific wavelengths. This assay can be tuned to include different fluorescent molecules, allowing multicolor visualization of PAD-dependent citrullination.
Figure 4.
Lawrence and colleagues developed a fluorescence quencher assay to detect citrullination. This continuous assay utilizes a substrate bound fluorophore (FL) that is activated upon release of a quencher when protein citrullination occurs.
The peptide backbone of the fluorescent probe is Gly-Arg-Gly-Ala, a derivative of a previously reported PAD4 substrate.20 Several fluorophores were tested, with the majority of studies being performed using the rhodamine derivative TAMRA attached to the N-terminus of the peptide (TAMRA-(Gly-Arg-Gly-Ala)3. Substrates were screened against a library of commercially available dyes, and the negatively charged dye Acid Green 27 was identified as the most efficient quencher. Among the synthesized PAD substrates, TAMRA-(Gly-Arg-Gly-Ala)4 in combination with the quencher Acid Green 27 exhibits the most potent readout, with a 166 ± 10 fold increase in fluorescence upon citrullination. To demonstrate the possibility of monitoring multiple biological processes at the same time, a PAD4 TAMRA-(GRGA)3 sensor was analyzed in parallel with a (cAMP)-dependant protein kinase (PKA) peptide based sensor, Ac-GRTGRRDap(pyrene)SYP-NH2.37 Upon monitoring the fluorescence of the PAD4 and PKA substrates at 590 and 400 nm, respectively, increases in fluorescence were only observed in the presence of the respective enzymes at their wavelengths, thereby monitoring both the enzymatic activities simultaneously. This assay is an excellent example of the power of chemical probes to understand the biology of arginine citrullination.
Biological role of methyl-arginine
Protein arginine methylation was identified in 1970, and over the past 40 years has been found to regulate a number of cellular processes including gene transcription, RNA splicing, differentiation, and cell division.38-41 Methylation of arginines is carried out by the protein arginine methyltransferases (PRMTs), a family of proteins that catalyze the methylation of numerous cytoplasmic and nuclear proteins, using S-adenosylmethionine (SAM) as the methyl donor.42,43 Arginine methylation comes in three different varieties including the enzymatic addition of either a single methyl group (NG-monomethyl arginine, MMA) to a sidechain ω-nitrogen, or the addition of two methyl groups on either the same nitrogen (ω-NG, NG-asymmetric dimethyl arginine, ADMA) or one on both ω-nitrogens (ω-NG, N’G symmetric dimethyl arginine, SDMA) (Figure 1). While both mono- and dimethylated arginine are detected in vitro, almost all in vivo modifications have been identified as dimethylarginine.44 Although arginine methylation plays a number of critical cellular roles, its best characterized physiological role is the methylation of histone tails within chromatin, as well as modification of transcription factors, to help regulate the overall chromatin architecture and thereby facilitate the activation or repression of specific genes.42,45 Given the important biological roles that this protein family plays in cell signaling, epigenetic homeostasis, and human disease (PRMT activity is dysregulated in heart disease and cancer46,47,48,43,49,42), a greater understanding of PRMT biology will undoubtedly facilitate the development of therapies targeting these enzymes. In the following sections we describe a number of methylarginine detection methods using radiolabeled SAM, antibodies, mass spectrometry, and a recently reported method to chemically modify protein methyltransferase substrates with an alkyne handle.
Radioactive S-adenosylmethionine (SAM) assays
Radiolabeled SAM (3[H] or 14[C]) has classically been used to detect PRMT activity. Typically, 3[H]-SAM or 14[C]-SAM are incubated with recombinant PRMTs in the presence of cell lysates or purified substrate proteins, and the radiolabeled methyl group detected after SDS-PAGE by in-gel fluorography or phosphorimage analysis.50,51 This assay format can also be used to quantitatively detect peptide methylation by using Tris-Tricine gels with an internal 14[C] standard.51 Although this method is quite effective with purified proteins, it is less effective at identifying substrates in cell lysates because of competition for the radiolabeled SAM. Another disadvantage of this method is that methylated proteins are not readily isolated and identified by MS and MS2.
Methylarginine antibodies
Similar to the anti-citrulline antibodies, a number of antibodies (e.g., SYM10, SYM11, ASYM24 from EMD Millipore or ab412, ab413, ab414, ab415 from Abcam) have been developed to bind and discriminate between mono- and dimethylarginine. Antibodies are advantageous because they are relatively specific and sensitive for the desired modification, but have the disadvantage of being expensive when purchased from commercial sources. Anti-methylarginine antibodies are generated by replacing the arginines in RGG or GRG target sequences, which are known PRMT substrates, with mono-or dimethylated residues.52 Antibodies for methylarginines in histones have also been developed by creating histone specific inoculation peptides in which the native arginines have likewise been replaced with a mono- (e.g., ABE10 from EMD Millipore) or dimethylated arginine (e.g., 04-808 from EMD Millipore).
Chemical labeling and detection of protein methyltransferase (PMT) substrates
Building on work in the DNA methyltransferase field,53,54 a number of groups have shown that protein methyltransferases can use a number of SAM analogues as alternative substrates. In one such example, we demonstrated that PRMT1 could catalyze the transfer of an aziridine containing analog to the substrate arginine residue to generate a bisubstrate analog.55 Subsequently, several groups showed that both the PRMTs and the lysine methyltransferases could catalyze the transfer of a number of different groups, including alkynes and allylic groups.56,57 A key limitation of this approach has been that the transfer rates are quite slow, likely due to steric hindrance within the SAM binding pocket. To overcome this limitation, Luo and colleagues mutated the SAM binding pockets in several enzymes to accommodate the large size of the functional group being transferred, and showed that the mutations increase the rates of transfer.58-60. This simple yet elegant approach is a beautiful extension of the bump-and-hole strategy developed by Shokat and colleagues for the kinases.61
More recently, Luo and colleagues synthesized a selenium containing SAM analogue (ProSeAM (propargylic Se-adenosyl-L-selenomethionine)62 (Figure 5) and demonstrated that this compound efficiently incorporated an alkyne tag onto both arginines and lysines. The alkyne was subsequently clicked to biotin to facilitate the isolation of methylated proteins. This method marks a major step forward in chemical probe development, and will be a useful resource in elucidating the role of methylarginine in human health and disease.
Figure 5.
Luo and colleagues developed a reporter assay to detect substrates of protein methyltransferases (PMTs) by creating a SAM cofactor analogue (ProSeAM, Se-Adenosyl-L-selenomethionine) that labels methylation sites with an alkyne handle. This handle can be derivatized by azide containing tags (fluorescent or affinity) to facilitate the detection of protein methylation (fluorophore), or the enrichment, purification, and identification of PMT substrates (biotinylation).
Biological role of Phosphoarginine
Although the phosphorylation of arginine residues in histone H3 was first reported two decades ago,63 the first direct evidence that arginines are phosphorylated came only recently from Fuhrmann and colleagues.64 Here, the authors demonstrated that the bacterial kinase McsB phosphorylates arginines present in numerous proteins substrates,65 including the global heat shock regulator CtsR. CtsR is a transcriptional regulator of the bacterial stress response system, which controls the expression of chaperones of the HSP100/Clp family and the protease ClpP.66 Phosphorylation of CtsR in its winged HTH domain results in its release from DNA and is thought to increase the expression of stress response genes.64,67 This effect on DNA binding is unsurprising because the addition of a phosphate group to a guanidinium nitrogen, forming a phosphoramidate N-P bond, effectively changes the net charge from the +1 of arginine to −1 of phospho-arginine. Although a phosphoarginine is highly reactive and unstable under acidic conditions, bacteria encode phosphoarginine phosphatases, and these enzymes bear striking homology to the low molecular weight protein tyrosine phosphatases (LMW PTPases). To enrich the phosphoarginine proteome, Elsholz et al. mutated the B. subtilis arginine phosphatase ywlE and then isolated the phosphorylated proteins. Demonstrating that arginine phosphorylation plays a pivotal role in bacterial physiology, the authors identified more than 80 arginine phosphorylated proteins that play important roles in DNA binding, transcriptional regulation, protein degradation, motility, competence, and the stress responses.65
Although genome searches for enzymes similar to McsB have only uncovered potential homologues in gram-positive bacteria, it will be important to develop phosphoarginine antibodies and other chemical tools to confirm that arginine specific protein kinases and phosphatases are also present in eukaryotes.
Detection of peptidyl-phosphoarginine
The original, indirect, detection of protein embedded phosphoarginines was done using a combination of ATP [γ-32P] radiolabeling of histone H3 incubated with nuclear cell extracts and Edman degradation, observing missing signals for the arginine sites.63 Currently, MS and 31P NMR are the methods of choice. Phosphoarginine can be detected through multiple MS methods; however, care has to be taken to preserve the acid labile modification during sample preparation and to apply gentle fragmentation methods such as electron transfer dissociation (ETD) or electron-capture dissociation (ECD) that are superior towards collision-induced dissociation (CID) fragmentations and provide more reliable site localizations during MS data interpretation.68,69 Initial studies of phospho-CtsR uncovered a phosphorylated amino acid in the winged HTH domain, and analysis of a CtsR peptide I57VESKpRGGGGYIRIM71 confirmed the presence of a phosphoarginine at Arg62 through ECD MS/MS analysis. Corresponding c- and z-fragment ions revealed a 236 dalton fragment, which represents a phosphoryl group attached to an arginine residue. The identification of phosphoarginine containing proteins on a proteome scale is further complicated by endogenous phosphoarginine phosphatases. Nevertheless, as mentioned above, it is possible to identify such proteins by knocking out the endogenous phosphatases, and then digesting the cell lysates with trypsin. Phosphoarginine containing peptides can then be enriched with TiO2 chromatography, and then separated and analyzed by LC-MS/MS.
Biological role of arginine ADP-Ribosylation
Another arginine PTM is mono-ADP-ribosylation, which occurs in bacteria and vertebrates and is catalyzed mainly by bacterial toxins to alter host processes such as actin polymerization, protein-protein interactions, and conformational changes in gated ion channels 70. The addition of the ADP-ribose group of NAD+ to arginine results in a 540 dalton mass increase, and a change in the net charge of arginine from +1 to −1 due to the two phosphate groups in ADP. This reaction is catalyzed by arginine-specific ADP-ribosyltransferases (ARTs), which are similar to ribosyltransferases that modify different amino acids and nucleotides.71 The reaction can be reversed enzymatically by ADP-ribosylarginine specific hydrolases (ARHs) that completely remove the ADP-ribose moiety. The ADP-ribose can also be removed from peptidyl-arginine in non-enzymatic reactions that result in the complete reversion to arginine, partial removal by phosphodiesterases resulting in phosphoribosylated arginine,72 or hydrolysis at the guanidine carbon resulting in an ornithine residue.73 The biological role of phosphoribosylated arginine and ornithine have yet to be fully characterized.
Detection of peptidyl-ADP-ribosylarginine
ADP-ribosylation can be analyzed using radioactively labeled [32P]-NAD+ to facilitate substrate identification and to characterize the properties of the ARTs.71 For example, proteins from whole cell lysates are labeled and separated by SDS-PAGE radiography, and the modified proteins identified by MS. Another method for substrate identification is by the addition of a biotin or etheno-group tag to NAD+, which facilitates detection (western blot and flow cytometry) and enrichment of proteins with streptavidin or etheno-adenosine-specific monoclonal antibodies.74 Although ADP-ribosylation is a bulky group that has previously been used to create antibodies,75 anti-ribosylarginine antibodies are cross-reactive with other mono- or poly-ADP-ribosylated residues, thereby limiting their utility. ADP-ribosyl peptides from biological samples can be enriched through selective binding to a titanium oxide affinity resin,76 or in a novel approach, ‘macro domains’ that specifically bind ADP-ribosyl moieties were used to selectively purify mono-ADP-ribosylated proteins.77 The latter study, which resulted in the identification of several new human substrates, has the potential to revolutionize the field.
Biological role of Non-enzymatic arginine modifications
Peptidyl-arginine is also susceptible to non-enzymatic modifications, including advanced glycation end-products (AGEs) and carbonylation (Figure 1). AGEs are formed by Malliard reactions between aldehyde groups on reducing sugars and either proteins, nucleic acids, or lipids. The major arginine AGEs are Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1), Nω-carboxymethyl-arginine (CMA), and Nω-carboxyethyl-arginine (CEA). These modifications increase during oxidative stress and cellular aging, and hyperglycemia accelerates AGE formation.78 Glycation of human LDL by methylglyoxal was also found to increase atherosclerosis by altering LDL morphology in individuals with diabetes and cardiovascular disease.79
Carbonylation is an age-associated, irreversible oxidative process that is the result of metal catalyzed oxidative (MCO) cleavage of amino acid sidechains. There is evidence that upregulated carbonylation is present in patients with Alzheimer’s disease,80 rheumatoid arthritis,81 and atherosclerosis.82 Carbonylation either marks proteins for degradation by the proteasome and Lon protease, or modified residues can escape proteolysis to be processed further to glutamate semialdehyde (arginine and proline) or aminoadipate semialdehyde (lysine).83
Detection of AGEs and carbonylation
The three major AGEs of arginine result in a 53-73 dalton increase that is detectable by protein digestion and subsequent LC-MS/MS.84 Antibodies have been developed for CMA (e.g., CosmoBio, CAC-AGE-M04)85 and MG-H1,79,86 but not CEA. These antibodies have been used for immunohistochemistry and ELISA based methods to determine AGE levels in renal failure,87 cardiovascular disease,79 and diabetes.86 MG-H1 can also be detected by reaction with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) resulting in products detectable by HPLC separation and fluorimetry.88 This method was used to determine that the levels of MG-H1 increased with age in human lens proteins, likely leading to protein aggregation and cataract formation.89
Arginine carbonylation is a non-enzymatic sidechain hydroxylation, which then collapses to release the guanidinium group to form a glutamate semialdehyde.90 Glutamate semialdehyde can be readily detected by MS,91 and the carbonyl can be chemically derivatized with 2,4-dinitrophenol hydrazine, which can be detected by ultraviolet spectrophotometry.92 The 2,4-dinitrophenolhydrazone modified proteins can also be detected with an anti-2,4-dinitrophenolhydroazone antibody, which facilitates detection by western blotting,93,94 immunocytochemistry,95 and ELISA.96 Millipore commercialized this process as the OxyBlot protein oxidation detection kit (Catalog No. S7150), which is similar to their ACM kit for the detection of modified citrulline,
Conclusions and future perspectives
Posttranslational modifications of arginine play several key roles in human health and disease. To better understand current and as yet unidentified PTMs, it is critical that new chemical and biological tools be developed to detect and analyze arginine derivatives. The emergence of chemical biology over the past decade has expanded our toolbox past antibodies and MS for PTM analysis. This renaissance has resulted in new methods for the isolation and identification of enzyme substrates and modifications, and we hope the methods described in this review inspire the design of novel chemical biology tools for further elucidating the biology of PTMs.
Acknowledgements
Work in the Thompson lab is funded by TSRI and NIH grants CA151304 and GM079357. JF is funded by an EMBO postdoctoral fellowship.
References
- 1.Cuthbert GL, Daujat S, Snowden AW, Erdjument-Bromage H, Hagiwara T, Yamada M, Schneider R, Gregory PD, Tempst P, Bannister AJ, Kouzarides T. Cell. 2004;118:545–553. doi: 10.1016/j.cell.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Thompson PR, Fast W. ACS Chem Biol. 2006;1:433–441. doi: 10.1021/cb6002306. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Bolt M, Guertin MJ, Chen W, Zhang S, Cherrington BD, Slade DJ, Dreyton CJ, Subramanian V, Bicker KL, Thompson PR, Mancini MA, Lis JT, Coonrod SA. Proc Natl Acad Sci U S A. 2012;109:13331–13336. doi: 10.1073/pnas.1203280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney PL, Bhatia M, Jones NG, Yuan L, Glascock MC, Catchings KL, Yamada M, Thompson PR. Biochemistry. 2005;44:10570–10582. doi: 10.1021/bi050292m. [DOI] [PubMed] [Google Scholar]
- 5.Jones JE, Causey CP, Knuckley B, Slack-Noyes JL, Thompson PR. Curr Opin Drug Discov Devel. 2009;12:616–627. [PMC free article] [PubMed] [Google Scholar]
- 6.Hidaka Y, Hagiwara T, Yamada M. FEBS Lett. 2005;579:4088–4092. doi: 10.1016/j.febslet.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Raijmakers R, Zendman AJ, Egberts WV, Vossenaar ER, Raats J, Soede-Huijbregts C, Rutjes FP, van Veelen PA, Drijfhout JW, Pruijn GJ. J Mol Biol. 2007;367:1118–1129. doi: 10.1016/j.jmb.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 8.Bicker KL, Subramanian V, Chumanevich AA, Hofseth LJ, Thompson PR. J Am Chem Soc. 2012;134:17015–17018. doi: 10.1021/ja308871v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, Holers VM. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, Davis T, Matesic LE, Thompson PR, Hofseth LJ. Am J Physiol Gastrointest Liver Physiol. 2011;300:G929–938. doi: 10.1152/ajpgi.00435.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange S, Gogel S, Leung KY, Vernay B, Nicholas AP, Causey CP, Thompson PR, Greene ND, Ferretti P. Dev Biol. 2011;355:205–214. doi: 10.1016/j.ydbio.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McElwee JL, Mohanan S, Griffith OL, Breuer HC, Anguish LJ, Cherrington BD, Palmer AM, Howe LR, Subramanian V, Causey CP, Thompson PR, Gray JW, Coonrod SA. BMC Cancer. 2012;12:500. doi: 10.1186/1471-2407-12-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Li P, Wang S, Hu J, Chen XA, Wu J, Fisher M, Oshaben K, Zhao N, Gu Y, Wang D, Chen G, Wang Y. J Biol Chem. 2012;287:25941–25953. doi: 10.1074/jbc.M112.375725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slack JL, Causey CP, Thompson PR. Cell Mol Life Sci. 2011;68:709–720. doi: 10.1007/s00018-010-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscarello MA, Lei H, Mastronardi FG, Winer S, Tsui H, Li Z, Ackerley C, Zhang L, Raijmakers R, Wood DD. Dis Model Mech. 2013 doi: 10.1242/dmm.010520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewallen DM, Steckler CJ, Knuckley B, Chalmers MJ, Thompson PR. Mol Biosyst. 2012;8:1701–1706. doi: 10.1039/c2mb25053e. [DOI] [PubMed] [Google Scholar]
- 17.Grammel M, Luong P, Orth K, Hang HC. J Am Chem Soc. 2011;133:17103–17105. doi: 10.1021/ja205137d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm A, Rise F, Sessler N, Sollid LM, Undheim K, Fleckenstein B. Anal Biochem. 2006;352:68–76. doi: 10.1016/j.ab.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Knipp M, Vasak M. Anal Biochem. 2000;286:257–264. doi: 10.1006/abio.2000.4805. [DOI] [PubMed] [Google Scholar]
- 20.Knuckley B, Causey CP, Jones JE, Bhatia M, Dreyton CJ, Osborne TC, Takahara H, Thompson PR. Biochemistry. 2010;49:4852–4863. doi: 10.1021/bi100363t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moelants EA, Van Damme J, Proost P. PLoS One. 2011;6:e28976. doi: 10.1371/journal.pone.0028976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slack JL, Jones LE, Jr., Bhatia MM, Thompson PR. Biochemistry. 2011;50:3997–4010. doi: 10.1021/bi200309e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senshu T, Sato T, Inoue T, Akiyama K, Asaga H. Anal Biochem. 1992;203:94–100. doi: 10.1016/0003-2697(92)90047-b. [DOI] [PubMed] [Google Scholar]
- 24.Makrygiannakis D, Hermansson M, Ulfgren AK, Nicholas AP, Zendman AJ, Eklund A, Grunewald J, Skold CM, Klareskog L, Catrina AI. Ann Rheum Dis. 2008;67:1488–1492. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 25.Senshu T, Akiyama K, Kan S, Asaga H, Ishigami A, Manabe M. J Invest Dermatol. 1995;105:163–169. doi: 10.1111/1523-1747.ep12317070. [DOI] [PubMed] [Google Scholar]
- 26.Matsuo K, Xiang Y, Nakamura H, Masuko K, Yudoh K, Noyori K, Nishioka K, Saito T, Kato T. Arthritis Res Ther. 2006;8:R175. doi: 10.1186/ar2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabushi Y, Nakanishi T, Takeuchi T, Nakajima M, Ueda K, Kotani T, Makino S, Shimizu A, Hanafusa T, Takubo T. Ann Clin Biochem. 2008;45:413–417. doi: 10.1258/acb.2007.007205. [DOI] [PubMed] [Google Scholar]
- 28.Tilleman K, Van Steendam K, Cantaert T, De Keyser F, Elewaut D, Deforce D. Rheumatology (Oxford) 2008;47:597–604. doi: 10.1093/rheumatology/ken077. [DOI] [PubMed] [Google Scholar]
- 29.Guo Q, Bedford MT, Fast W. Mol Biosyst. 2011;7:2286–2295. doi: 10.1039/c1mb05089c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Q, Fast W. J Biol Chem. 2011;286:17069–17078. doi: 10.1074/jbc.M111.230961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozdag M, Dreker T, Henry C, Tosco P, Vallaro M, Fruttero R, Scozzafava A, Carta F, Supuran CT. Bioorg Med Chem Lett. 2013;23:715–719. doi: 10.1016/j.bmcl.2012.11.102. [DOI] [PubMed] [Google Scholar]
- 32.Nicholas AP, Whitaker JN. Glia. 2002;37:328–336. [PubMed] [Google Scholar]
- 33.Tutturen AE, Holm A, Jorgensen M, Stadtmuller P, Rise F, Fleckenstein B. Anal Biochem. 2010;403:43–51. doi: 10.1016/j.ab.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Causey CP, Thompson PR. Tetrahedron Lett. 2008;49:4383–4385. doi: 10.1016/j.tetlet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, Arita K, Bhatia M, Knuckley B, Lee YH, Stallcup MR, Sato M, Thompson PR. Biochemistry. 2006;45:11727–11736. doi: 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Priestman MA, Lawrence DS. Angew Chem Int Ed Engl. 2013;52:2323–2325. doi: 10.1002/anie.201208464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reimann EM, Walsh DA, Krebs EG. J Biol Chem. 1971;246:1986–1995. [PubMed] [Google Scholar]
- 38.Paik WK, Kim S. J Biol Chem. 1970;245:88–92. [PubMed] [Google Scholar]
- 39.Lin WJ, Gary JD, Yang MC, Clarke S, Herschman HR. J Biol Chem. 1996;271:15034–15044. doi: 10.1074/jbc.271.25.15034. [DOI] [PubMed] [Google Scholar]
- 40.Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M. Nature. 2007;445:214–218. doi: 10.1038/nature05458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friesen WJ, Massenet S, Paushkin S, Wyce A, Dreyfuss G. Mol Cell. 2001;7:1111–1117. doi: 10.1016/s1097-2765(01)00244-1. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y, Bedford MT. Nat Rev Cancer. 2013;13:37–50. doi: 10.1038/nrc3409. [DOI] [PubMed] [Google Scholar]
- 43.Bedford MT, Clarke SG. Mol Cell. 2009;33:1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith BC, Denu JM. Biochim Biophys Acta. 2009;1789:45–57. doi: 10.1016/j.bbagrm.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Lorenzo A, Bedford MT. FEBS Lett. 2011;585:2024–2031. doi: 10.1016/j.febslet.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang J, Frankel A, Cook RJ, Kim S, Paik WK, Williams KR, Clarke S, Herschman HR. J Biol Chem. 2000;275:7723–7730. doi: 10.1074/jbc.275.11.7723. [DOI] [PubMed] [Google Scholar]
- 47.Tran CT, Leiper JM, Vallance P. Atheroscler Suppl. 2003;4:33–40. doi: 10.1016/s1567-5688(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 48.Vallance P, Leiper J. Arterioscler Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimatsu M, Toyokawa G, Hayami S, Unoki M, Tsunoda T, Field HI, Kelly JD, Neal DE, Maehara Y, Ponder BA, Nakamura Y, Hamamoto R. Int J Cancer. 2011;128:562–573. doi: 10.1002/ijc.25366. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Cheng D, Bedford MT. Methods Mol Biol. 2004;284:195–208. doi: 10.1385/1-59259-816-1:195. [DOI] [PubMed] [Google Scholar]
- 51.Osborne TC, Obianyo O, Zhang X, Cheng X, Thompson PR. Biochemistry. 2007;46:13370–13381. doi: 10.1021/bi701558t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan P, Xu Y, Birkaya B, Myers J, Pelletier M, Read LK, Guarnaccia C, Pongor S, Denman RB, Aletta JM. J Immunol Methods. 2007;320:132–142. doi: 10.1016/j.jim.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalhoff C, Lukinavicius G, Klimasauskas S, Weinhold E. Nat Protoc. 2006;1:1879–1886. doi: 10.1038/nprot.2006.253. [DOI] [PubMed] [Google Scholar]
- 54.Peters W, Willnow S, Duisken M, Kleine H, Macherey T, Duncan KE, Litchfield DW, Luscher B, Weinhold E. Angew Chem Int Ed Engl. 2010;49:5170–5173. doi: 10.1002/anie.201001240. [DOI] [PubMed] [Google Scholar]
- 55.Osborne T, Roska RL, Rajski SR, Thompson PR. J Am Chem Soc. 2008;130:4574–4575. doi: 10.1021/ja077104v. [DOI] [PubMed] [Google Scholar]
- 56.Binda O, Boyce M, Rush JS, Palaniappan KK, Bertozzi CR, Gozani O. Chembiochem. 2011;12:330–334. doi: 10.1002/cbic.201000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willnow S, Martin M, Luscher B, Weinhold E. Chembiochem. 2012;13:1167–1173. doi: 10.1002/cbic.201100781. [DOI] [PubMed] [Google Scholar]
- 58.Islam K, Zheng W, Yu H, Deng H, Luo M. ACS Chem Biol. 2011;6:679–684. doi: 10.1021/cb2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Islam K, Bothwell I, Chen Y, Sengelaub C, Wang R, Deng H, Luo M. J Am Chem Soc. 2012;134:5909–5915. doi: 10.1021/ja2118333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang R, Islam K, Liu Y, Zheng W, Tang H, Lailler N, Blum G, Deng H, Luo M. J Am Chem Soc. 2013;135:1048–1056. doi: 10.1021/ja309412s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 62.Bothwell IR, Islam K, Chen Y, Zheng W, Blum G, Deng H, Luo M. J Am Chem Soc. 2012;134:14905–14912. doi: 10.1021/ja304782r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakim BT, Aswad GD. J Biol Chem. 1994;269:2722–2727. [PubMed] [Google Scholar]
- 64.Fuhrmann J, Schmidt A, Spiess S, Lehner A, Turgay K, Mechtler K, Charpentier E, Clausen T. Science. 2009;324:1323–1327. doi: 10.1126/science.1170088. [DOI] [PubMed] [Google Scholar]
- 65.Elsholz AK, Hempel K, Michalik S, Gronau K, Becher D, Hecker M, Gerth U. J Bacteriol. 2011;193:3887–3893. doi: 10.1128/JB.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Derre I, Rapoport G, Msadek T. Mol Microbiol. 1999;31:117–131. doi: 10.1046/j.1365-2958.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 67.Kruger E, Zuhlke D, Witt E, Ludwig H, Hecker M. EMBO J. 2001;20:852–863. doi: 10.1093/emboj/20.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt A, Ammerer G, Mechtler K. Proteomics. 2012 doi: 10.1002/pmic.201200240. [DOI] [PubMed] [Google Scholar]
- 69.Engholm-Keller K, Larsen MR. Proteomics. 2013 [Google Scholar]
- 70.Laing S, Unger M, Koch-Nolte F, Haag F. Amino Acids. 2011;41:257–269. doi: 10.1007/s00726-010-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koch-Nolte F, Kernstock S, Mueller-Dieckmann C, Weiss MS, Haag F. Front Biosci. 2008;13:6716–6729. doi: 10.2741/3184. [DOI] [PubMed] [Google Scholar]
- 72.Zolkiewska A, Moss J. J Biol Chem. 1995;270:9227–9233. doi: 10.1074/jbc.270.16.9227. [DOI] [PubMed] [Google Scholar]
- 73.Stevens LA, Levine RL, Gochuico BR, Moss J. Proc Natl Acad Sci U S A. 2009;106:19796–19800. doi: 10.1073/pnas.0910633106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krebs C, Koestner W, Nissen M, Welge V, Parusel I, Malavasi F, Leiter EH, Santella RM, Haag F, Koch-Nolte F. Anal Biochem. 2003;314:108–115. doi: 10.1016/s0003-2697(02)00640-1. [DOI] [PubMed] [Google Scholar]
- 75.Schwab CJ, Colville MJ, Fullerton AT, McMahon KK. Proc Soc Exp Biol Med. 2000;223:389–396. doi: 10.1046/j.1525-1373.2000.22355.x. [DOI] [PubMed] [Google Scholar]
- 76.Lang AE, Schmidt G, Schlosser A, Hey TD, Larrinua IM, Sheets JJ, Mannherz HG, Aktories K. Science. 2010;327:1139–1142. doi: 10.1126/science.1184557. [DOI] [PubMed] [Google Scholar]
- 77.Dani N, Stilla A, Marchegiani A, Tamburro A, Till S, Ladurner AG, Corda D, Di Girolamo M. Proc Natl Acad Sci U S A. 2009;106:4243–4248. doi: 10.1073/pnas.0900066106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brownlee M, Cerami A, Vlassara H. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 79.Rabbani N, Godfrey L, Xue M, Shaheen F, Geoffrion M, Milne R, Thornalley PJ. Diabetes. 2011;60:1973–1980. doi: 10.2337/db11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chapman ML, Rubin BR, Gracy RW. J Rheumatol. 1989;16:15–18. [PubMed] [Google Scholar]
- 82.Uchida K, Toyokuni S, Nishikawa K, Kawakishi S, Oda H, Hiai H, Stadtman ER. Biochemistry. 1994;33:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- 83.Nystrom T. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rabbani N, Chittari MV, Bodmer CW, Zehnder D, Ceriello A, Thornalley PJ. Diabetes. 2010;59:1038–1045. doi: 10.2337/db09-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mera K, Fujiwara Y, Otagiri M, Sakata N, Nagai R. Ann N Y Acad Sci. 2008;1126:155–157. doi: 10.1196/annals.1433.000. [DOI] [PubMed] [Google Scholar]
- 86.Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Diabetologia. 2010;53:989–1000. doi: 10.1007/s00125-010-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yamada K, Miyahara Y, Hamaguchi K, Nakayama M, Nakano H, Nozaki O, Miura Y, Suzuki S, Tuchida H, Mimura N, et al. Clin Nephrol. 1994;42:354–361. [PubMed] [Google Scholar]
- 88.Ahmed N, Argirov OK, Minhas HS, Cordeiro CA, Thornalley PJ. Biochem J. 2002;364:1–14. doi: 10.1042/bj3640001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM. Invest Ophthalmol Vis Sci. 2003;44:5287–5292. doi: 10.1167/iovs.03-0573. [DOI] [PubMed] [Google Scholar]
- 90.Levine RL. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 91.Chavez JD, Bisson WH, Maier CS. Anal Bioanal Chem. 2010;398:2905–2914. doi: 10.1007/s00216-010-4289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 93.Shacter E, Williams JA, Lim M, Levine RL. Free Radic Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 94.Keller RJ, Halmes NC, Hinson JA, Pumford NR. Chem Res Toxicol. 1993;6:430–433. doi: 10.1021/tx00034a007. [DOI] [PubMed] [Google Scholar]
- 95.Smith MA, Sayre LM, Anderson VE, Harris PL, Beal MF, Kowall N, Perry G. J Histochem Cytochem. 1998;46:731–735. doi: 10.1177/002215549804600605. [DOI] [PubMed] [Google Scholar]
- 96.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Free Radic Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]