Abstract
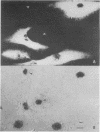
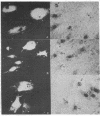
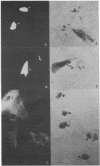
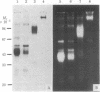
The in vivo function of defined chromosomal proteins was examined by microinjecting purified antibody and antibody fragments into living fibroblasts. The involvement of histones and chromosomal high mobility group proteins HMG-1, 2, and 17 in transcription was visualized by studying the [3H]uridine incorporation in KD human fibroblasts after microinjection of fluoresceinated antibodies to these proteins. Nuclear uridine incorporation was not affected by microinjection of control antibodies or by the presence of immune complexes formed after microinjection of antibodies to chromosomal proteins that are not involved in transcription. In contrast, injection of anti-histone IgG, F(ab')2, or Fab and anti-HMG-17 IgG causes a significant reduction in transcription. The reduction is proportional to the amount of antibody introduced into the cell. We conclude that histones and protein HMG-17 are present on transcribed regions of the genome and that passage of RNA polymerase along the chromatin fiber is prevented by antibody binding to these proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antman K. H., Livingston D. M. Intracellular neutralization of SV40 tumor antigens following microinjection of specific antibody. Cell. 1980 Mar;19(3):627–635. doi: 10.1016/s0092-8674(80)80039-0. [DOI] [PubMed] [Google Scholar]
- Baer B. W., Rhodes D. Eukaryotic RNA polymerase II binds to nucleosome cores from transcribed genes. Nature. 1983 Feb 10;301(5900):482–488. doi: 10.1038/301482a0. [DOI] [PubMed] [Google Scholar]
- Barsoum J., Levinger L., Varshavsky A. On the chromatin structure of the amplified, transcriptionally active gene for dihydrofolate reductase in mouse cells. J Biol Chem. 1982 May 10;257(9):5274–5282. [PubMed] [Google Scholar]
- Bustin M. Binding of E. coli RNA polymerase to chromatin subunits. Nucleic Acids Res. 1978 Mar;5(3):925–932. doi: 10.1093/nar/5.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Hopkins R. B., Isenberg I. Immunological relatedness of high mobility group chromosomal proteins from calf thymus. J Biol Chem. 1978 Mar 10;253(5):1694–1699. [PubMed] [Google Scholar]
- Bustin M. Immunological approaches to chromatin and chromosome structure and function. Curr Top Microbiol Immunol. 1979;88:105–142. doi: 10.1007/978-3-642-67331-3_3. [DOI] [PubMed] [Google Scholar]
- Bustin M., Neihart N. K. Antibodies against chromosomal HMG proteins stain the cytoplasm of mammalian cells. Cell. 1979 Jan;16(1):181–189. doi: 10.1016/0092-8674(79)90199-5. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd Studies on repair of adenovirus 2 by human fibroblasts using normal, xeroderma pigmentosum, and xeroderma pigmentosum heterozygous strains. Cancer Res. 1974 Aug;34(8):1965–1970. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Goldblatt D., Bustin M. Exposure of histone antigenic determinants in chromatin. Biochemistry. 1975 Apr 22;14(8):1689–1695. doi: 10.1021/bi00679a022. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Mueller C. Microinjection of early SV40 DNA fragments and T antigen. Methods Enzymol. 1980;65(1):816–825. doi: 10.1016/s0076-6879(80)65076-9. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Keene M. A., Elgin S. C. Micrococcal nuclease as a probe of DNA sequence organization and chromatin structure. Cell. 1981 Nov;27(1 Pt 2):57–64. doi: 10.1016/0092-8674(81)90360-3. [DOI] [PubMed] [Google Scholar]
- Lacy E., Axel R. Analysis of DNA of isolated chromatin subunits. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3978–3982. doi: 10.1073/pnas.72.10.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Feramisco J. R. Disruption of the in vivo distribution of the intermediate filaments in fibroblasts through the microinjection of a specific monoclonal antibody. Cell. 1981 Apr;24(1):185–193. doi: 10.1016/0092-8674(81)90514-6. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. doi: 10.1146/annurev.bi.49.070180.005343. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Bustin M., Miller O. L., Jr Electron microscopic analysis of chromosome metabolism in the Drosophila melanogaster embryo. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):741–754. doi: 10.1101/sqb.1978.042.01.075. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Sommerville J., Bustin M. Injected histone antibodies interfere with transcription of lampbrush chromosome loops in oocytes of Pleurodeles. J Cell Sci. 1979 Dec;40:1–20. doi: 10.1242/jcs.40.1.1. [DOI] [PubMed] [Google Scholar]
- Tahourdin C. S., Neihart N. K., Isenberg I., Bustin M. Immunochemical detection of chromosomal protein HMG-17 in chromatin subunits. Biochemistry. 1981 Feb 17;20(4):910–915. doi: 10.1021/bi00507a040. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. Transcription by eukaryotic RNA polymerases A and B of chromatin assembled in vitro. Eur J Biochem. 1979 Aug 1;98(2):317–327. doi: 10.1111/j.1432-1033.1979.tb13191.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W. Method of examining viral RNA metabolism in cells in culture: metabolism of vesicular stomatitis virus RNA. J Virol. 1975 Nov;16(5):1340–1344. doi: 10.1128/jvi.16.5.1340-1344.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson P., Felsenfeld G. Transcription of histone-covered T7 DNA by Escherichia coli RNA polymerase. Biochemistry. 1978 Dec 26;17(26):5695–5705. doi: 10.1021/bi00619a015. [DOI] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Yamaizumi M., Uchida T., Mekada E., Okada Y. Antibodies introduced into living cells by red cell ghosts are functionally stable in the cytoplasm of the cells. Cell. 1979 Dec;18(4):1009–1014. doi: 10.1016/0092-8674(79)90213-7. [DOI] [PubMed] [Google Scholar]