Abstract
Study Objectives:
We examined associations between pubertal maturation and sleep in early adolescence, at age 12 y, and continuity and change in actigraphy-based sleep and parent-reported sleep disorders from age 8 to 12 y. We also explored longitudinal associations between actigraph estimates of sleep and sleep disorders.
Design:
A cohort study of children born in 1998 and tested at ages 8 y (standard deviation [SD] = 0.3) and 12 y (SD = 0.5).
Participants:
A total of 348 children participated in cross-sectional analyses. We had longitudinal actigraphy data for 188 children and repeated parent reports of sleep disorders for 229 children.
Measurements and Results:
At age 8 y, participants wore actigraphs for 7.1 nights (SD = 1.2, range 3-14) on average and at age 12 y for 8.4 nights (SD = 1.7, range 3-11). Sleep disorders were parent-rated based on the Sleep Disturbance Scale for Children. Pubertal maturity was self-reported at age 12 y using the continuous Pubertal Development Scale and the picture-assisted categorical Tanner scales.
Results:
Significant mean-level changes toward shorter but higher quality sleep occurred over time. Sleep variables had low to high rank-order stability over time. Sleep disorders were highly stable from age 8 to 12 y. Actigraphy-based sleep and parent-rated sleep disorders showed no association either in cross-section or longitudinally. Pubertal maturation was not associated with worse sleep.
Conclusions:
Sleep in early adolescence can be anticipated from childhood sleep patterns and disorders, but is not associated with pubertal maturity. Although sleep duration becomes shorter, sleep quality may improve during early adolescence. Parent-rated sleep disorders are distinct from actigraph estimates of sleep.
Citation:
Pesonen AK; Martikainen S; Heinonen K; Wehkalampi K; Lahti J; Kajantie E; Räikkönen K. Continuity and change in poor sleep from childhood to early adolescence. SLEEP 2014;37(2):289-297.
Keywords: Actigraph, adolescents, children, cohort, longitudinal, puberty, sleep disorder
INTRODUCTION
Adolescence has been associated with alterations in sleep-wake organization. For instance, changes in pubertal maturation have been associated with later circadian phase preference,1 lower melatonin secretion amplitude,2 and a decline in delta (1-4 Hz) and theta (4-8 Hz) nonrapid eye movement sleep.3 Although the need for sleep is argued to remain unchanged through adolescence,4 greater intrinsic and environmental pressures to later bedtimes, coupled with early school starts, easily lead to daytime sleepiness.5 An estimated 9% to 42% of adolescents worldwide suffer from insufficient sleep.6 Compensating for this accumulating sleep loss with longer catch-up sleep periods during the weekend promotes further irregular sleep patterns in adolescence.6
However, existing epidemiological sleep studies in adolescence are based mostly on cross-sectional study designs and subjective or parent-reported sleep complaints. Consequently, they cannot answer the question of whether altered sleep, as reflected in late sleep onset time and/or short sleep, poor sleep quality, or sleep problems, is a product of pubertal maturation, or whether variations in childhood sleep patterns or problems precede them. It is noteworthy that not all adolescents experience sleep problems, and trajectories how pubertal maturation affects sleep vary considerably between subjects.3
Longitudinal studies with data spanning from childhood through adolescence are urgently needed to address these questions. Yet, follow-up studies with objective sleep measurements from childhood to adolescence are rare. Continuity and change in non-categorical, continuous measures of sleep can be addressed by studying both mean-level stability, referring to the absolute consistency of sleep parameters over time, and rank-order stability, referring to the maintenance of the individual's relative position within the study group as defined by these sleep parameters. In an actigraphy-based study, Sadeh et al.7 followed 94 children across three ages, 10.5, 11.5 and 12.5 y, and found significant mean-level changes during this period: sleep duration decreased by 37 min and sleep onset was delayed by 50 min, but there were no significant changes in sleep efficiency. Sleep duration, sleep onset time, and sleep efficiency showed high rank-order stability over time. Interestingly, pubertal maturity was not associated with the sleep variables at either baseline or during follow-up. However, later sleep onset time, shorter sleep duration, and lower sleep efficiency at age 10.5 y predicted faster pubertal maturation from age 10.5 to 11.5 y, but not from age 11.5 to 12.5 y. Based on a cross-lagged model, it was concluded that in the early stages of pubertal development, sleep-wake patterns preceded pubertal changes, whereas no similar influence occurred in the opposite direction, i.e., pubertal changes showed no prospective association with worse sleep.
In another actigraphy-based study, Kelly and El-Sheikh8 also reported high rank-order stability for sleep duration from age 8.7 to 10.7 y among 176 healthy children. The main focus of their study, however, was on the reciprocal associations between sleep, cognitive development, family environment, and emotional security: they controlled for pubertal development but did not report its associations with the sleep variables.8–11
In addition to mean-level and rank-order continuity, other studies have addressed whether categorical sleep disorders, or sleep problems/disturbances treated as categorical, show continuity across development. These studies seek to show which proportion of children with sleep disorders/problems exhibit them in later assessments, or what the odds are for having a persistent disorder/problem. For example, experiencing frequent bad dreams at the age of 29 mo was associated a 2.9-fold greater risk for having bad dreams at age 6 y.12 Approximately 60% of 9-y-old children with a problem in initiating sleep it persisted over 2 y,13 and after a 4-y follow-up period from age 7 to 11 y, 35% of children experienced persistent sleep disturbance.14 However, the evidence for continuing sleep disorders from childhood to adolescence is still lacking.
Consequently, this study aims to increase our understanding of continuity and change in sleep over a 4-y period, from childhood (8 y) to early adolescence (12 y). Our study had three aims. First, we examined the contributions of pubertal maturation to actigraphy-based sleep and parent-reported sleep disorders in early adolescence. Second, we examined the mean-level and rank-order continuity and change in actigraph estimates of sleep from age 8 to 12 y, and the continuation of parent-rated sleep disorders from childhood to early adolescence. Third, because the associations between parent-rated sleep disorders and actigraph estimates of sleep are poorly understood, we investigated their associations both in cross-section and longitudinally.
METHODS
Participants
The children came from an urban community-based cohort comprising 1,049 infants born between March and November 1998 in Helsinki, Finland.15 In 2006, we invited a subsample of the cohort to a follow-up study that included actigraphy-based and parent-reported measures of sleep. Of the 431 children invited, 321 (77.7%) participated at a mean age of 8.1 y (standard deviation [SD] = 0.3, range 7.4-8.9 y).16,17 In 2009-2011, all initial cohort members who had given permission to be contacted and whose addresses were traceable were invited to a further follow-up that re-tested measures of sleep. Of the 920 adolescents invited, 451 (49.0%) participated at a mean age of 12.3 y (SD = 0.5, range 11.0-13.2 y). The average time difference between the two measurements was 4.2 y (SD = 0.7, range 2.6-5.6 years).
Of the 451 children participating in the follow-up at age 12 y, complete sleep and pubertal data were available for 348 adolescents. Complete sleep actigraphy data from both measurement points (ages 8 and 12 y) were available for 188 adolescents (64% of those for whom we had successful actigraphy data at age 8 y), and complete parent-reported sleep disorder data from both measurement points were available for 229 adolescents (74% of those for whom we had parent-reports available at age 8 y). In the analyses we used the maximum sample size (n = 348 in the cross-sectional analyses, and n = 188 or 229 in the longitudinal analyses).
Our previous papers have addressed the representativeness of the sample at age 8 y in relation to the initial sample.16–19 The entire sample at age 12 y with valid sleep and pubertal data (n = 348), and the sample with actigraphy data (n = 188) and parent-rated sleep disorder data (n = 229) at ages 8 and 12 y showed no differences from the initial sample in terms of birth weight (P > 0.99; P > 0.40; P > 0.36), length at birth (P > 0.86; P > 0.38; P > 0.88), or gestational age (P > 0.83; P > 0.27; P > 0.75). The participants at age 12 y were less likely to have mothers who smoked during pregnancy (P > 0.03; for other samples P > 0.22; P > 0.07), respectively. Because the invited subsample in 2006 was weighted upon maternal licorice consumption during pregnancy,20 the current samples differed in prenatal liquorice exposure in relation to the initial cohort (P < 0.03). We also examined whether the dropout rate among children with complete sleep measurements at 8 y (n = 291) was related to any of the background variables, and found that attrition in families with low educational status (40.4% dropout rate) was greater than in families where either parent had a university degree (21.4 % dropout rate; P value in χ2 = 0.03).
Ethics Committees of the City of Helsinki Health Department and Children's Hospital in Helsinki University Central Hospital approved the study protocol. Each child and her or his parent(s) provided their written informed consent at both follow-ups.
Objective Assessment of Sleep by Actigraph
Sleep was objectively measured with actigraphs (Actiwatch AW4 and AW7, Cambridge Neurotechnology Ltd., UK). The devices were worn on the nondominant wrist for an average of 7.1 nights (SD = 1.2; range 3-14) at age 8 y, and 8.4 nights (SD 1.7, range 3-11) at age 12 y. Parents and children/adolescents were instructed to maintain a sleep log on bedtimes and waking times, temporary pauses in actigraph registration (e.g., while taking a shower), and significant events that might affect sleep quantity or quality (illness, pain, injury, travel, or other events likely to disturb sleep). The adolescent was instructed to press a button (event marker) in the actigraph at bedtime and waking times. All participants provided completed sleep logs, including both parent- or self-reported sleep logs and event markers of the bedtimes and waking times reported by the child. The activity data were visually inspected to detect significant discrepancies among the sleep logs, event markers, and activity patterns. If the same night had several event markers, the most recent was compared with the sleep log. If the sleep log was not synchronous with the event marker, the event marker served to define the bedtime.
Similarly to the recordings at age 8 y,16 we found high compliance in the sleep log registrations in relation to the event markers at age 12 y: for 67% of the participants, we found no discrepancies; for 28%, we found a discrepancy of more than 5 min for 1 or 2 nights; and for 5%, we found a discrepancy for 3 or more nights. We excluded nights from further sleep analysis if (1) the actigraph was not in use, (2) information on bedtimes was missing, (3) the data on reported bedtime indicated the child was already asleep (probably the bedtime was not correctly reported), (4) information on waking time was missing and the activity pattern was unclear, or (5) the parent reported a change in normal life due to, for example, illness or travel. Of the 348 participants in the actigraph study, 148 (43%) had no excluded nights, and 327 (94%) had five or more valid sleep registration nights available.
The scored sleep data for each study subject were averaged over the valid registration nights and separately for weekday and weekend nights. Sleep duration refers to actual time asleep. We used the validated Actiwatch algorithm21 (validated in adults), which defines “Sleep start” as 10 min of consecutively recorded immobile data, with no more than 1 epoch of movement within that time period. For “Sleep end”, the algorithm looks backward from the last sample in the analysis window for a specific consecutive period (6 min) of activity below the threshold (≤ six counts) and classifies the last epoch in this period as Sleep end. Sleep efficiency was defined as actual time asleep divided by time in bed. Sleep latency is the time difference between bedtime and sleep onset time. The fragmentation index, an indication of restlessness, is an addition of percentages of moving minutes after sleep onset and percentages of minutes in immobility. The duration of catch-up sleep during the weekend was calculated as the difference between sleep duration weekends minus sleep duration on weekdays. If the child slept more on weekdays than on weekends, the duration of catch-up sleep was scored as zero.
Data were scored with Actiwatch Activity & Sleep Analysis version 7.38 software (Cambridge Neurotechnology, UK) with medium sensitivity and a 1-min epoch duration, as recommended by the manufacturer. Ward et al.22 compared polysomnography (PSG) and actigraphy (using Actiwatch) with different thresholds in assessing total sleep time, wake after sleep onset, and sleep efficiency in healthy (9- to 11-y-old) children as well as in children with asthma or idiopathic arthritis. They found that actigraphy was most accurate in healthy children and that medium sensitivity led to the least overestimation or underestimation of total sleep time or wake after sleep onset. They also conducted an epoch-by-epoch comparison between actigraphy and PSG using 30-sec epochs, and found that sensitivity, specificity, and accuracy for healthy children with medium sensitivity were high: 0.95, 0.69, and 0.90, respectively, which were among the highest values in comparison with those of other threshold and participant groups. Meltzer et al.23 used 1-min epochs in their validation study with the same brand as ours, and found sensitivity of 0.93, specificity of 0.69, and accuracy of 0.89 against PSG in epoch-by-epoch comparisons. In healthy children, actigraph was found to underestimate total sleep time by 5.5 min and sleep efficiency by 1%. Few validation studies have examined the fragmentation index and sleep latency. A recent study with Actiwatch found a an 0.82 sensitivity and an 0.51 specificity between the fragmentation index and the arousal index (PSG) in 2- to 18-y-old children referred for investigation of a sleep breathing disorder.24 The comparisons of sleep latency (measured by the Actiwatch) against PSG have revealed an underestimation of sleep latency by 4.5 to 6.9 min in healthy adults and children.25–27 In addition, the studies by Chae et al.28 in adults and by Spruyt et al.26 in children showed that actigraphy tended to underestimate sleep latencies only when PSG latencies were short, and to greatly overestimate when PSG latencies were long. Chae et al.28 suggested that a shorter threshold for immobile data (5 min) than the 10 min inherent in the algorithm would be more accurate for defining sleep latency. The concordance between actigraph and PSG in sleep latency was rather weak (0.33) in healthy school-aged children.26 Sleep Problems/Disorders
The parents completed a 26-item sleep questionnaire (Sleep Disturbance Scale for Children, SDSC).29 Each sleep behavior was scored on a five-point scale: never, once or twice per month,once or twice per week, three to five times per week, or every night. Following Spruyt et al.,30 we defined a sleep problem as a sleep behavior occurring at least 3 nights per week during the past 6 mo, with the exception of the items for disorders of arousal and disorders of excessive daytime somnolence, which had to be present for at least 1 or 2 nights per week.30 In the current study then, having a specific sleep disorder (1 = yes, 0 = no) was defined as having at least one sleep problem within the respective disorder scale (Disorder in initiating and maintaining sleep, Sleep breathing disorder, Disorder of arousal, Sleep wake transition disorder, Daytime excessive somnolence, Sleep hyperhydrosis). A variable named “any sleep disorder” was defined as scoring positive on at least one of the six sleep disorder subscales.
Pubertal Development
We used two scales to assess pubertal development. The scales were self-completed with the assistance of the research nurse during the clinical visit at age 12 y. The Tanner staging of pubertal development31 is a categorical self-reported measure of pubertal maturation, based on line drawings of pubic hair and genitals and where the adolescent chooses the picture most closely resembling her or his own body. Following previous studies,2 we used only the pubic hair scale, rather than the scale based on genital drawings. The Pubertal Development Scale (PDS)32 is a five-item self-reported questionnaire assessing body hair, growth spurt, and skin changes, and serves as a continuous measure of pubertal maturation. For girls, two additional items measured menarche and breast changes, whereas for boys, two additional items measured facial hair and voice change. The development of each characteristic was rated on a three-point scale from 1 (no changes yet) to 3 (clear changes), except for menarche, which was scored as binary (1 = no, 4 = has occurred). Following Mustanski et al.,33 we omitted the fourth option (= development is complete) in all other scales because of the young age distribution of our sample. We used the mean score of PDS items as a continuous index of pubertal development. The PDS score correlated significantly with the Tanner pubic hair scale (r = 0.65, P < 0.001).
Socioeconomic Status
We classified the socioeconomic status of the family, used for the attrition analyses only, according to the highest self-reported level of education of either parent in 2006, at the child's age of 8 y (1 = secondary or lower; 2 = lower-level tertiary; 3 = upper-level tertiary).
Statistical Analyses
To test whether pubertal maturation is related to actigraphy-based sleep variables, we used analyses of covariance (ANCOVAs) to compare adolescents in categorical Tanner stages and partial correlations to test associations with continuous PDS score. Associations between pubertal maturation and dichotomized parent-rated sleep disorders were tested using logistic regression analyses with odds ratios (OR) and 95% confidence intervals (95% CI). All of these analyses were adjusted first for sex, and thereafter for sex and age.
To study the second research question related to continuity of sleep from age 8 to 12 y, we used t-tests to study mean-level stability and change, and correlations to study rank-order stability. We then divided the actigraphy-based sleep variables into tertiles separately for girls and boys, and used logistic regressions to define the OR for staying in either the highest or the lowest tertile in sleep duration, efficiency, fragmentation, and latency from age 8 to 12 y. Logistic regressions also served to define the odds for having a persistent sleep disorder from age 8 to 12 y. These analyses were adjusted for sex, age, time between the two measurements, and PDS score.
To study the third research question related to associations between sleep disorders and actigraphy-based sleep variables both in cross-section and longitudinally (persistent sleep disorder versus no sleep disorder or transient sleep disorder), we used ANCOVAS, with the categorical sleep disorder variable serving as predictor, and the outcomes being the continuous actigraphy-based variables. We adjusted the analyses for sex, age, time between the two assessments (in longitudinal analyses), and the PDS score.
RESULTS
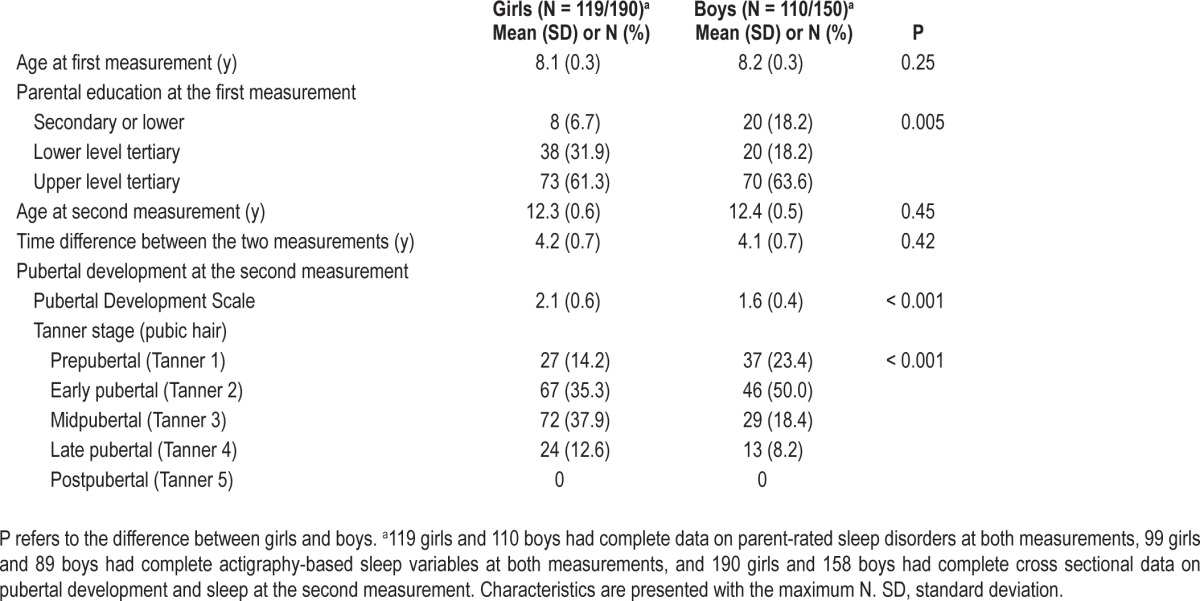
Table 1 shows the characteristics of the samples by sex. We found no age differences between the girls and boys at ages 8 and 12 y; at age 12 y, the boys lagged significantly behind the girls in pubertal development when measured with continuous PDS and categorical Tanner stages. As expected, at age 12 y, older children displayed more advanced pubertal status (PDS: r = -0.24; Tanner stage: r = -0.27; P < 0.001).
Table 1.
Characteristics of the participants
Associations between Pubertal Maturation and Sleep: 12-y Follow-up
Pubertal status and actigraph measurement of sleep
Pubertal status, measured as a continuous variable with the PDS, was associated with shorter sleep duration, especially on weekdays, in partial correlation analyses adjusted for sex (r = -0.14, P < 0.01), but when further adjusted for age, the association became nonsignificant (P > 0.14). We used ANCOVA to compare the actigraph estimates of the sleep of children in the late pubertal stage (Tanner 4 in pubic hair growth, 10.6% of the participants) to other children (Tanner 1-3), and found a 10-min shorter sleep duration when adjusted for sex (P = 0.04; P values for other variables > 0.28), but the difference became nonsignificant when controlling for sex and age (P > 0.14). Older age at assessment correlated with shorter sleep duration, especially on weekdays (r's > -0.19, P = 0.001) and later bedtimes on both weekdays and at weekends (r's > 0.18, P < 0.01) when controlling for sex or both sex and pubertal status.
Pubertal status and parent-rated sleep disorders
We used logistic regressions to compare parent-rated sleep disorders between adolescents in the late pubertal stage (Tanner 4 in pubic hair growth) and others (Tanner 1-3), but found no significant differences between the groups (P > 0.21 when controlling for sex; P > 0.17 when controlling for sex and age).
Continuity of Actigraph Estimates of Sleep from Age 8 to 12 y
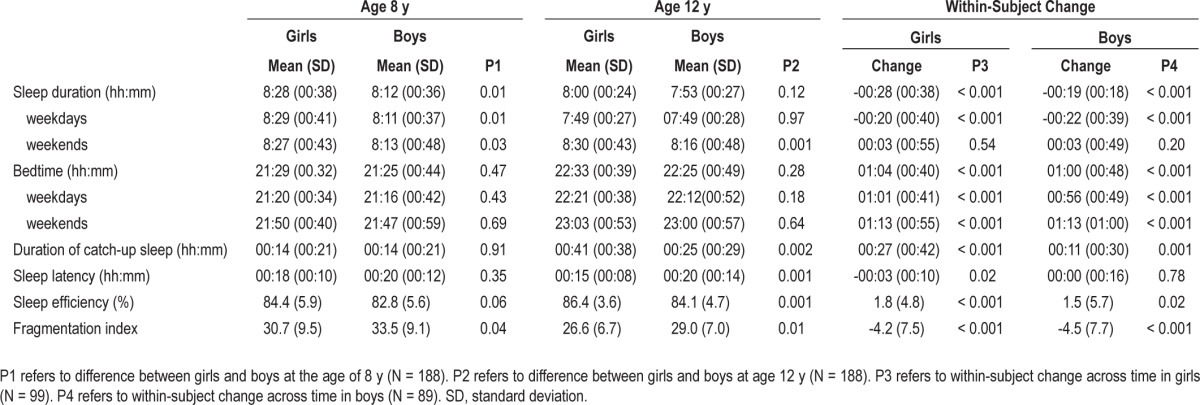
Table 2 describes actigraphy-based sleep variables at both measurement points. As reported in our previous paper,34 at age 8 y, girls slept significantly longer on both weekdays and weekends, and had less fragmented sleep than did boys. Similarly, at age 12 y, girls slept longer than boys, but on weekends only, and their sleep was less fragmented than that of boys. In addition, new differences became apparent: at age 12 y, girls had 16 min longer catch-up sleep, 5 min shorter sleep latency, and 2.3 percentage units higher sleep efficiency than boys.
Table 2.
Means, standard deviations (SD) and within-subject changes of actigraphy-based sleep variables from the age 8 to 12 y
Mean level stability and change in actigraphy-based sleep variables from age 8 to 12 y
Sleep duration decreased by 28 min in girls and 19 min in boys over the follow-up period. When analyzed separately for weekdays and weekends, the change was significant for week-days only. Bedtime was delayed for 1 h on both weekdays and weekends. The duration of catch-up sleep increased by 27 min in girls and by 11 min in boys. Also, sleep efficiency increased by 1.8 and by 1.5 percentage units in girls and boys. The fragmentation index decreased significantly in both girls and boys. Sleep latency decreased by 3 min in girls, and remained unchanged in boys.
Rank-order continuity and change in actigraphy-based sleep variables from age 8 to 12 y
Table 3 shows the Pearson correlation coefficients of actigraph estimates from age 8 to 12 y. The actigraph estimates were significantly correlated across time, except for the duration of catch-up sleep.
Table 3.
Correlation coefficients between actigraphy-based sleep variables at age 8 and 12 y
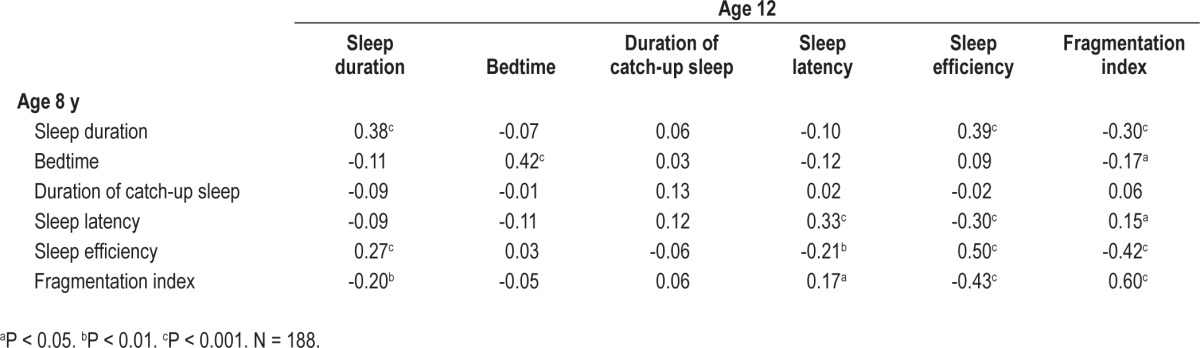
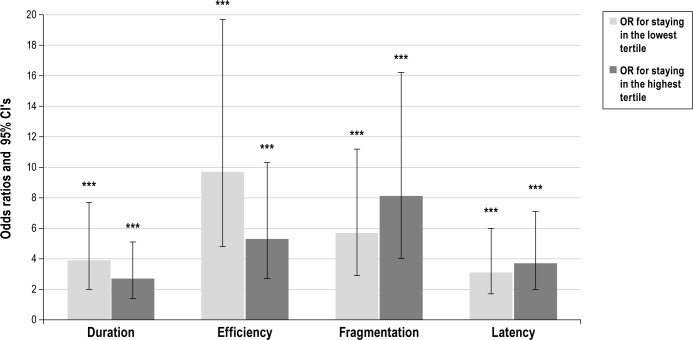
Figure 1 shows the OR for remaining in either the lowest or the highest tertile in sleep duration, efficiency, the fragmentation index, or sleep latency from age 8 to 12 y. All categories showed significant (P ≤ 0.001) stability. The risks for poor sleep at age 12 y (i.e. having short sleep duration, low sleep efficiency, high sleep fragmentation, or long sleep latency) were 3.9-, 9.7-, 8.1-and 3.6-fold higher for those who had poor sleep at age 8 y, respectively. The likelihood of having good sleep (i.e., having long sleep duration, high sleep efficiency, low sleep fragmentation, and short sleep latency) were 2.7-, 5.3-, 5.7-, and 3.1-fold higher for those who had good sleep at age 8 y, respectively. These ORs were adjusted for sex, age, PDS score, and time between assessments. In terms of raw percentages, of those who slept the shortest or had the poorest sleep efficiency at age 8 y, 52% and 65.1% belonged to the same category at age 12 y, respectively. Of those who slept the longest or had the highest sleep efficiency at age 8 y, 46.9% and 58.7% belonged to the same category at age 12 y, respectively.
Figure 1.
Adjusted odds ratios (OR) (bars) and 95% confidence intervals (95% CI) (error bars) for staying in the lowest and in highest tertiles in actigraphy-based sleep variables from age 8 to 12 y. Adjustments were made for sex, pubertal status, time difference between the two measurement points, and age at first measurement. ***P ≤ 0.001.
Continuity and Change in Sleep Disorders from Age 8 to 12 y
There were only a few cases of 8-y-old children with disorder of arousal (n = 6) and sleep breathing disorder (n = 5), and only seven 12-y-olds had sleep hyperhydrosis. Consequently, we chose not to use these scales individually in further analyses. Table 4 shows that at age 8 y, boys' and girls' sleep disorders showed no substantial differences. At the age of 12 y, boys had more excessive daytime somnolence and disorders of initiating and maintaining sleep than did girls. Table 4 shows the crude, unadjusted percentages of girls and boys who met the criteria of sleep disorders at both ages 8 and 12 y. The stabilities in girls and boys were 27% and 44% for disorder of initiating and maintaining sleep, 42% and 25% for sleep-wake transition disorder, 50% and 78% for daytime excessive somnolence, and 56% and 66% for any sleep disorder, respectively. Of those who had no sleep disorders at age 8 y, 32% had one at age 12 y.
Table 4.
Frequency of sleep disorders (symptom[s] of each scale occurring at least three times per week) at age 8 and 12 y
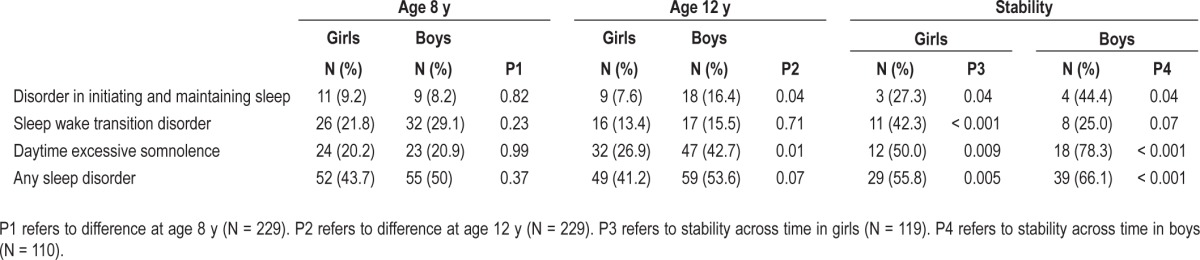
Children who met the criteria for disorder of initiating and maintaining sleep at age 8 y were 6.9-fold (95% CI 2.2 to 21.2) more likely to meet the criteria again at age 12 y. For those who met the criteria for daytime excessive somnolence at age 8 y, the odds of meeting the criteria again at age 12 y were 6.4 (95% CI 2.9 to 13.8); for daytime excessive somnolence, the odds were 6.0 (95% CI 2.6 to 13.6); for sleep wake transition disorders, the odds were 4.0 (95% CI 2.3 to 7.4); for any sleep disorders, the odds were 4.0 (95% CI 2.3 to 7.4) (all P < 0.001). These ORs were adjusted for sex, age, PDS score, and time between assessments.
Associations between Sleep Problems and Actigraph Estimates of Sleep
In comparison with children with no sleep disorder, the presence of any parent-rated sleep disorder showed no association with actigraphy-based sleep at either age 8 y (P > 0.64) or at age 12 y (P > 0.33), nor were the individual sleep disorder subscales at the ages of 8 or 12 y associated with actigraphy-based sleep (P > 0.09), except for one: 8-y-old children with difficulty initiating and maintaining sleep had shorter sleep duration (8.1 h) than did those without (8.4 h, P = 0.048). Children with a persistent sleep disorder from age 8 to 12 y did not differ in their actigraphy-based sleep at age 12 y from those with no sleep disorder or with a transient sleep problem (P > 0.10).
DISCUSSION
The current study showed that among 11- to 13-y-old children, the stage of pubertal maturity was not associated with alterations in sleep, whether measured with actigraphs or with parent reports of sleep disorders. Instead, childhood sleep patterns showed both significant continuity and change toward early adolescence. Over the 4-y follow-up period of the current study, from age 8 to 12 y, significant, paralleled mean-level changes occurred in the sleep of girls and boys: sleep duration decreased by 28 and 19 min, the duration of catch-up sleep increased by 27 and 11 min, and bedtime was delayed by 64 and 60 min in girls and boys, respectively. However, although sleep duration decreased, sleep quality, as indicated by sleep efficiency and fragmentation, improved slightly.
All actigraph estimates of sleep showed significant, low to medium rank-order continuity throughout the follow-up period. The longitudinal correlations of sleep duration (r = 0.38) and sleep efficiency (r = 0.50) corresponded roughly to the magnitude reported in earlier longitudinal studies with actigraphy (r = 0.47 and 0.53 for sleep duration, 0.67 for sleep efficiency).7,10 The fact that our follow-up period was 2 y longer than that of earlier studies7,10 may explain the somewhat lower correlations found in the current study. We found the highest correlation (r = 0.60) over the follow-up period for the fragmentation index, which reflects restlessness of sleep.
Many sleep studies have approached sleep in adolescence in isolation without taking into account adolescents' longitudinal, developmental sleep profiles. Accordingly, our main contribution was to demonstrate that, despite the expected mean-level changes in sleep patterns, both sleep patterns measured with actigraphs and sleep disorder reports by parents showed rank-order continuity over the transition from childhood to early adolescence, independently of pubertal maturation. We showed that the risks for poor sleep at the age of 12 y (i.e., having short sleep duration, low sleep efficiency, high sleep fragmentation, or long sleep latency) were 3.9- to 9.7-fold for those who had poor sleep at age 8 y. In addition, the odds for having a stable, parent-rated sleep disorder from age 8 to 12 y were more than six-fold for initiating and maintaining sleep, daytime excessive somnolence, and sleep-wake transition disorders. Notably, while earlier studies have focused mainly on the development and implications of poor sleep, we showed that not only poor but also good sleep has significant stability: the odds of having long sleep duration, high sleep efficiency, low sleep fragmentation, and short sleep latency were 2.7- to 5.7-fold for those who had good sleep at age 8 y.
Adolescent sleep has generated considerable interest in recent years. Studies have shown that pubertal maturation leads to a progressive decline in slow wave sleep,4,35 a change that should be reflected in lower sleep quality as measured with actigraphy. However, cross-sectional studies have failed to find associations between pubertal maturity and actigraph estimates of sleep quality,7 or have found only modest associations in boys.36 This study found no associations between sleep and pubertal maturation. Despite the neurodevelopmental changes in adolescent sleep patterns, stability estimates are high in sleep electroencephalogram (EEG) patterns also. A recent sleep EEG study reported a highly significant, trait-like consistency in sleep both in children from mean age 10 to 12 y and in teens from mean age 16 to 18 y, with intraclass correlations exceeding 0.7.37 This high continuation of sleep from childhood to adolescence and during adolescence has been overlooked in adolescent sleep discourse.
The continuity of sleep patterns and disorders can be mediated both by genetic factors and continuation in the environment. Studies of twins, as recently reviewed by Barclay and Gregory,38 have revealed modest or nonexistent genetic influence on parent-rated sleep duration in school-aged children; in adulthood, however, genetic influence on sleep duration has been determined in twins.39 Thus, environmental continuity could explain the rank-order continuity in sleep duration and in other actigraphy-based sleep parameters in the current study or the parent assessment method of sleep duration used in studies of twins is not accurate enough to detect the genetic influence. As recently shown, and as discussed in detail in the following paragraphs, the rate of agreement between actigraphy and parental questionnaires is low.40
With regard to a broad category of childhood “sleep problems,” including symptoms of parent- or self-rated dyssomnias and parasomnias, estimates indicate that the effect of genetics is strong (71% and 50%, respectively) in school-aged children.41 However, shared environmental influences (42%) contributed to sleep problems more than genetics (30%) during adolescence,42 suggesting that the magnitude of environmental effects on sleep is highest during adolescence.42 In studies focusing on specific childhood parasomnias, such as bruxism, nightmares, sleeptalking, and sleepwalking, heritability estimates are roughly 40-50%.43–47 Genetic factors also likely underlie a significant proportion of our findings: in terms of raw percentages, of those who had any sleep disorder at age 8 y, more than 60% had a sustained disorder to age 12 y. Of those who had no sleep disorder at age 8 y, 32% had one at age 12 y.
Significantly, actigraphy-based sleep variables correlated with parent-rated sleep disorders neither in cross-section nor longitudinally. Only one modest association was found between disorders of initiating and maintaining sleep and shorter sleep duration in 8-y-old children. This confirms earlier observations of modest or even no associations between sleep-related complaints or diagnosed sleep disorders and actigraph estimates of sleep in children.48–50 However, previous evidence on this observation is thus far quite slim. As a limitation to our study, we had so few children with disorders of arousal, sleep hyperhydrosis, and sleep breathing disorder that they were not analyzed separately against actigraphy. In addition, we did not assess circadian rhythm disturbances, which can be detected with actigraph.49,50
Our study has several strengths. We used a longitudinal, community-based cohort of adequate size and methods for studying sleep from both an objective and parental perspective. With an average of 7 to 8 nights of actigraph data, we had a sufficient measurement period in both of our follow-ups. We defined pubertal maturation as both a continuous and categorical variable with two different methods. The major limitation of our study is that attrition was greater in families with low educational status than in families in which either parent had an upper tertiary-level education. In addition, self-reported sleep disorders would also have provided the measurement greater validity, as parents may sometimes be unaware of the sleep problems of their children. This may have diminished the potential to find correlations between actigraph estimates of sleep and sleep disorders, but in addition, measuring continuation requires repeated measurement with the same method. We also acknowledge that the validity estimates of the fragmentation index and sleep latency have been reported to be significantly weaker than those reported for sleep duration and sleep efficiency in studies with various actigraph systems. Although actigraphy can provide information about sleep patterns over several days, PSG would have provided more reliable estimates of sleep quality. Finally, peer-reviewed cross-validation studies do not exist between the two models of Actiwatch we used. However, their manufacturers have compared the devices and found no differences for any of the sleep statistics between the models.51
In summary, our study demonstrates that sleep in early adolescence should be examined not as an isolated phenomenon, but rather as a continuum from childhood: despite the mean-level changes, both good and poor sleep show high temporal rank-order continuity. Thus, children with short sleep duration in childhood are especially at risk for inadequate sleep during early adolescence. Pubertal maturity was not associated with sleep in early adolescence, but older age, irrespective of pubertal status, showed a modest association. Parent-rated sleep disorders showed high stability over the 4-y assessment period, but had no association with the actigraph estimates of sleep.
DISCLOSURE STATEMENT
This was not an industry supported study. Financial support was provided by the Academy of Finland, Ministry of Education and Culture, Signe and Ane Gyllenberg Foundation, Juho Vainio Foundation, Yrjö Jahnsson Foundation, and Finnish Medical Foundation. The study was performed at the University of Helsinki, Institute of Behavioural Sciences. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–62. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- 2.Crowley SJ, Acebo C, Carskadon MA. Human puberty: salivary melatonin profiles in constant conditions. Dev Psychobiol. 2012;54:468–73. doi: 10.1002/dev.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell IG, Grimm KJ, de Bie E, Feinberg I. Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc Natl Acad Sci U S A. 2012;109:5740–3. doi: 10.1073/pnas.1120860109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2:453–60. doi: 10.1093/sleep/2.4.453. [DOI] [PubMed] [Google Scholar]
- 5.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–12. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–8. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Sadeh A, Dahl RE, Shahar G, Rosenblat-Stein S. Sleep and the transition to adolescence: a longitudinal study. Sleep. 2009;32:1602–9. doi: 10.1093/sleep/32.12.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly RJ, El-Sheikh M. Marital conflict and children's sleep: reciprocal relations and socioeconomic effects. J Fam Psychol. 2011;25:412–22. doi: 10.1037/a0023789. [DOI] [PubMed] [Google Scholar]
- 9.Bub KL, Buckhalt JA, El-Sheikh M. Children's sleep and cognitive performance: a cross-domain analysis of change over time. Dev Psychol. 2011;47:1504–14. doi: 10.1037/a0025535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Sheikh M, Kelly RJ, Bagley EJ, Wetter EK. Parental depressive symptoms and children's sleep: the role of family conflict. J Child Psychol Psychiatry. 2012;53:806–14. doi: 10.1111/j.1469-7610.2012.02530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller P, El-Sheikh M. Children's emotional security and sleep: longitudinal relations and directions of effects. J Child Psychol Psychiatry. 2011;52:64–71. doi: 10.1111/j.1469-7610.2010.02263.x. [DOI] [PubMed] [Google Scholar]
- 12.Simard V, Nielsen TA, Tremblay RE, Boivin M, Montplaisir JY. Longitudinal study of preschool sleep disturbance: the predictive role of maladaptive parental behaviors, early sleep problems, and child/mother psychological factors. Arch Pediatr Adolesc Med. 2008;162:360–7. doi: 10.1001/archpedi.162.4.360. [DOI] [PubMed] [Google Scholar]
- 13.Fricke-Oerkermann L, Pluck J, Schredl M, et al. Prevalence and course of sleep problems in childhood. Sleep. 2007;30:1371–7. doi: 10.1093/sleep/30.10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simola P, Laitalainen E, Liukkonen K, et al. Sleep disturbances in a community sample from preschool to school age. Child Care Health Dev. 2012;38:572–80. doi: 10.1111/j.1365-2214.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 15.Strandberg TE, Järvenpää AL, Vanhanen H, McKeigue PM. Birth outcome in relation to licorice consumption during pregnancy. Am J Epidemiol. 2001;153:1085–8. doi: 10.1093/aje/153.11.1085. [DOI] [PubMed] [Google Scholar]
- 16.Pesonen AK, Räikkönen K, Matthews K, et al. Prenatal origins of poor sleep in children. Sleep. 2009;32:1086–92. doi: 10.1093/sleep/32.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Räikkönen K, Matthews KA, Pesonen AK, et al. Poor sleep and altered hypothalamic-pituitary-adrenocortical and sympatho-adrenal-medullary system activity in children. J Clin Endocrinol Metab. 2010;95:2254–61. doi: 10.1210/jc.2009-0943. [DOI] [PubMed] [Google Scholar]
- 18.Pesonen AK, Räikkönen K, Paavonen EJ, et al. Sleep duration and regularity are associated with behavioral problems in 8-year-old children. Int J Behav Med. 2010;17:298–305. doi: 10.1007/s12529-009-9065-1. [DOI] [PubMed] [Google Scholar]
- 19.Pesonen AK, Sjösten NM, Matthews KA, et al. Temporal associations between daytime physical activity and sleep in children. PLoS One. 2011;6:e22958. doi: 10.1371/journal.pone.0022958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Räikkönen K, Pesonen AK, Heinonen K, et al. Maternal licorice consumption and detrimental cognitive and psychiatric outcomes in children. Am J Epidemiol. 2009;170:1137–46. doi: 10.1093/aje/kwp272. [DOI] [PubMed] [Google Scholar]
- 21.Oakley NR. Validation with Polysomnography of the Sleepwatch Sleep/ Wake Scoring Algorithm used by the Actiwatch Activity Monitoring System: Cambridge. Neurotechnology. 1996 [Google Scholar]
- 22.Ward TM, Lentz M, Kieckhefer GM, Landis CA. Polysomnography and actigraphy concordance in juvenile idiopathic arthritis, asthma and healthy children. J Sleep Res. 2012;21:113–21. doi: 10.1111/j.1365-2869.2011.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meltzer LJ, Walsh CM, Traylor J, Westin AM. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep. 2012;35:159–66. doi: 10.5665/sleep.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Driscoll DM, Foster AM, Davey MJ, Nixon GM, Horne RS. Can actigraphy measure sleep fragmentation in children? Arch Dis Child. 2010;95:1031–3. doi: 10.1136/adc.2009.166561. [DOI] [PubMed] [Google Scholar]
- 25.Rupp TL, Balkin TJ. Comparison of Motionlogger Watch and Actiwatch actigraphs to polysomnography for sleep/wake estimation in healthy young adults. Behav Res Methods. 2011;43:1152–60. doi: 10.3758/s13428-011-0098-4. [DOI] [PubMed] [Google Scholar]
- 26.Spruyt K, Gozal D, Dayyat E, Roman A, Molfese DL. Sleep assessments in healthy school-aged children using actigraphy: concordance with polysomnography. J Sleep Res. 2011;20:223–32. doi: 10.1111/j.1365-2869.2010.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonetti L, Pasquini F, Fabbri M, Belluzzi M, Natale V. Comparison of two different actigraphs with polysomnography in healthy young subjects. Chronobiol Int. 2008;25:145–53. doi: 10.1080/07420520801897228. [DOI] [PubMed] [Google Scholar]
- 28.Chae KY, Kripke DF, Poceta JS, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–5. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Bruni O, Ferini-Strambi L, Russo PM, et al. Sleep disturbances and teacher ratings of school achievement and temperament in children. Sleep Med. 2006;7:43–8. doi: 10.1016/j.sleep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Spruyt K, O'Brien LM, Cluydts R, Verleye GB, Ferri R. Odds, prevalence and predictors of sleep problems in school-age normal children. J Sleep Res. 2005;14:163–76. doi: 10.1111/j.1365-2869.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 31.Tanner J. Growth in adolescence. Oxford: Blackwell; 1962. [Google Scholar]
- 32.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolescence. 1988;17:117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 33.Mustanski BS, Viken RJ, Kaprio J, Pulkkinen L, Rose RJ. Genetic and environmental influences on pubertal development: longitudinal data from Finnish twins at ages 11 and 14. Dev Psychol. 2004;40:1188–98. doi: 10.1037/0012-1649.40.6.1188. [DOI] [PubMed] [Google Scholar]
- 34.Paavonen EJ, Räikkönen K, Lahti J, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009;123:e857–64. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- 35.Tarokh L, Van Reen E, LeBourgeois M, Seifer R, Carskadon MA. Sleep EEG provides evidence that cortical changes persist into late adolescence. Sleep. 2011;34:1385–93. doi: 10.5665/SLEEP.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Short MA, Gradisar M, Lack LC, Wright H, Carskadon MA. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med. 2012;13:378–84. doi: 10.1016/j.sleep.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Tarokh L, Carskadon MA, Achermann P. Trait-like characteristics of the sleep EEG across adolescent development. J Neurosci. 2011;31:6371–8. doi: 10.1523/JNEUROSCI.5533-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barclay NL, Gregory AM. Quantitative genetic research on sleep: a review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Med Rev. 2013;17:29–40. doi: 10.1016/j.smrv.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 40.Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children's sleep patterns. Arch Pediatr Adolesc Med. 2008;162:350–8. doi: 10.1001/archpedi.162.4.350. [DOI] [PubMed] [Google Scholar]
- 41.Gregory AM. A genetic decomposition of the association between parasomnias and dyssomnias in 8-year-old twins. Arch Pediatr Adolesc Med. 2008;162:299–304. doi: 10.1001/archpedi.162.4.299. [DOI] [PubMed] [Google Scholar]
- 42.Moore M, Slane J, Mindell JA, Burt SA, Klump KL. Genetic and environmental influences on sleep problems: a study of preadolescent and adolescent twins. Child Care Health Dev. 2011;37:638–41. doi: 10.1111/j.1365-2214.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hublin C, Kaprio J, Partinen M, Heikkila K, Koskenvuo M. Prevalence and genetics of sleepwalking: a population-based twin study. Neurology. 1997;48:177–81. doi: 10.1212/wnl.48.1.177. [DOI] [PubMed] [Google Scholar]
- 44.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Sleeptalking in twins: epidemiology and psychiatric comorbidity. Behav Genet. 1998;28:289–98. doi: 10.1023/a:1021623430813. [DOI] [PubMed] [Google Scholar]
- 45.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Nocturnal enuresis in a nationwide twin cohort. Sleep. 1998;21:579–85. doi: 10.1093/sleep/21.6.579. [DOI] [PubMed] [Google Scholar]
- 46.Hublin C, Kaprio J, Partinen M, Koskenvuo M. Sleep bruxism based on self-report in a nationwide twin cohort. J Sleep Res. 1998;7:61–7. doi: 10.1046/j.1365-2869.1998.00091.x. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen BH, Perusse D, Paquet J, et al. Sleep terrors in children: a prospective study of twins. Pediatrics. 2008;122:e1164–7. doi: 10.1542/peds.2008-1303. [DOI] [PubMed] [Google Scholar]
- 48.Anders T, Iosif AM, Schwichtenberg AJ, Tang K, Goodlin-Jones B. Sleep and daytime functioning: a short-term longitudinal study of three preschool-age comparison groups. Am J Intellect Dev Disabil. 2012;117:275–90. doi: 10.1352/1944-7558-117.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 50.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30:519–29. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 51.Equivalence of activity recordings and derived sleep statistics 2008. [cited 31.7.2013]; Available from: http://actigraphy.respironics.com/downloads/AWDataEquivalenceReport_0309_final.pdf.