Abstract
Background
Apolipoprotein ε4 genotype has been recommended as a potential inclusion or exclusion criterion in targeted clinical trials for Alzheimer disease and MCI due to AD, and implemented in trials of immunotherapeutic agents.
Methods
We tested this recommendation with clinical trials simulations using participants from a meta-database of 19 studies to create trials samples with APOE ε4 proportions ranging from 0% (all noncarriers) to 100% (all carriers). For each percentage of APOE ε4 carriers, we randomly resampled the database for 1000 trials for each trials scenario, planning for 18 or 24 month trials with samples from 50 to 400 patients per treatment or placebo group, up to 40% dropouts, outcomes on the AD Assessment Scale – cognitive subscale (ADAS-cog) with effect sizes from 0.15 to 0.75, and calculated statistical power.
Findings
Enrichment of clinical trials participants based on APOE ε4 carrier status resulted in minimal increases in power compared to enrolling participants with APOE ε3 genotype only or enrolling patients without regard to APOE genotype. Increased screening requirements to enhance the sample would offset gains in power.
Interpretations
Although samples enriched for APOE ε4 carriers in AD or MCI clinical trials showed slightly more cognitive impairment and greater decline using number APOE ε4 alleles as an inclusion criterion most likely would not result in more efficient trials; and trials would take longer because fewer patients would be available. APOE ε4/εX (where x= 2, 3 or 4) genotype could be useful however as an explanatory variable or covariate if warranted by a drug’s action.
Keywords: Alzheimer disease, mild cognitive impairment, apolipoprotein ε4, clinical trials, clinical trials simulations, biomarkers, Alzheimer’s Disease Neuroimaging Initiative (ADNI), Alzheimer’s Disease Cooperative Study (ADCS), Alzheimer’s Disease Assessment Scale
BACKGROUND
The apolipoprotein E ε4 (APOE ε4) genotype is the major genetic risk factor for Alzheimer’s disease (AD), associated with both increased risk for developing AD and earlier age of onset [1]. It is also associated with increased risk for developing mild cognitive impairment (MCI) due to AD and progression from MCI to dementia [2]. APOE has a primary role as a lipid transport protein in the central nervous system and as a cholesterol transport protein in the periphery [3]. In addition, APOE is a major transporter for the amyloid β (Aβ) proteins that compose the plaques of AD, and thus may play a direct role in the neuropathogenesis of this disorder [4].
Because of its central role as a risk factor for AD, many clinical trials include post-hoc analyses of the effect of APOE ε4 on trial outcomes, but results have been mixed. The clinical trials for tacrine, performed in the late 1980s, suggested that subjects with an APOE ε4 allele were more likely to respond, but similar studies with newer cholinesterase inhibitors did not show a differential APOE genotype effect [5, 6, 7, 8]. Two retrospective analyses of clinical trials of subjects with MCI due to AD suggested lower rates of progression to dementia for patients receiving active therapy with rivastigmine or donepezil occurred selectively among APOE ε4 carriers [9, 10]. More recently, experts have considered enrichment of clinical trials in AD dementia and MCI due to AD based on APOE ε4 status, in which number of APOE ε4 alleles (or lack thereof) would be an entry criterion for the study [11]. Such enrichment is expected to lead to reduced sample sizes and greater efficiency for clinical trials by directing therapies more specifically to the underlying neuropathological changes. This recommendation was implemented in the industry-sponsored trial of bapineuzumab, in which subjects are enrolled in one of two parallel trials depending on their APOE genotype [12], with the hypothesis that APOE ε4 non-carriers would more likely benefit from bapineuzumab perhaps due to lower Aβ burden or to fewer cerebrovascular adverse effects.
We empirically tested the potential efficiency of these recommendations by statistically simulating clinical trials scenarios of MCI due to AD or AD patients across a broad percentage of APOE ε4 carriers enrolled, using a recently developed meta-database of studies from the Alzheimer’s Disease Cooperative Study (ADCS) [13] and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) [14].
METHODS
Study Overview and Participants
Participants for the simulations were drawn from a meta-database consisting of 18 ADCS studies and ADNI, representing both clinical trials and observational studies in AD, MCI, and normal individuals (National Institutes of Health grant R01 AG037561; details available from the authors). Of the available studies, 8 had both relevant clinical ratings and APOE genotyping, 6 for AD (DHA, CE, HC, LL, PR, SL, and ADNI) and 2 for MCI (MIS, ADNI) (Table 1). The primary outcome measure was the ADAS-cog [15], which evaluates memory, reasoning, orientation, praxis, language, and word finding difficulty, and is scored from 0 to 70 errors. Clinical assessments were done at 6-month intervals over the first 2 years.
Table 1. Placebo-controlled and observational studies included in the analyses.
Studies were drawn from the Alzheimer’s Disease Cooperative Study (ADCS; http://www.adcs.org) and the Alzheimer’s Disease Neuroimaging Initiative (ADNI; http://adni.loni.ucla.edu) and included those with MCI or dementia due to AD and APOE genotyping.
| Study (code), dates | Design | Intervention | N | Duration (months) |
|---|---|---|---|---|
| Selegiline, vitamin E, 1993–1996 | RCT, moderate to severe AD | Vitamin E, selegiline | 341 | 24 |
| Prednisone 1995–1998 | RCT, mild to moderate AD | Prednisone | 138 | 16 |
| Conjugated estrogens (CE) 1995–1999 | RCT, mild to moderate AD | Conjugated estrogens | 120 | 15 |
| Memory impairment study (MIS), donepezil, vitamin E, 1999–2004 | RCT, MCI | Donepezil, vitamin E | 769 | 36 |
| Simvastatin (LL) 2003–2008 | RCT, mild to moderate AD | Simvastatin | 406 | 18 |
| Vitamins B (HC) 2003–2007 | RCT, mild to moderate AD | B vitamins | 409 | 18 |
| DHA (DHA) 2006–2009 | RCT, mild to moderate AD | DHA | 402 | 18 |
| ADNI (ADNI) 2005–2010 | Observational, AD, MCI, normal | None | 800 | 36 (AD) 48 (MCI) 48 (NL) |
All diagnoses of dementia due to AD were based on NINDS-ARDRA criteria [16], with the additional requirement of a minimal severity based on clinical ratings. These were a Clinical Dementia Rating (CDR) [17] score of 2 or greater for the SL trial and Mini-Mental State Examination (MMSE) [18] scores between 14 and 26 (DHA, HC), between 12 and 28 (CE), between 12 and 26 (LL), and between 13 and 26 (PR). Diagnosis of amnestic MCI or MCI due to AD required a CDR score of 0.5 with the memory box scored at 0.5 or greater, and delayed recall from the Logical Memory II subscale of the Wechsler Memory Scale–Revised [19] to be ≤ 8 for 16 years of education, ≤ 4 for 8–15 years, or ≤ 2 for 0–7 years.[10, 14] Patients had to be largely intact with regard to general cognition and functional performance, and could not qualify for a dementia diagnosis. Participants with AD or MCI, as in most of the trials analyzed, could continue using marketed anti-dementia drugs if they had been on stable doses prior to entry.
Simulation Methods
Simulations were conducted under a detailed protocol [20], as described in this section, to reflect typical clinical trials of an experimental drug for amnestic MCI or dementia due to AD with one treatment and placebo group, 1:1 allocation ratio, and parameters selected to be consistent with previously published trials (e.g., [10], [21]). While use of standard formulae for sample size or power can be used, this simulation approach allows for relaxation of those assumptions giving a slightly more realistic assessment of power. For each trial scenario, a separate set of patients was constructed by randomly choosing from the meta-database with replacement, i.e., patients from the dataset could be present in the simulated groups more than once in the same or different treatment arm. Sample sizes of 50, 100, 200, and 400 per group were used; 12, 18, and 24 month long trials were considered; and the ADAS-cog was the primary outcome. The placebo group outcome was the score for the patient at the specified time point in the meta-database. For the treatment group, a range of effect sizes from 0.15 to 0.75 in 0.10 increments were used to compute an expected treatment effect (or slowing of decline) reflecting very small to moderately large effect sizes [22]. For each patient, an individual treatment effect was randomly generated from a chi-square distribution with a mean equal to the expected treatment effect. The individual treatment effect was shifted by subtracting 2 times the expected treatment effect, then adding to the patient’s score at the specified time point in the database. Thus, even when a patient was reused in the analysis, the actual value used would be modified by this randomly selected amount in the treatment arm. In the placebo arm use of the same patient would lead to a slight underestimate of the variance, slightly improving the statistical power to be examined below. Dropout rates of 20% and 40% in both the treatment and placebo groups were incorporated into the scenarios.
Selection Criteria
Patients were selected for the samples as though they were applying for clinical trials using differing levels of APOE ε4 enrichment, ranging from 0% to 100% in 20% increments. Patients were classified as APOE ε4 carriers if they had one or more copies of the APOE ε4 allele, i.e., having genotypes of ε2/ε4, ε3/ε4, or ε4/ε4; and APOE ε4 noncarriers if they had no copies of the APOE ε4 allele, i.e., having genotypes of ε2/ε2, ε2/ε3, or ε3/ε3. Thus a rate of 0% enrichment would correspond to a trial enrolling only APOE ε4 noncarriers, and a rate of 100% enrichment would correspond to a trial enrolling only APOE ε4 carriers, as defined above. Intermediate percentages would correspond to the typical clinical trial with 60% APOE ε4 carriers, with lesser percentages representing enrichment for APOE ε4 noncarriers and greater percentages enrichment for APOE ε4 carriers. Secondary analyses were performed excluding individuals with an APOE ε2 genotype, so that APOE ε4 carriers would have genotypes of ε3/ε4 or ε4/ε4 and APOE ε4 noncarriers would have a genotype of ε3/ε3; as well as restricting AD dementia samples to those having MMSE ≥ 16, i.e., milder impairment to better represent recent phase 2 and 3 AD trials. Simulations were also performed using only APOE ε4 carriers, with enrichment based on the number of ε4 alleles, to evaluate dosage effects. In this set of simulations, a rate of 0% enrichment would correspond to a trial enrolling only APOE ε4 heterozygote carriers with 1 copy of the ε4 allele and a rate of 100% enrichment would correspond to a trial enrolling only APOE ε4 homozygote carriers with 2 copies of the ε4 allele. Finally, because having one APOE ε2 allele appears particularly protective against AD, an exploratory simulation was performed using only the APOE ε2 carriers (i.e., those with genotypes ε2/ε2, ε2/ε3, or ε2/ε4), even though such a trial would not be pragmatic as only 10% of a sample with dementia due to AD would satisfy inclusion criteria that required APOE ε2 carriers.
Statistical Analysis
The primary analyses were conducted using a mixed effects linear model (random coefficients model) [23], which adjusts for missing data to test for differences in the slopes (rate of change) of the ADAS-cog between the treatment and placebo groups. The mixed effects model was employed as it utilizes data from all participants (rather than just completers) and minimizes bias and better controls for Type I error in the presence of missing data [24]. For each simulated trial, a full model was constructed with group effect, visit effect, and group by visit interactions, with age and gender as covariates, and a reduced model with visit, age, and gender effects. Thus, for participant i = 1,2, n at visit j = 1,2, ni, the full model was
and the reduced model was
where the model includes both fixed effects of time at the group level and random effects of time at the individual level. An unstructured covariance matrix was used to model the independence of the slope and intercept parameters. Parameters were estimated using maximum likelihood. P-values for the group (treatment) effect were found using -2 times the difference in the log likelihood of the full and reduced model, which follows a chi-squared distribution with the appropriate degrees of freedom. Secondary analyses examined last observation carried forward (LOCF) samples to impute missing values using the nonparametric Wilcoxon test to detect any differences between treatment and placebo groups due to the skewed distributions of the resultant outcomes. For all analyses, the missing data pattern present in the meta-database was used to realistically simulate dropouts; observations were missing in simulated datasets if they were originally missing in the meta-database. This approach would minimize bias arising from differential dropout among groups [25].
One thousand simulations were done for each scenario so that estimates of power could be obtained to 3 digits. Power was calculated as the proportion of 1000 simulated trials per trial scenario having an a error p-value ≤ 0.05. Analyses were performed using version 2.15.0 of the R programming environment [26]. Mixed model analyses were performed using version 3.1-89 of the nlme package for R [27].
Finally, we compared the results from simulations using standardized effect sizes to compute the change in ADAS-cog scores over time to the results using a percentage in slope reduction. The former assumes that the magnitude of the change, relative to the variability, is constant; while the latter assumes that the rate of change is constant.
RESULTS
Patient characteristics
For both AD dementia and MCI due to AD, the APOE ε4 carriers and noncarriers were similar on most demographic and clinical characteristics, being predominantly Caucasian, married and highly educated (Tables 2 and 3). Slightly more than half of the MCI participants were males, while slightly more than half of the AD participants were females. The APOE ε4 noncarriers were older than the carriers, although this only reached statistical significance in the AD group.
Table 2. Clinical characteristics and ratings among participants with dementia due to AD based on APOE ε4 carrier status.
Clinical characteristics are shown for individual studies, but pooled for calculating statistical significance of between-group differences.
| Study Name | ADNI | DHA | ES | HC | LL | PR | Overall | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε4− | ε4+ | ε4− | ε4+ | ε4− | ε4+ | ε4− | ε4+ | ε4− | ε4+ | ε4− | ε4+ | ε4− | ε4+ | P-value | ||
| N | (N=64) | (N=124) | (N=170) | (N=232) | (N=10) | (N=16) | (N=128) | (N=246) | (N=150) | (N=208) | (N=23) | (N=47) | (N=545) | (N=873) | ||
| Age, years | 1368 | 76.7 (8.5) | 74.4 (6.9) | 76.5 (9.3) | 75.5 (7.9) | 78.6 (4.1) | 76.6 (5.2) | 77.2 (8.6) | 75.4 (7.2) | 74.2 (10.4) | 73.4 (8.5) | 69.3 (8.9) | 72.4 (7.0) | 75.8 (9.5) | 74.7 (7.7) | <0.001 |
| Education, years | 1374 | 14.8 (3.3) | 14.6 (3.0) | 14.4 (3.0) | 14.2 (2.7) | 11.9 (2.5) | 11.9 (1.9) | 14.0 (3.3) | 13.9 (2.9) | 14.0 (3.5) | 14.6 (2.9) | 14.3 (4.1) | 14.1 (2.9) | 14.2 (3.3) | 14.2 (2.9) | 0.9 |
| Ethnicity, Hispanic | 1374 | 0 (0%) | 4 (3%) | 6 (4%) | 8 (3%) | 0 (0%) | 2 (12%) | 10 (8%) | 10 (4%) | 15 (10%) | 7 (3%) | 0 (0%) | 1 (2%) | 31 (6%) | 32 (4%) | 0.077 |
| Marital Status, Married | 1411 | 45 (70%) | 108 (88%) | 121 (71%) | 165 (71%) | 3 (30%) | 10 (62%) | 80 (62%) | 171 (70%) | 99 (66%) | 156 (75%) | 19 (83%) | 44 (94%) | 367 (67%) | 654 (75%) | 0.001 |
| Race, White | 1374 | 59 (94%) | 111 (93%) | 158 (93%) | 210 (91%) | 9 (90%) | 13 (81%) | 103 (85%) | 201 (87%) | 130 (90%) | 188 (93%) | 23 (100%) | 46 (98%) | 482 (91%) | 769 (91%) | 0.85 |
| Gender, Female | 1374 | 35 (56%) | 51 (43%) | 88 (52%) | 122 (53%) | 10 (100%) | 16 (100%) | 67 (55%) | 127 (55%) | 92 (63%) | 113 (56%) | 11 (48%) | 22 (47%) | 303 (57%) | 451 (53%) | 0.19 |
| APOE ε2 carrier | 1418 | 5 (8%) | 4 (3%) | 23 (14%) | 10 (4%) | 1 (10%) | 0 (0%) | 16 (12%) | 8 (3%) | 22 (15%) | 6 (3%) | 2 (9%) | 0 (0%) | 69 (13%) | 28 (3%) | <0.001 |
| Baseline CDRsb | 1057 | 4.4 (1.7) | 4.3 (1.6) | 5.6 (2.6) | 5.8 (2.6) | 6.0 (1.9) | 6.2 (2.8) | 5.6 (2.7) | 5.7 (2.7) | -- | -- | 5.3 (1.8) | 5.6 (2.7) | 5.4 (2.5) | 5.5 (2.6) | 0.65 |
| Baseline MMSE | 1418 | 23.2 (2.1) | 23.3 (2.0) | 21.0 (3.7) | 20.4 (3.5) | 21.4 (3.3) | 20.4 (3.9) | 21.4 (3.4) | 20.8 (3.5) | 20.5 (4.7) | 20.2 (4.7) | 22.1 (4.1) | 21.1 (4.8) | 21.3 (3.9) | 20.9 (3.9) | 0.06 |
| ADAS-Cog Scores | ||||||||||||||||
| Baseline | 1392 | 18.9 (7.1) | 18.6 (5.9) | 23.3 (9.2) | 23.8 (8.3) | 12.9 (4.9) | 15.3 (6.6) | 21.9 (8.2) | 22.7 (8.9) | 24.2 (10.3) | 24.1 (9.3) | 17.8 (8.2) | 16.0 (7.7) | 22.3 (9.2) | 22.2 (8.7) | 0.82 |
| 6 months | 1252 | 21.1 (8.4) | 20.7 (7.5) | 25.1 (10.3) | 26.4 (10.2) | 15.5 (6.9) | 18.4 (8.6) | 22.9 (9.5) | 24.5 (9.2) | 25.4 (11.1) | 26.2 (10.3) | 20.5 (8.4) | 17.6 (9.8) | 23.7 (10.1) | 24.3 (9.9) | 0.18 |
| 12 months | 1129 | 22.2 (9.1) | 22.5 (8.8) | 26.0 (11.1) | 27.4 (11.5) | 18.0 (10.9) | 17.7 (7.3) | 24.3 (9.6) | 26.9 (10.2) | 27.4 (12.3) | 28.4 (11.2) | 27.3 (12.0) | 19.8 (10.8) | 25 (11) | 27 (11) | 0.19 |
| 18 months | 793 | -- | -- | 28 (13) | 29 (12) | 25 (12) | 29 (12) | 29 (12) | 30 (12) | -- | -- | 27 (12) | 29 (12) | 0.042 | ||
| 24 months | 133 | 26.4 (9.9) | 28.8 (12.6) | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 26.4 (9.9) | 28.8 (12.6) | 0.57 |
Table 3. Clinical characteristics and ratings among participants with MCI due to AD based on APOΕ4 carrier status.
Clinical characteristics are shown for individual studies, but pooled for calculating statistical significance of between-group differences.
| Study Name | ADNI | MIS trial | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| ε4− | ε4+ | ε4− | ε4+ | ε4− | ε4+ | P-value | ||
| N | (N=187) | (N=215) | (N=357) | (N=433) | (N=544) | (N=648) | ||
| Age, years | 1134 | 75.7 (8.1) | 73.8 (6.7) | 72.2 (8.0) | 72.4 (6.5) | 73.4 (8.2) | 72.9 (6.6) | 0.054 |
| Education, years | 1134 | 15.7 (3.1) | 15.7 (3.0) | 14.6 (3.2) | 14.7 (3.0) | 15.0 (3.2) | 15.0 (3.1) | 0.73 |
| Ethnicity, Hispanic | 1134 | 8 (4%) | 6 (3%) | 19 (6%) | 9 (2%) | 27 (5%) | 15 (2%) | 0.013 |
| Marital status, married | 1182 | 143 (78%) | 172 (80%) | 251 (71%) | 353 (82%) | 394 (73%) | 525 (82%) | <0.001 |
| Race, White | 1134 | 165 (93%) | 196 (94%) | 303 (90%) | 384 (94%) | 468 (91%) | 580 (94%) | 0.046 |
| Gender, Female | 1134 | 60 (34%) | 77 (37%) | 146 (43%) | 194 (47%) | 206 (40%) | 271 (44%) | 0.18 |
| APOE ε2 Carrier | 1192 | 17 (9%) | 11 (5%) | 52 (15%) | 21 (5%) | 69 (13%) | 32 (5%) | <0.001 |
| Baseline CDRsb | 402 | 1.52 (0.85) | 1.68 (0.89) | 1.70 (0.77) | 1.90 (0.78) | 1.52 (0.85) | 1.68 (0.89) | 0.049 |
| Baseline MMSE | 1192 | 27.1 (1.8) | 26.9 (1.8) | 27.5 (1.9) | 27.1 (1.8) | 27.3 (1.8) | 27.1 (1.8) | 0.005 |
| ADAS-Cog Scores | ||||||||
| Baseline | 402 | 10.5 (4.4) | 12.4 (4.3) | 10.4 (4.1) | 12.0 (4.5) | 10.4 (4.2) | 12.1 (4.4) | <0.001 |
| 6 months | 1038 | 11.5 (5.5) | 13.0 (5.4) | 9.3 (4.8) | 11.8 (5.1) | 10.2 (5.2) | 12.2 (5.2) | <0.001 |
| 12 months | 972 | 11.6 (6.2) | 13.5 (6.1) | 9.9 (5.1) | 12.8 (5.8) | 10.6 (5.6) | 13.0 (5.9) | <0.001 |
| 18 months | 872 | 12.1 (6.7) | 14.8 (7.7) | 10.0 (5.1) | 13.6 (6.6) | 10.8 (5.8) | 14.0 (7.0) | <0.001 |
| 24 months | 814 | 12.6 (7.4) | 15.3 (7.3) | 9.6 (5.6) | 14.4 (7.3) | 10.7 (6.5) | 14.7 (7.3) | <0.001 |
For the MCI group, APOE ε4 carriers had worse performance on the ADAS-cog, which occurred at baseline and all subsequent time points. For the AD dementia group, APOE ε4 carriers also showed worse performance on the ADAS-cog, although these differences were statistically significant only at 18 months.
Outcomes
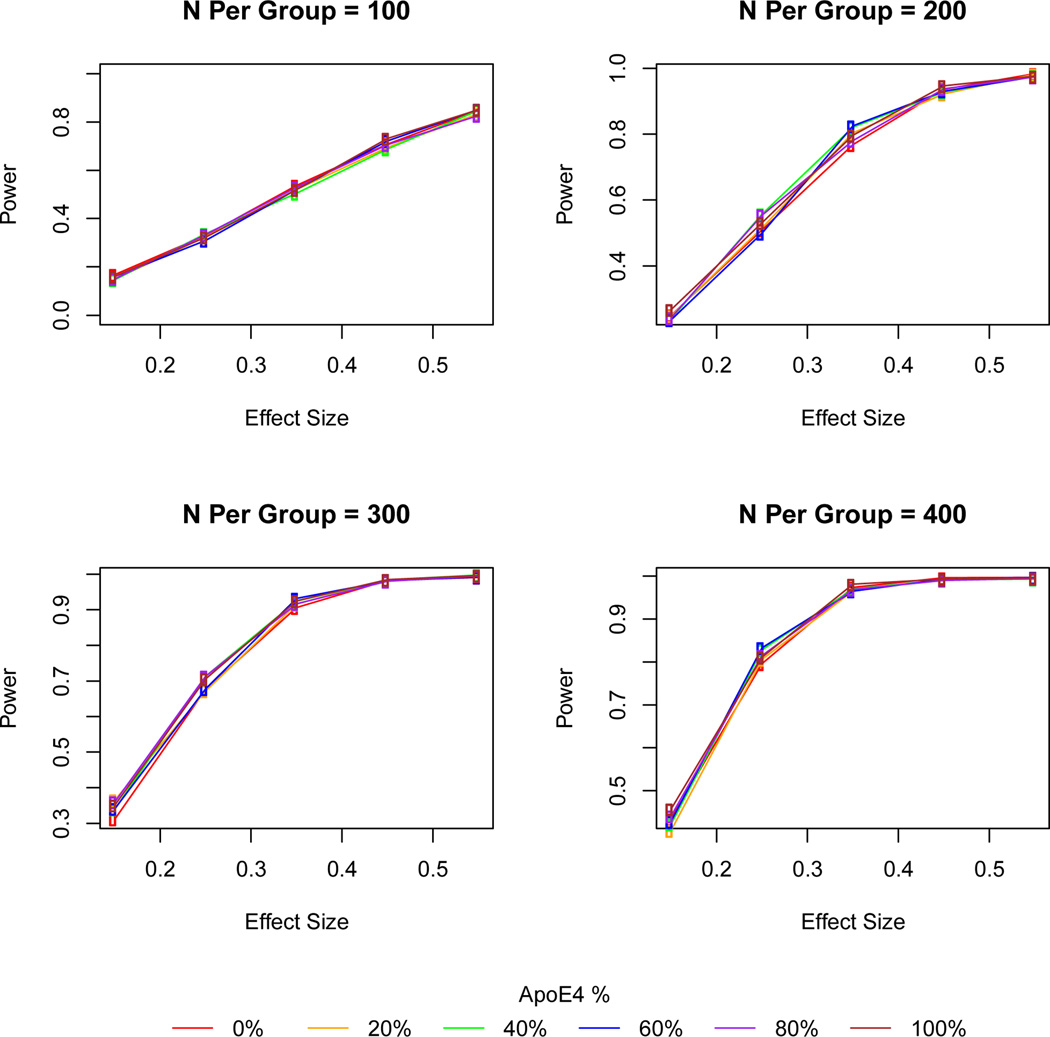
Power calculations for the mixed model analyses are shown across a range of effect and sample sizes providing for 40% dropouts and 18-month duration for AD and 24-month for MCI due to AD (Figures 1 and 2). Power increased with increasing effect size and sample size but there was generally little difference in power across the range of APOE ε4 carrier percentages - typically less than 2% increase in power (Tables 4 and 5; additional details in Supplemental Tables 1 and 2). Patients showed considerable heterogeneity in their clinical course and within each diagnostic group. Although there were greater mean differences between placebo and treatment groups among APOE ε4 carriers, there were also greater increases in variability that tended to offset these differences in computing power and sample size.
Figure 1. Power for ADAS-cog outcomes in 24 month long trials of participants with MCI due to AD.
Power calculations for the ADAS-cog by effect size and sample size across with APOE ε4 carriers ranging from 0% to 100% of the sample. Enrichment based on APOE ε4 carrier status did not appreciably increase power under any of the scenarios. Simulation parameters included α=0.05, Chi-squared random errors, and 40% dropouts with mixed model analysis for participants with missing data.
Figure 2. Power for ADAS-cog outcomes in 18 month long trials of participants with dementia due to AD.
Power calculations for the ADAS-cog by effect size and sample size across with APOE ε4 carriers ranging from 0% to 100% of the sample. Enrichment based on APOE ε4 carrier status did not appreciably increase power under any of the scenarios. Simulation parameters included α=0.05, Chi-squared random errors, and 40% dropouts with mixed model analysis for participants with missing data.
Table 4. Power for ADAS-cog outcomes in 24 month long trials of participants with MCI due to AD based on APOE ε4 carrier status.
To ensure an approximate power of 80% to 90% for the mixed model analysis in a trial of 100% APOE ε4 carriers, simulations show that for small effects of 0.25, typical to that of cholinesterase inhibitors, somewhat greater than 400 patients per group are needed with a dropout rate of 40%. Enrichment based on APOE ε4 carrier status resulted in very small increases in statistical power across most scenarios. Simulation parameters included α=0.05, effect sizes of 0.25 to 0.45 with Chi-squared random errors, and 40% dropouts with mixed model analysis for participants with missing data.
| N per group |
Effect Size |
Apo ε4 % |
Treatment group mean |
Placebo group mean |
Treatment group SD |
Placebo group SD |
Power mixed model |
|---|---|---|---|---|---|---|---|
| 50 | 0.45 | 0 | −1.15 | 0.96 | 5.22 | 4.85 | 0.363 |
| 50 | 0.45 | 0.6 | −0.55 | 1.71 | 5.89 | 5.48 | 0.367 |
| 50 | 0.45 | 1 | −0.12 | 2.24 | 6.16 | 5.75 | 0.376 |
| 100 | 0.45 | 0 | −1.14 | 0.96 | 5.36 | 4.96 | 0.600 |
| 100 | 0.45 | 0.6 | −0.57 | 1.76 | 6.02 | 5.60 | 0.613 |
| 100 | 0.45 | 1 | −0.10 | 2.29 | 6.24 | 5.86 | 0.590 |
| 200 | 0.35 | 0 | −0.71 | 0.91 | 5.36 | 5.00 | 0.691 |
| 200 | 0.35 | 0.6 | −0.07 | 1.75 | 5.95 | 5.59 | 0.722 |
| 200 | 0.35 | 1 | 0.35 | 2.26 | 6.25 | 5.95 | 0.713 |
| 200 | 0.45 | 0 | −1.17 | 0.94 | 5.44 | 5.00 | 0.860 |
| 200 | 0.45 | 0.6 | −0.60 | 1.77 | 6.00 | 5.63 | 0.876 |
| 200 | 0.45 | 1 | −0.16 | 2.26 | 6.31 | 5.97 | 0.879 |
| 300 | 0.35 | 0 | −0.72 | 0.96 | 5.40 | 5.07 | 0.866 |
| 300 | 0.35 | 0.6 | −0.07 | 1.79 | 5.98 | 5.66 | 0.867 |
| 300 | 0.35 | 1 | 0.35 | 2.28 | 6.26 | 6.00 | 0.859 |
| 400 | 0.25 | 0 | −0.24 | 0.96 | 5.28 | 5.05 | 0.745 |
| 400 | 0.25 | 0.6 | 0.44 | 1.78 | 5.87 | 5.66 | 0.742 |
| 400 | 0.25 | 1 | 0.89 | 2.29 | 6.22 | 6.03 | 0.726 |
| 400 | 0.35 | 0 | −0.73 | 0.94 | 5.37 | 5.07 | 0.937 |
| 400 | 0.35 | 0.6 | −0.10 | 1.75 | 5.95 | 5.68 | 0.940 |
| 400 | 0.35 | 1 | 0.37 | 2.25 | 6.27 | 5.99 | 0.928 |
Table 5. Power for ADAS-cog outcomes in 18 month long trials of participants with dementia due to AD based on APOE ε4 carrier status.
To ensure an approximate power of 80% to 90% for the mixed model analysis, simulations show that for small effects of 0.25, typical to that of cholinesterase inhibitors, somewhat fewer than 400 patients per group are needed with a dropout rate of 40%, and for medium size effects of 0.45, somewhat greater than 100 per group are needed with a dropout rate of 40%. Enrichment based on APOE ε4 carrier status resulted in very small increases in statistical power. Simulation parameters included α=0.05, effect sizes of 0.25 to 0.45 with Chisquared random errors, and 40% dropouts with mixed model analysis for participants with missing data.
| N per group |
Effect Size |
Apo ε4 % |
Treatment group mean |
Placebo group mean |
Treatment group SD |
Placebo group SD |
Power mixed model |
|---|---|---|---|---|---|---|---|
| 50 | 0.45 | 0.0 | 2.43 | 5.63 | 8.03 | 7.61 | 0.437 |
| 50 | 0.45 | 0.6 | 3.02 | 6.35 | 7.99 | 7.63 | 0.474 |
| 50 | 0.45 | 1.0 | 3.55 | 6.82 | 7.91 | 7.54 | 0.454 |
| 100 | 0.45 | 0.0 | 2.29 | 5.69 | 8.18 | 7.83 | 0.709 |
| 100 | 0.45 | 0.6 | 2.96 | 6.32 | 8.04 | 7.69 | 0.722 |
| 100 | 0.45 | 1.0 | 3.37 | 6.66 | 7.95 | 7.60 | 0.732 |
| 200 | 0.35 | 0.0 | 3.06 | 5.69 | 8.20 | 7.87 | 0.768 |
| 200 | 0.35 | 0.6 | 3.68 | 6.31 | 8.03 | 7.74 | 0.825 |
| 200 | 0.35 | 1.0 | 4.11 | 6.66 | 7.95 | 7.65 | 0.797 |
| 200 | 0.45 | 0.0 | 2.27 | 5.67 | 8.23 | 7.82 | 0.937 |
| 200 | 0.45 | 0.6 | 2.90 | 6.29 | 8.11 | 7.71 | 0.931 |
| 200 | 0.45 | 1.0 | 3.36 | 6.65 | 8.03 | 7.64 | 0.947 |
| 300 | 0.25 | 0.0 | 3.81 | 5.68 | 8.09 | 7.86 | 0.676 |
| 300 | 0.25 | 0.6 | 4.44 | 6.29 | 7.98 | 7.75 | 0.678 |
| 300 | 0.25 | 1.0 | 4.83 | 6.66 | 7.88 | 7.65 | 0.706 |
| 300 | 0.35 | 0.0 | 3.03 | 5.68 | 8.19 | 7.88 | 0.906 |
| 300 | 0.35 | 0.6 | 3.68 | 6.32 | 8.09 | 7.79 | 0.932 |
| 300 | 0.35 | 1.0 | 4.13 | 6.69 | 7.95 | 7.65 | 0.925 |
| 400 | 0.25 | 0.0 | 3.77 | 5.68 | 8.14 | 7.90 | 0.796 |
| 400 | 0.25 | 0.6 | 4.41 | 6.30 | 7.98 | 7.77 | 0.833 |
| 400 | 0.25 | 1.0 | 4.85 | 6.69 | 7.89 | 7.68 | 0.811 |
| 400 | 0.35 | 0.0 | 3.04 | 5.72 | 8.20 | 7.91 | 0.974 |
| 400 | 0.35 | 0.6 | 3.71 | 6.32 | 8.06 | 7.75 | 0.965 |
| 400 | 0.35 | 1.0 | 4.08 | 6.68 | 7.96 | 7.68 | 0.981 |
Analyses of other trial durations using the scenarios above also did not show a meaningful difference in power for the outcomes across APOE ε4 carrier percentages (data not shown). Secondary analyses using samples excluding individuals with the ε2 genotype showed similar, very small differences between diagnostic groups. (Supplemental Tables 3 and 4 and Supplemental Figures 1 and 2). Analyses using only milder dementia participants with MMSE ≥ 16 did not differ from results using all dementia participants (Supplemental Table 5 and Supplemental Figure 3). Simulations using only ε2 carriers (8.5% and 6.8% of the MCI due to AD sample and AD sample, respectively) did show slower progression and decreased variability compared to simulations predominantly involving ε3 and ε4, leading to slightly greater power to detect treatment differences (Supplemental Tables 6 and 7 and Supplemental Figures 4 and 5). Simulations of trials with enrichment based on the number of APOE ε4 alleles showed little difference in power when selecting those with 1 or with 2 copies of the ε4 allele, echoing the results when simply selecting based on presence or absence of the ε4 allele (Supplemental Tables 8 and 9 and Supplemental Figures 6 and 7).
By contrast, power calculations using a fixed reduction in slope showed an increase in power as the percentage of APOE ε4 carriers increased (Table 6). However, increases in the mean and, to a lesser extent, the variance in ADAS-cog scores also meant that the effect size was large among APOE ε4 carriers.
Table 6.
Sample size calculations based on effect sizes and slope reductions. All scenarios are based on a sample size of 200 per group and 40% dropouts. For each effect size, simulations were used to compute the corresponding slope reduction, which was calculated as (placebo – treatment change) / placebo change * 100, and power. For each slope reduction, effect sizes were calculated as (treatment – placebo change) / (standard deviation of placebo) and power was calculated using a two−sample t−test. Although power did increase with APOE ε4 enrichment in the slope reduction approach, this also corresponds to a greater differential effect in the APOE ε4 group.
| Effect Size | 0.25 | 0.35 | 0.45 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APOE ε4 % | Baseline ADAS-cog |
Placebo Change |
Treatment Change |
Drug-placebo difference |
% Slope reduction |
Power | Placebo Change |
Treatment Change |
Drug-placebo difference |
% Slope reduction |
Power | Placebo Change |
Treatment Change |
Drug-placebo difference |
% Slope reduction |
Power |
| 0% | 10.4 (4.2) | 0.97 (5.04) | −0.22 (5.24) | 1.19 | 123 | 0.466 | 0.97 (5.04) | −0.71 (5.36) | 1.62 | 167 | 0.691 | 0.94 (5.00) | −1.17 (5.44) | 2.11 | 224 | 0.860 |
| 60% | 11.3 (4.3) | 1.77 (5.66) | 0.45 (5.88) | 1.32 | 75 | 0.467 | 1.77 (5.66) | −0.07 (5.95) | 1.82 | 103 | 0.722 | 1.77 (5.66) | −0.6 (6.00) | 2.37 | 134 | 0.876 |
| 100% | 12.1 (4.4) | 2.29 (5.95) | 0.93 (6.14) | 1.36 | 59 | 0.458 | 2.29 (5.95) | 0.35 (6.25) | 1.91 | 83 | 0.713 | 2.26 (5.97) | −0.16 (6.31) | 2.10 | 107 | 0.879 |
| Slope Reduction | 40% | 50% | 60% | |||||||||||||
| APOE ε4 % | Baseline ADAS-cog |
Placebo Change |
Treatment Change |
Drug-placebo difference |
Effect Size | Power | Placebo Change |
Treatment Change |
Drug-placebo difference |
Effect Size | Power | Placebo Change |
Treatment Change |
Drug-placebo difference |
Effect Size | Power |
| 0% | 10.4 (4.2) | 0.97 (5.04) | 0.582 (5.04) | 0.388 | 0.08 | 0.117 | 0.97 (5.04) | 0.485 (5.04) | 0.485 | 0.10 | 0.159 | 0.94 (5.00) | 0.376 (5.00) | 0.564 | 0.11 | 0.202 |
| 60% | 11.3 (4.3) | 1.77 (5.66) | 1.062 (5.66) | 0.708 | 0.13 | 0.236 | 1.77 (5.66) | 0.885 (5.66) | 0.885 | 0.16 | 0.345 | 1.77 (5.66) | 0.708 (5.66) | 1.062 | 0.19 | 0.465 |
| 100% | 12.1 (4.4) | 2.29 (5.95) | 1.374 (5.95) | 0.916 | 0.15 | 0.336 | 2.29 (5.95) | 1.145 (5.95) | 1.145 | 0.19 | 0.484 | 2.26 (5.97) | 0.904 (5.97) | 1.356 | 0.23 | 0.620 |
DISCUSSION
This study provides an empirical evaluation through the use of simulations of recent recommendations for the incorporation of biomarkers, specifically APOE ε4, in clinical trials of AD dementia and MCI due to AD [20]. Enrichment of the clinical trial population based on APOE ε4 carrier status did not result in meaningful increases in the efficiency of the trials compared to the typical approach of enrolling AD or MCI participants regardless of APOE ε4 status. For example, for an MCI trial with an expected small effect size of 0.25, typical to that of cholinesterase inhibitors, an un-enriched trial with only 60% APOE ε4 carriers would require approximately 432 participants to achieve 80% power. By comparison, note that this is about 15% lower than the 506 participants that would be required based on a t-test of differences, and illustrates the advantages of using simulations with longitudinal data. Furthermore, the simulations can be adapted to model more complex trials while avoiding the assumptions needed for fixed formulas using repeated measures. An enriched trial of 100% APOE ε4 carriers would require essentially the same number of participants, approximately 440 patients, to achieve 80% power. However, assuming 60% of otherwise eligible clinical trials applicants are APOE ε4 carriers, approximately 734 subjects would need to be screened to reach this goal of 440. Similar results would apply for enrichment based on APOE ε4 noncarrier status, but here assuming 40% are noncarriers, 1075 would have to be screened. Thus, the small gains in power based on APOE ε4 enrichment are almost certain to be offset by the time and effort required in screening.
As with our previous evaluation of CSF Aβ1–42 and Aβ1–42 /t-tau biomarkers in simulated clinical trials [28], APOE ε4 carrier status has several drawbacks that limit its usefulness as an inclusion criterion for the selection of participants. The utility of biomarkers in clinical trials depends on the effectiveness of the drug in both the biomarker positive and negative groups, the proportion of biomarker positive patients in the sample, and the accuracy of the assay [29]. Enrichment strategies are generally effective when less than 50% of applicants are biomarker positive and the drug has little benefit for biomarker negative patients [29]. As approximately 60% of clinical trial participants are APOE ε4 carriers and 40% are non-carriers [30], selecting for APOE ε4 status fails to satisfy the former. (Even selection of APOE ε4 non-carriers, the minority of patients who enter trials, may not screen out a sufficient number of subjects to make it an effective biomarker.) Furthermore, the latter criterion is not satisfied as no differential response based on APOE ε4 status has been convincingly demonstrated to date, although it has sometimes been argued so based on theoretical grounds or post hoc analyses of completed trials. (For example, rosiglitazone has been postulated to have preferential effects in APOE ε4 non-carriers, based on reduction of amyloid plaque burden and amyloid-associated inflammation in animal models [31].) Indeed, even the theoretical basis for differential response has been conflicted, with some arguing for better response among APOE ε4 carriers based on more rapid rate of progression, while others arguing for better response among APOE ε4 non-carriers based on different metabolism and the lower accumulation of Aβ plaques in the brain [11]. APOE ε4 carriage is strongly predictive of positive Aβ biomarker status. For example, in ADNI among participants with MCI due to AD and mild AD who were APOE ε4 carriers, 88% and 98%, respectively, had a low CSF Aβ level less than 192 pG/mL. The issue of differential response must also be borne in mind when estimating sample sizes for the planning of clinical trials. For example, basing sample sizes on expected percentage of slope reduction may indirectly imply an assumption of differential response when this was not intended.
Thus, APOE ε4 carriers who are ultimately enrolled in clinical trials may be more likely to be positive on CSF and PET Aβ biomarkers [32, 33, 34]. This may, however, reflect past Aβ accumulation and not be a further predictor of clinical response [12]. Another potential explanation for the minimal increase in power with APOE ε4 in clinical trial selection is the considerable heterogeneity among APOE ε4 carriers. Although multiple studies [35, 36, 37, 38, 39, 40] including ADNI [41, 42] have demonstrated more rapid progression of cognitive impairment in MCI and AD among APOE ε4 carriers and increased risk of conversion from MCI to dementia, such studies have also demonstrated substantial variability among the participants within a group [43, 44]. Such variation is likely to carry over into clinical trials and adversely affect trial outcomes, as demonstrated by our simulations. Despite greater clinical worsening of about 0.5 ADAS-cog points in the APOE ε4 carriers, the standard deviations for the outcomes were larger, decreasing the power to detect drug-placebo differences, i.e., the within group effect sizes were about the same.
A third consideration in the utility of APOE ε4 carrier status in the design of clinical trials is that, although carrier status is associated with more rapid decline, it is also associated with greater impairment at baseline assessment. This is consistent with previous studies showing that APOE ε4 primarily exerts its effect in AD by influencing age of onset [45]. Thus, it appears that APOE ε4 carrier status -- when determined after a diagnosis of MCI has been made, as in ADNI and the MIS trial [10] -- may mainly identify more advanced disease or more impaired cognitive scores at baseline, so that cognitive severity is the more pragmatic predictor of decline [46, 47]. Under these circumstances, APOE ε4 status would have utility in the diagnosis of MCI due to AD or AD at a single time point, but this does not necessarily translate into utility in treatment trials that focus on rates of change over time. In a similar vein, it must be recognized that participants in clinical trials and ADNI are samples of convenience with their own prevailing characteristics, so that trends observed in population studies (such as the effect of genotype on disease progression or as a prognostic biomarker) may differ from trends observed in clinical trials. Particularly for trials with small sample sizes, trends contradictory to population studies may be seen [48].
Based on these considerations, APOE ε4 status may have greater utility as an explanatory covariate or stratification variable than as an inclusion criteria for clinical trials, especially in pre-planned subanalyses incorporated into the trial design, and when warranted by the hypothesized actions of the drug treatment. In this context, APOE ε4 may serve as a proxy for disease severity (as it is associated with earlier age of onset, a measure of disease severity that can often only be approximated) or for unmeasured biological processes targeted by an intervention (such as fibrillar Aβ deposition). In addition, stratification could be useful with agents that show a strong differential response or adverse effect profile based on APOE ε4 status. Enrichment based on APOE ε4 status may also show benefits in such circumstances, which were not examined in this study, but may preclude further analyses of differential effect of APOE status within the trial if the number of noncarriers becomes sufficiently small.
Finally, it is important to note that this study did not address the utility of enrichment for APOE ε4 carrier status in prevention trials among participants who have not yet manifested illness, but only the utility of enrichment in therapeutic trials among individuals with a diagnosis of MCI or dementia due to AD. Thus, further research is needed before conclusions can be reached about APOE ε4 enrichment in the former type of design. Particularly if APOE ε4 affects age of onset rather than rate of decline, selecting APOE ε4 carriers for intervention prior to symptom onset may lead to very different results. It is also likely that the prevalence of APOE ε4 carriers in prevention trials would be lower – approaching the population rate of 25% rather than the 50 – 60% observed in the samples of convenience used in therapeutic clinical trials – and thus the utility of screening would be greater.
A particular strength of this study is that it expands previous clinical trials simulations based on the ADNI dataset [28] by using a meta-database of several clinical trials and observational studies across a population of more than 5500 individuals. The inclusion of a large number of participants across several studies greatly increases generalizability, as the design of the ADNI study may limit the conclusions that can be drawn. Meta-databases may be rich resources not only for simulation studies but also for meta-analyses and other investigations into the design and analysis of clinical trials in dementia and MCI due to AD.
One limitation to these results is that the utility of the APOE ε4 biomarker was assessed in isolation, and its usefulness in conjunction with other biomarkers was not evaluated. However, the primary goal of this study was to evaluate enrichment strategies for clinical trials, and APOE ε4 status is an inexpensive and readily available marker for such purposes. Another limitation is that the primary analyses only stratified based on the presence or absence of the APOE ε4 allele, and did not investigate the specific effects of each of the 6 possible genotypes. This was also consistent with the goal of evaluating enrichment strategies for clinical trials, where more extensive stratification is not likely to be feasible. Similarly, this study did not evaluate the effects of different statistical model parameterizations, but instead utilized a single model with age and education as covariates. The random slope model has been recommended for the analysis of clinical trial data in Alzheimer’s disease and MCI, and has flexibility and merits. Although the model selection is a key aspect of trial design that can influence the overall results, it should also be noted that the goal of this study was not to investigate the absolute power attained under a specific modeling strategy, but the relative power (or difference in power) afforded by enrichment strategies.
A third limitation is this study assumed equal response between APOE ε4 carriers and noncarriers, and did not investigate potential gains or losses in power if APOE ε4 carrier status (or not) is associated with a preferential response to treatment. Such an approach could be interpreted as artificially inducing a lack of difference between groups, but it is also reflective of current clinical trial design and analysis, which in general have not included a differential response based on genotype as none has been consistently shown to date. However, if a therapy that is preferentially effective by APOE ε4 carrier status becomes available in the future, then enrichment of clinical trials based on APOE status may be more attractive. Previous simulation studies, however, have indicated that biomarker positive status must be associated with a marked difference in response between groups for biomarker selection to be effective [29]. Therefore, even a moderate degree of preferential response due to APOE carrier status may not be sufficient to justify such screening strategies. Finally, although resampling strategies incorporate the variability from sample to sample that is usually not adequately contained in parametric models, even resampling may not capture all of the relevant characteristics of clinical trial participants. The use of a meta-database rather than a single study for the simulations tends to mitigate this problem by including a larger sample likely to be representative of clinical trial participants in general.
In sum, selecting patients with MCI or dementia due to AD for a clinical trial on the basis of APOE ε4 carrier status (which also predicts amyloid-beta biomarker positivity) will most likely not enhance the trials’ statistical power. Results of these simulations likely will be confirmed in the near future in ongoing clinical trials that utilize APOE ε4 status as an inclusion criterion. In the absence of a strong scientific rationale positing a relationship between APOE ε4 status and therapeutic drug actions, it may be more practical and clinically relevant to not require APOE ε4 genotyping for trials entry, but to restrict their use as explanatory or stratification variables when there are reasons to do so.
Supplementary Material
Acknowledgments
Funding acknowledgments: Funding for this reported was provided by NIH R 01 AG037561 (LSS, REK, GRC), NIH P50 AG05142, ADRC (LSS), Data used in the preparation of this study were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI, NIA U01 AG024904) database (www.loni.ucla.edu/ADNI), and from the ADCS (NIH AG10483).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors participated in the writing and editing of the manuscript.
Conflict of interest statement
During the 36 month window before submission
Dr Schneider reports being an editor on the Cochrane Collaboration Dementia and Cognitive Improvement Group, which oversees systematic reviews of drugs for cognitive impairment and dementia; receiving a grant from the Alzheimer’s Association for a registry for dementia and cognitive impairment trials; receiving grant or research support from Baxter, Eli Lilly, Genentech, Novartis, and Pfizer; and having served as a consultant for or receiving consulting fees from AC Immune, Accera, Allon, AstraZeneca, Baxter, Biogen Idec, Chiesi, Elan, Eli Lilly, En Vivo, GlaxoSmithKline, Ipsen, Johnson & Johnson, Lundbeck, Merck, Pfizer, Roche, Takeda, Toyama, and Zinfandel.
REK reports receiving grant support from NIA, NINDS, NHLBI, NIDDK, and the Department of Education.
GRC reports receiving grant or research support from Participation of Data and Safety Monitoring Committees: All of the below organizations are focused on medical research: Apotek, Biogen-Idec, Cleveland Clinic, Glaxo Smith Kline Pharmaceuticals, Gilead Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Neuren, Revalesio, Sanofi-Aventis, Teva , Vivus, NHLBI (Bone Marrow Transplant Protocol Review Committee), NINDS, NMSS, NICHD (OPRU oversight committee). Consulting, Speaking fees & Adviosry Boards: Alexion, Allozyne, Bayer, Celgene, Coronado Biosciences, Consortium of MS Centers (grant), Diogenix, Klein-Buendel Incorporated, Medimmune, Novartis, Nuron Biotech, Receptos, Spiniflex Pharmaceuticals, Teva pharmaceuticals.
Dr. Cutter is employed by the University of Alabama at Birmingham and President of Pythagoras, Inc. a private consulting company located in Birmingham, AL.
REFERENCES
- 1.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farlow MR, He Y, Tekin S, Xu J, Lane R, Charles HC. Impact of APOE in mild cognitive impairment. Neurology. 2004;63(10):1898–1901. doi: 10.1212/01.wnl.0000144279.21502.b7. [DOI] [PubMed] [Google Scholar]
- 3.Linton MF, Gish R, Hubl ST, Bütler E, Esquivel C, Bry WI, et al. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J Clin Invest. 1991;88(1):270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, et al. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer's amyloid beta peptides. Biochem J. 2000;348(Pt 2):359–365. [PMC free article] [PubMed] [Google Scholar]
- 5.Aerssens J, Raeymaekers P, Lilienfeld S, Geerts H, Konings F, Parys W. APOE genotype: no influence on galantamine treatment efficacy nor on rate of decline in Alzheimer's disease. Dement Geriatr Cogn Disord. 2001;12(2):69–77. doi: 10.1159/000051238. [DOI] [PubMed] [Google Scholar]
- 6.Blesa R, Aguilar M, Casanova JP, Boada M, Martínez S, Alom J, et al. Relationship between the efficacy of rivastigmine and apolipoprotein E (epsilon4) in patients with mild to moderately severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(4):248–254. doi: 10.1097/01.wad.0000213880.93665.c7. [DOI] [PubMed] [Google Scholar]
- 7.Poirier J, Delisle MC, Quirion R, Aubert I, Farlow M, Lahiri D, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92(26):12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rigaud A, Traykov L, Latour F, Couderc R, Moulin F, Forette F. Presence or absence of at least one epsilon 4 allele and gender are not predictive for the response to donepezil treatment in Alzheimer's disease. Pharmacogenetics. 2002;12(5):415–420. doi: 10.1097/00008571-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Lane R, Feldman HH, Meyer J, He Y, Ferris SH, Nordberg A, et al. Synergistic effect of apolipoprotein E epsilon4 and butyrylcholinesterase K-variant on progression from mild cognitive impairment to Alzheimer's disease. Pharmacogenet Genomics. 2008;18(4):289–298. doi: 10.1097/FPC.0b013e3282f63f29. [DOI] [PubMed] [Google Scholar]
- 10.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 11.Cummings JL. Alzheimer's disease clinical trials: changing the paradigm. Curr Psychiatry Rep. 2011;13(6):437–442. doi: 10.1007/s11920-011-0234-y. [DOI] [PubMed] [Google Scholar]
- 12.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thal L. Development of the Alzheimer's Disease Cooperative Study. Int. J. Geriatr. Psychopharmacol. 1997;1:6–9. [Google Scholar]
- 14.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer's Disease Assessment Scale that broaden its scope. Alzheimer Dis. Assoc. Disord. 1997;11(Suppl 2):S13–S21. [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein S, McHugh P. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 20.Burton A, Altman DG, Royston P, Holder RL. The design of simulation studies in medical statistics. Stat. Med. 2006;25(24):4279–4292. doi: 10.1002/sim.2673. [DOI] [PubMed] [Google Scholar]
- 21.Doody RS, Ferris SH, Salloway S, Sun Y, Goldman R, Watkins WE, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology. 2009;72(18):1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 23.Brown H, Prescott R. Applied Mixed Models in Medicine. 2nd ed. Chichester: Wiley; 2006. [Google Scholar]
- 24.Siddiqui O, Hung HMJ, O'Neill R. MMRM vs. LOCF: a comprehensive comparison based on simulation study and 25 NDA datasets. J. Biopharm. Stat. 2009;19(2):227–246. doi: 10.1080/10543400802609797. [DOI] [PubMed] [Google Scholar]
- 25.Schneider LS, Kennedy RE, Cutter GR. Estimating power with effect size versus slop differences: Both means and variance matter. Alzheimers Dement. 2011;7(2):247–249. [Google Scholar]
- 26.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. http://www.R-project.org/ [Google Scholar]
- 27.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York: Springer; 2000. [Google Scholar]
- 28.Schneider LS, Kennedy RE, Cutter GR Alzheimer's Disease Neuroimaging Initiative. Requiring an amyloid-beta$_1-42$ biomarker for prodromal Alzheimer's disease or mild cognitive impairment does not lead to more efficient clinical trials. Alzheimers Dement. 2010;6(5):367–377. doi: 10.1016/j.jalz.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitournam A, Simon R. On the efficiency of targeted clinical trials. Stat Med. 2005;24(3):329–339. doi: 10.1002/sim.1975. [DOI] [PubMed] [Google Scholar]
- 30.Schneider LS, Sano M. Current Alzheimer's disease clinical trials: methods and placebo outcomes. Alzheimers Dement. 2009;5(5):388–397. doi: 10.1016/j.jalz.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer's disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement. Geriatr. Cogn. Disord. 2010;30(2):131–146. doi: 10.1159/000318845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67(3):308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleisher AS, Chen K, Liu X, Ayutyanont N, Roontiva A, Thiyyagura P, et al. Apolipoprotein E ε4 and age effects on florbetapir positron emission tomography in healthy aging and Alzheimer disease. Neurobiol Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 34.Drzezga A, Grimmer T, Henriksen G, Mühlau M, Perneczky R, Miederer I, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72(17):1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 35.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66(6):828–832. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 37.Lopez OL, Jagust WJ, Dulberg C, Becker JT, DeKosky ST, Fitzpatrick A, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60(10):1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 38.Luck T, Riedel-Heller SG, Luppa M, Wiese B, Wollny A, Wagner M, et al. Risk factors for incident mild cognitive impairment--results from the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe) Acta Psychiatr Scand. 2010;121(4):260–272. doi: 10.1111/j.1600-0447.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 39.Petersen RC, Waring SC, Smith GE, Tangalos EG, Thibodeau SN. Predictive value of APOE genotyping in incipient Alzheimer's disease. Ann N Y Acad Sci. 1996;802:58–69. doi: 10.1111/j.1749-6632.1996.tb32599.x. [DOI] [PubMed] [Google Scholar]
- 40.Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- 41.Risacher SL, Shen L, West JD, Kim S, McDonald BC, Beckett LA, et al. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2010;31(8):1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, et al. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132(Pt 4):1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Devanand DP, Pelton GH, Zamora D, Liu X, Tabert MH, Goodkind M, et al. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62(6):975–980. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- 44.Mungas D, Beckett L, Harvey D, Farias ST, Reed B, Carmichael O, et al. Heterogeneity of cognitive trajectories in diverse older persons. Psychol Aging. 2010;25(3):606–619. doi: 10.1037/a0019502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khachaturian AS, Corcoran CD, Mayer LS, Zandi PP, Breitner JCS Cache County Study Investigators. Apolipoprotein E epsilon4 count affects age at onset of Alzheimer disease, but not lifetime susceptibility: The Cache County Study. Arch Gen Psychiatry. 2004;61(5):518–524. doi: 10.1001/archpsyc.61.5.518. [DOI] [PubMed] [Google Scholar]
- 46.Schmand B, Huizenga HM, van Gool WA. Meta-analysis of CSF and MRI biomarkers for detecting preclinical Alzheimer's disease. Psychol Med. 2010;40(1):135–145. doi: 10.1017/S0033291709991516. [DOI] [PubMed] [Google Scholar]
- 47.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund L, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8(7):619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 48.Stone DJ, Molony C, Suver C, Schadt EE, Potter WZ. ApoE genotyping as a progression-rate biomarker in phase II disease-modification trials for Alzheimer's disease. Pharmacogenomics J. 2010;10(3):161–164. doi: 10.1038/tpj.2009.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.