Abstract
Study Objectives:
Previous studies have demonstrated short-wavelength sensitivity for the acute alerting response to nocturnal light exposure. We assessed daytime spectral sensitivity in alertness, performance, and waking electroencephalogram (EEG).
Design:
Between-subjects (n = 8 per group).
Setting:
Inpatient intensive physiologic monitoring unit.
Participants:
Sixteen healthy young adults (mean age ± standard deviation = 23.8 ± 2.7 y).
Interventions:
Equal photon density exposure (2.8 × 1013 photons/cm2/s) to monochromatic 460 nm (blue) or 555 nm (green) light for 6.5 h centered in the middle of the 16-h episode of wakefulness during the biological day. Results were compared retrospectively to 16 individuals who were administered the same light exposure during the night.
Measurements and Results:
Daytime and nighttime 460-nm light exposure significantly improved auditory reaction time (P < 0.01 and P < 0.05, respectively) and reduced attentional lapses (P < 0.05), and improved EEG correlates of alertness compared to 555-nm exposure. Whereas subjective sleepiness ratings did not differ between the two spectral conditions during the daytime (P > 0.05), 460-nm light exposure at night significantly reduced subjective sleepiness compared to 555-nm light exposure at night (P < 0.05). Moreover, nighttime 460-nm exposure improved alertness to near-daytime levels.
Conclusions:
The alerting effects of short-wavelength 460-nm light are mediated by counteracting both the circadian drive for sleepiness and homeostatic sleep pressure at night, but only via reducing the effects of homeostatic sleep pressure during the day.
Citation:
Rahman SA; Flynn-Evans EE; Aeschbach D; Brainard GC; Czeisler CA; Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. SLEEP 2014;37(2):271-281.
Keywords: Alertness, circaidian, homeostatic, light, short-wavelength light, sleep
INTRODUCTION
Ocular light exposure induces a range of circadian, neuroendocrine, and neurobehavioral responses (sometimes termed ‘nonvisual’ or ‘nonimage-forming responses), including circadian phase resetting,1 melatonin suppression,1 and enhancement of alertness and performance.2 These responses are most sensitive to short-wavelength (blue) visible light (450 to 480 nm).3–8 This short-wavelength sensitivity corresponds closely to the spectral sensitivity of melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs λmax ∼480 nm) that primarily mediate these responses, and not to the spectral sensitivity of the rod and cone visual photoreceptors.3,4 These nonvisual responses persist in some totally visually blind humans lacking outer retina function, demonstrating that rod and cone photoreceptors are not required to detect light for these responses,9–11 although other studies have demonstrated that rods and cones can modulate nonvisual responses via the ipRGCs.12–16 The relative contribution of the rods and cones depend on the properties of the light exposure, such that at low intensities and short durations, visual photoreceptors have a relatively greater contribution to nonvisual responses.16,17
Nighttime light exposure in humans acutely increases alertness2 and suppresses melatonin1 in a dose-dependent manner and both of these nonvisual responses are short-wavelength sensitive.5–7 Daytime white light exposure has also been shown to increase alertness,18–20 independent of melatonin suppression (as melatonin is not released during the day) but the spectral sensitivity of this response is not well characterized.
Subjective alertness is significantly increased in response to very short duration (20 sec) pulses of blue light over ∼10 min, as compared to duration and intensity-matched yellow light pulses during the day.21 Recent functional magnetic resonance imaging (fMRI) studies confirm these results showing that very short duration (50 sec) 470-nm blue light exposure induces greater neural activation as compared to 555-nm green light exposure.8 Similarly, a 2-h daytime (evening) exposure to monochromatic blue light (456 nm) induced greater subjective alertness and reduced sleepiness as compared to intensity-matched 548-nm exposure, although this difference did not reach statistical significance.22 In contrast, a 4-h daytime (early morning) exposure to monochromatic blue light (420 nm) induced significantly greater subjective alertness than 470- or 600-nm light, although alertness was not objectively assessed.23
Although previous results suggest a short-wavelength sensitivity for daytime alerting effects of light, most studies used a relatively short duration (≤ 2 h). Given that daytime light exposures are generally much longer than several hours,24–26 we examined the daytime spectral sensitivity in alertness, performance, and the waking electroencephalogram (EEG) to longer duration (6.5 h) light exposure in humans as a starting point for assessing longer duration exposures more generally, given the interaction between photoreceptor recruitment and duration of light exposure.17,27
METHODS
Participants and Prestudy Conditions: Daytime Light Exposure
We studied 16 healthy males (mean age ± standard deviation [SD] = 24.8 ± 2.6 y; range 20-30 y) in the Intensive Physiology Monitoring Unit in the Center for Clinical Investigation (CCI) at Brigham and Women's Hospital between November 2010 and September 2011. The study was approved by the Partners Human Research Committee, and participants provided written informed consent prior to the study. All had comprehensive but unremarkable physical, psycho logical, and ophthalmologic examinations, including a negative Ishihara color blindness test. For at least 3 w prior to entering the Intensive Physiologic Monitoring Unit, participants maintained a self-selected, constant 8-h sleep/rest/dark schedule confirmed with calls to a time- and date-stamped voicemail at bedtime and wake time. Throughout the ≥ 3-w screening process, participants were asked to refrain from use of any prescription or nonprescription medications, supplements, recreational drugs, caffeine, alcohol, or nicotine. Compliance was verified by urine and blood toxicology during screening and urine toxicology upon entry to the unit. For at least 7 days prior to entering the unit, participants' schedules were monitored with actigraphy (Actiwatch-L, Minimitter, Inc., Bend, OR).
Study Protocol: Daytime Light Exposure
Participants were studied for 7 days in an environment free of time cues (no access to windows, clocks, watches, live television, radio, internet, telephones, and newspapers and continually supervised by staff trained not to reveal information about the time of day). The schedule consisted of a 3-day baseline (8-h:16-h sleep:wake cycle based on average sleep times in the 7 days prior to study entry), an initial 40-h constant routine, a 16-h light exposure day, followed by 8 h of sleep and then discharge (Figure 1A). During the constant routine procedures, participants were asked to remain awake while supervised in constant dim light in a semirecumbent posture, with daily nutritional intake divided into hourly portions (150 mEq Na+/100 mEq K+ (± 20%) controlled nutrient, isocaloric [basal energy expenditure × 1.3] diet, 2,500 mL fluids/24 h).
Figure 1.
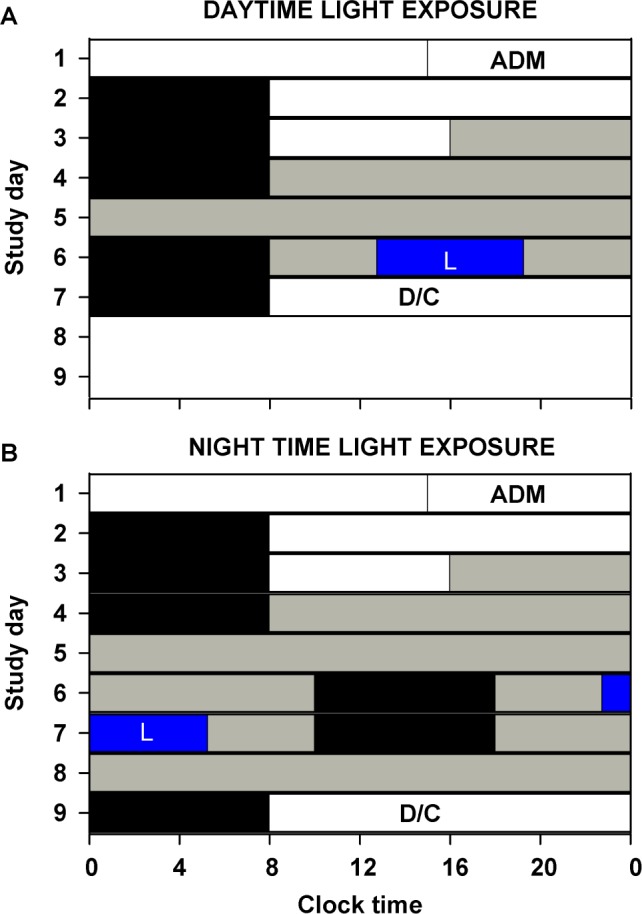
Study protocol to assess the acute alerting effects of daytime and nighttime monochromatic light exposure. Participants completed a (A) 7-day (daytime light exposure; 460 nm, n = 8; 555 nm, n = 8) or (B) 9-day (nighttime light exposure; 460 nm, n = 8; 555 nm, n = 8) inpatient protocol in an environment free of time cues. White bars indicate exposure to ambient fluorescent white light at 23 μW/cm2 (∼90 lux) and gray bars indicate exposure to dim ambient light at < 0.4 μW/cm2 (< 3 lux). Black bars show scheduled sleep episodes, and the blue bar with an L (light) inscribed indicates the 6.5-h 460-nm or 555-nm monochromatic light exposure. The schedule consisted of a 3-day baseline (8-h:16-h sleep:wake cycle based on average sleep times in the 7 days prior to admission [ADM]), (A) an initial 40 h (Daytime exposure) or (B) 50 h 10 min (Nighttime exposure) constant routine, a 16-h light exposure day, followed by (A) 8 h of sleep and then discharge (D/C) or (B) a second 29 h 50 min constant routine followed by an 8-h sleep episode and then discharge.
During the first 2.5 baseline days, maximum ambient light during scheduled wake was 48 μW/cm2 (∼190 lux) maximum when measured in the horizontal plane at a height of 187 cm and 23 μW/cm2 (∼88 lux) when measured in the vertical plane (137 cm). Midway through day 3, maximum ambient light was decreased to < 0.4 μW/cm2 to approximately 0.1 μW/cm2 (< 3 lux [∼1.5 lux]) when measured in the vertical plane at 137 cm and maintained at that level for the remainder of the study. Room light was switched off during monochromatic light exposure and scheduled sleep episodes. Ambient room lighting was generated using ceiling-mounted 4,100 K fluorescent lamps (F96T12/41U/HO/EW, 95W; F32T8/ADV841/A, 32W; F25T8/ TL841, 25W; Philips Lighting, The Netherlands) with digital ballasts (Hi-Lume 1% and Eco-10 ballasts, Lutron Electronics Co., Inc., Coopersburg, PA) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, Pittsfield, MA). Routine illuminance and irradiance measures were conducted using an IL1400 radiometer/powermeter with an SEL-033/Y/W or SEL-033/F/W detector, respectively (International Light, Inc., Newburyport, MA).
Monochromatic Light Exposure: Daytime Light Exposure
The monochromatic light exposure (LE) occurred on day 6 (Figure 1), and the 6.5-h exposure was timed to start 4.75 h after scheduled wake time based on each participants' baseline days, centered within the 16-h wake episode. Participants remained in dim < 0.4 μW/cm2 (< 3 lux) white polychromatic light before and after the 6.5-h monochromatic light exposure. Monochromatic light was generated using a 1,300 W xenon arc lamp and grating monochromator and administered via a modified Ganzfeld source coated with 96% to 99% reflective paint.3 The monochromatic light wavelength was confirmed using a PR-650 SpectraScan Colorimeter with a CR-650 cosine receptor (Photo Research Inc., Chatsworth, CA). Routine power measures were conducted using an IL1400 radiometer/power-meter with an SEL 033/F/W detector and fixed at the front of the dome at approximate eye level using a clear plastic holder.
Participants were randomized to either 460 nm (n = 8) or 555 nm (n = 8) monochromatic light (both < 15 nm half-peak bandwidth). The target irradiance at the level of the eye was 10.0 μW/cm2 and 12.1 μW/cm2 for 555 nm and 460 nm, respectively, generating an equal photon density of 2.8 × 1013 photons/ cm2/s for both exposures. The measured values, averaged between the start and end of each 90-min fixed-gaze episode, were 10.0 μW/cm2 (555 nm) and 12.1 μW/cm2 (460 nm). Ninety minutes prior to and throughout the light exposure, participants were seated, and, 15 min prior to exposure, a pupil dilator was administered to each eye (ophthalmologic preparation of 0.5% cyclopentolate hydrochloride, 1 drop per eye; Cyclogyl, Alcon, TX), after which participants wore blackout goggles until the start of the light exposure. During monochromatic light exposure, participants were supervised continually and asked to maintain a fixed gaze for 90 min in the Ganzfeld dome before a free gaze for 10 min while remaining seated. This sequence was repeated throughout the exposure. Participants received a 10-min free gaze episode after each 90 min of exposure throughout the 6.5 h. During free gazes, eye level irradiance was approximately 1.0 μW/cm2. Participants completed their lunch ∼30 min before light exposure started and began their dinner ∼30 min after the light exposure ended. Participants did not eat during the light exposure session but were allowed to sip water if requested during the free gaze episodes.
Sleepiness and Performance Assessments: Daytime Light Exposure
Subjective sleepiness was rated using the Karolinska Sleepiness Scale (KSS),28 a nine-point scale from 1-“very alert” to 9-“very sleepy, fighting sleep”. During monochromatic light exposure participants respond ed verbally after having been read the identical instructions and options presented during the rest of the protocol. During the light exposure day, the KSS was presented every 30-60 min throughout the 16-h wake episode, including the start of the monochromatic light exposure, every subsequent hour, and immediately upon lights off. Objective measures of performance included the auditory 10-min psycho-motor vigilance task (PVT-10A) assessed every 60 min through the 16-h wake episode, including the start of the monochromatic light exposure, every subsequent hour, and immediately upon lights off. During the PVT-10A an auditory signal was presented at random intervals (1-9 sec) and the participant was asked to press a button as soon as possible after hearing the sound. No simultaneous visual stimulus was presented.
Waking EEG Recordings: Daytime Light Exposure
Polysomnographic recordings were made continuously throughout the constant routine and light exposure episodes using a portable, modular, battery-operated, ambulatory, digital polysomnographic recorder (Vitaport-3 digital recorder, TEMEC Instruments B.V., Kerkrade, The Netherlands). Recordings consisted of EEG, electrooculogram, and a two-lead electrocardiogram. Electrodes were positioned according to the International 10-20 System, with linked mastoid references (Ax) used for wake recordings from the z-line, Fz-Ax, Cz-Ax, Pz-Ax, and Oz-Ax. Only data from the Cz-Ax derivation (central position on the nasion-inion midline) are presented in this report. All EEG signals were high-pass filtered (time constant: 0.33 seconds), low-pass filtered (-6 dB at 70 Hz, 24 dB/octave), and digitized (resolution: 12-bit, sampling rate: 256 Hz, storage rate: 128 Hz). The raw signals were stored on a Flash RAM Card (SanDisk, Sunnyvale, CA) and downloaded off-line. Electrode impedances were checked using a GRASS F-EZM4 impedance meter (Grass-Telefactor, Astro-Med, Inc., West Warwick, RI) at the beginning and end of the light exposure and every 8 h throughout the constant routine. Electrode impedances were documented, and electrode applications were repeated until the impedances were all < 10 kΩ. Participants were also asked to complete the Karolinska Drowsiness Test (KDT) hourly throughout the constant-routine and light exposure episode, after completing the alertness and performance battery.28,29 During the KDT, participants were instructed to relax and fixate on a 5-cm black dot 1 m away attached to a computer screen for 3 min with their eyes open. During monochromatic light exposure, participants were asked to focus on a 30-mm spot at the back of the modified Ganzfeld source approximately 20 cm from eye level.
Comparison of Responses to Daytime Versus Nighttime Light Exposure
In order to compare the spectral sensitivity of the acute alerting effects of light exposure during the daytime versus during the nighttime, the responses of the participants exposed to daytime light were compared to the previously reported responses of the participants to nighttime light exposure under comparable conditions.7 The protocol for nighttime light exposure and the protocol differences between the daytime and nighttime groups are as follows.
The methodology for nighttime light exposure has been described in detail previously.7 We studied 16 healthy participants (eight women; mean age ± SD = 23.3 ± 2.4 y; range 19-27 y) in the same facility. Participants were studied for 9 instead of 7 days and completed two constant routines (CRs) instead of one. The first CR was 50 h 10 min and the second CR was 29 h 50 min (Figure 1B). Caloric composition for hourly meals during CR was identical between protocols except the nighttime light exposure group participants received 2000 mL fluids/24 h. In the nighttime light exposure group monochromatic light exposure occurred 9.25 h before scheduled wake time (14.75 h after scheduled wake time) corresponding on average to approximately 6.75 h before core body temperature minimum, a phase at which white light expo sure induces robust melatonin suppression, phase-delay shifts, and acute alerting effects.1,2 The measured irradiance values during monochromatic light exposure, averaged between the start and end of each 90-min fixed-gaze episode were 9.9 μW/cm2 (555 nm) and 11.8 μW/cm2 (460 nm). One individual in the nighttime light exposure group had an extended free gaze lasting 40 min, starting 3 h and 20 min into the 555 nm monochromatic light exposure.7
Data Analysis
Data from all 32 participants (16 daytime and 16 nighttime) were analyzed together for the current study. KSS ratings and performance parameters (mean reaction time and lapses [defined as responses > 500 ms]) measured during monochromatic light exposure were z-transformed and subjected to one-, two- and four-way repeated measures with random intercepts mixed-model analysis of variance using the restricted maximum likelihood estimation method (REML) for analyzing data with missing values or unbalanced groups; and generalized linear models for balanced groups with no missing values. Fixed effects were set as spectral condition, exposure duration, test duration, and diurnality (day versus night) as appropriate. If a significant main or interaction effect was observed with mixed-model analysis, data were further subjected to Dunnett post hoc multiple comparison test when comparing between groups. If a significant main or interaction effect was observed with generalized linear model analysis, data were further subjected to Student-Newman-Keuls post hoc multiple comparison test when comparing between groups. Mean reaction time under constant routine and light exposure conditions were fitted with a dual harmonic cosinor regression model [Y = μ + (A*cos*((2π(x − Φ))/24)) + (B*cos*((2π(x − φ))/12))], where μ = Mesor, A = 24-h component amplitude, B = 12-h component amplitude, Φ = 24-h component acrophase, φ = 12-h component acrophase. Residual error was assumed to be independent and to have a normal distribution εi ∼ N(0,σ2).30 Linear trends were assessed using robust regression models with M-estimation.
Waking EEG signals derived from Cz/Ax during the KDT were visually inspected, and 2-sec epochs containing muscle artifact, eye blinks, and eye movements were discarded from further analysis. Artifact-free 2-sec epochs were subjected to offline spectral analysis using a fast-Fourier transform and a 10% cosine window. Data were reduced by removing spectra above 20 Hz and analyzed between 0.5-20.0 Hz in 0.5-Hz bins. Power densities were log-transformed and weighted for the number of artifact-free epochs available. Because absolute power density varies greatly among individuals, values were expressed for each participant and light condition as a percentage of power density during dim fluorescent light (< 0.4 μW/cm2 or 3 lux) during an interval of equal clock time in the constant routine 48 h prior to the light exposure. Resultant relative percentage power density values across individual frequency bins were subjected to generalized extreme studentized deviate test for detecting multiple outliers in the daytime light exposure group. Outliers were removed from four individuals (n = 2, 460 nm; n = 2, 555 nm) who were identified to have poor signal quality prior to data analysis. Outliers were not removed from any of the other individuals. Relative percentage power density data were log-transformed and subjected to two-way mixed model analysis with REML estimation with spectral condition and frequency as fixed effects. If a significant main or interaction effect was observed, data were further subjected to Dunnett post hoc multiple comparison test when comparing between two groups. For EEG time course analysis of specific frequency ranges, the log-transformed power density from each KDT during the monochromatic light exposure session was expressed relative to the average power density of the total 6.5-h duration during the constant routine on the day prior to the light exposure. Relative percentage power density data were log-transformed and subjected to two-way repeated measures with random intercepts mixed-model analysis of variance with REML estimation with spectral condition and exposure duration as fixed effects. If a significant main or interaction effect was observed, data were further subjected to Dunnett post hoc multiple comparison test when comparing between two groups. Significance was set to P < 0.05. All analyses were performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA). Missing data due to equipment malfunction were treated by truncation and were limited to 5.5% for auditory PVT, 0.4% for KSS, and 8.6% for KDT during light exposure sessions. Data loss due to statistical outlier removal by generalized extreme studentized deviate test was 5.9% of the total EEG data. In addition, 63.8% of total 2-sec EEG epochs contained artifacts that were removed from the final analysis.
RESULTS
Subjective Sleepiness
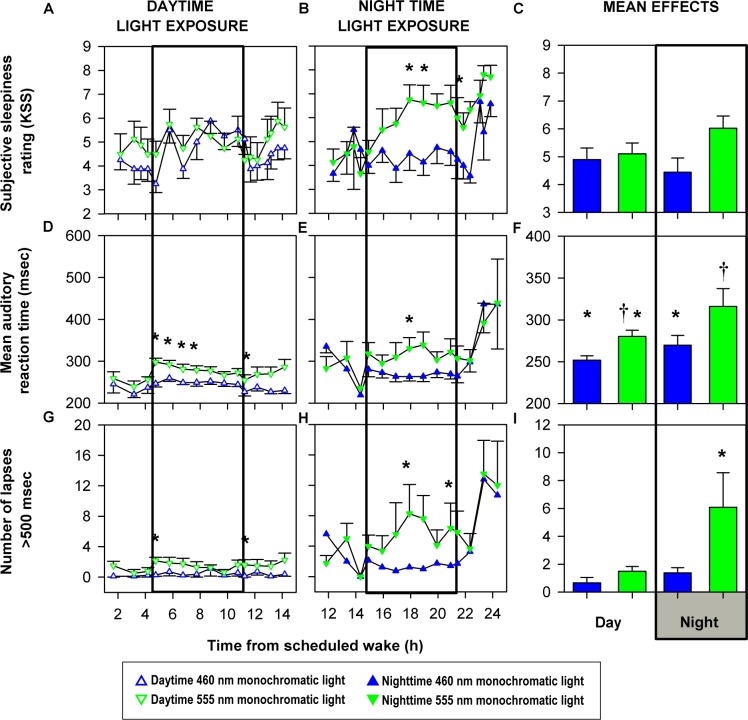
In the daytime light exposure group, there was no significant difference in KSS ratings between the two spectral conditions at any point during the 6.5-h monochromatic light exposure session or immediately after the light exposure ended (Figure 2A). In the nighttime monochromatic light exposure group, 460-nm exposure significantly (P < 0.05) reduced KSS scores throughout the 6.5-h exposure as compared to 555-nm light including immediately after the light exposure ended (Figure 2B). An effect of exposure duration was not detected (P > 0.05), nor an effect of the interaction between exposure duration and spectral condition (P > 0.05) for either daytime or nighttime KSS ratings. Analysis restricted to the tests conducted only during the light exposure session revealed no significant differences between any of the four groups (P > 0.05; Figure 2C).
Figure 2.
Effects of daytime and nighttime monochromatic light exposure on behavioral measures. Mean (± standard error of the mean) profiles of subjective sleepiness (A-C), auditory reaction time (D-F), and auditory attentional lapses (G-I) before, during, and after exposure to monochromatic light for 6.5 hours (open box). Behavioral test data were analyzed during light exposure including the first cognitive test immediately after the end of light exposure (A, B, D, E, G, H) and only during the light exposure (C, F, I). Karolinska Sleepiness Scale (KSS) ratings and performance parameters (mean reaction time and lapses [defined as responses > 500 ms]) measured during monochromatic light exposure were z-transformed and subjected to two-way repeated measures with random intercepts mixed model analysis of variance with restricted maximum likelihood estimation method followed by Dunnett post hoc multiple comparison test for comparing between groups (A, B, D, E, G, H) and one-way generalized linear model analysis followed by Student-Newman-Keuls post hoc multiple comparison test for comparing between groups (C, F, I). Fixed effects were set as spectral condition and/or exposure duration as appropriate. Exposure to 460 nm monochromatic light during the day did not change subjective sleepiness ratings (A) but improved reaction times (D) and reduced attentional lapses (G) relative to 555-nm exposure. Exposure to 460 nm monochromatic light at night significantly reduced subjective sleepiness ratings (B), reaction time (E), and attentional lapses (H) as compared with 555-nm light. Subjective sleepiness was not different between any of the four groups during light exposure (C). Reaction times were lowest under daytime 460-nm exposure, followed by daytime 555 nm and nighttime 460 nm and highest under nighttime 555 nm (F). Attentional lapses were not different between daytime 460 nm, daytime 555 nm, and nighttime 460 nm and all three were significantly lower than nighttime 555 nm (I). Significant differences between condition means are represented by * and † (P < 0.05). Panels B, E and H were published previously in reference 7.
Auditory Psychomotor Vigilance Test
Daytime and nighttime monochromatic 460-nm light exposure significantly reduced mean reaction time as compared to 555-nm light throughout the 6.5-h light exposure session as well as immediately after the daytime light exposure ended (P < 0.01 and P < 0.05, respectively; Figures 2D and 2E). Analysis of the tests conducted only during the light exposure session revealed significantly reduced reaction time under 460-nm exposure as compared to 555-nm exposure during the night (Figure 2F). Moreover, there was no significant difference in mean reaction time under nighttime 460-nm exposure and daytime reaction times whereas nighttime 555-nm exposure was significantly slower than daytime 460-nm exposure (Figure 2F). Reaction times were quickest under daytime 460-nm exposure, followed by daytime 555 nm and nighttime 460 nm and slowest under nighttime 555 nm (P < 0.01; Figure 2F). There was a significant effect (P < 0.0001) of exposure duration on reaction time in the daytime group (Figure 2D) indicating a reduction in reaction time but not in the nighttime group (Figure 2E), and there was no significant effect of the interaction of spectral condition and exposure duration on reaction time in either daytime or nighttime groups (P > 0.05). Similar to mean reaction time, daytime and nighttime attentional lapses were significantly lower in 460-nm groups than in 555-nm groups (P < 0.05; Figure 2G-2I). There was no significant effect of exposure duration or the interaction of spectral condition and exposure duration on attentional lapses in either daytime or nighttime groups (P > 0.05). Attentional lapses only during the light exposure session were not different between daytime 460 nm, daytime 555 nm, and nighttime 460 nm but all three were significantly lower than nighttime 555 nm (P < 0.05; Figure 2I).
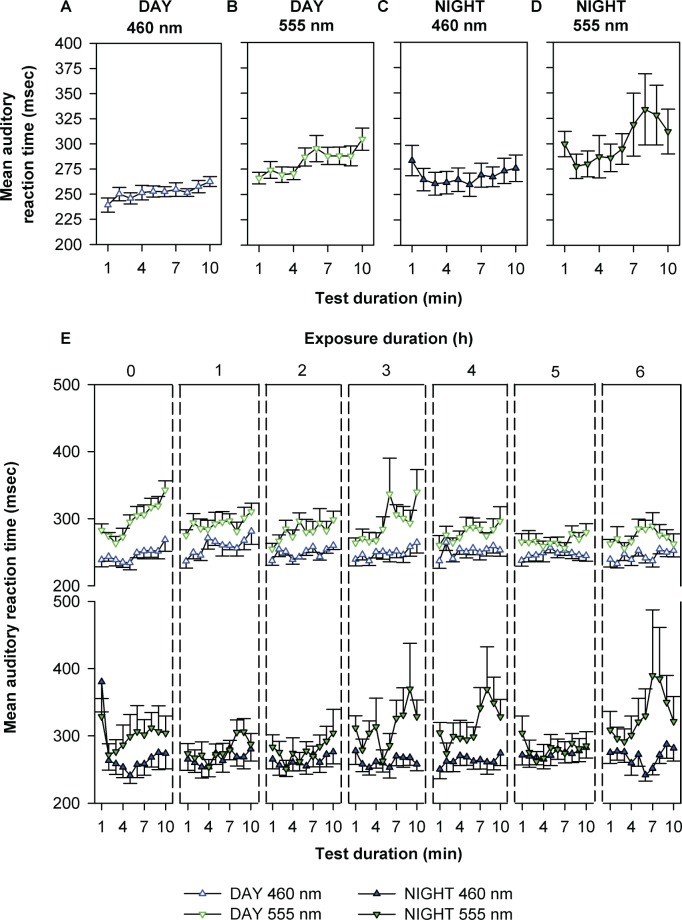
In addition, we examined the time course of reaction times per min during each 10-min PVT session during light exposure. There was a significant effect of test duration on reaction time for all four conditions with worsening performance over time (Figures 3A-3D). Moreover, all four conditions demonstrated a significant linear trend over the 10-min test duration as determined by robust regression (P < 0.0001) and revealed significant differences (P < 0.05) in the rate of deterioration in reaction time over the 10-min test duration. The rates of reaction time deterioration under daytime and nighttime 460 nm were 1.8 ± 0.4 and 1.7 ± 0.4 msec/ min, respectively, whereas under daytime and nighttime 555-nm exposure the rates were 3.6 ± 0.7 and 5.2 ± 1.7 msec/min, respectively. Furthermore, a four-way mixed model analysis of variance revealed significant main effects (P < 0.01) of spectral condition, exposure duration, and test duration, but not time of day (night or day) nor interactions between the factors on per-minute fluctuation in reaction time (Figure 3E). Attentional lapses showed similar profiles (data not shown).
Figure 3.
Temporal dynamics in auditory reaction time. The top panel shows the mean (± standard error of the mean [SEM]) reaction times for each min of the 10-min auditory performance task averaged over the entire light exposure during monochromatic daytime 460 nm (A), daytime 555 nm (B), nighttime 460 nm (C), and nighttime 555 nm (D) exposure. Data were subjected to one-way mixed-model analysis with restricted maximum likelihood estimation method (REML) with test duration as a fixed effect. Reaction times became significantly slower with increasing test-duration in all spectral conditions (A: P < 0.001; B: P < 0.0001; C: P < 0.003; D: P < 0.001). The rate of performance impairment, as determined by the slope of the linear regression of the profiles presented in A-D, were significantly different (P < 0.05) between conditions, with greater rates of impairment under daytime and nighttime 555-nm conditions than under daytime and nighttime 460-nm conditions. The bottom panel shows the effects of spectral condition, exposure duration, test duration, and diurnality (day versus night) on per min mean (± SEM) reaction times throughout the entire 6.5 h monochromatic light exposure period (E). Data were subjected to four-way repeated measures with random intercepts mixed-model analysis of variance with REML estimation. There was a significant effect of spectral condition, exposure duration, and test duration (P < 0.01). There was no significant effect of diurnality on reaction time or an interaction between spectral condition, exposure duration, and test duration on reaction time (P > 0.05; E). Performance appeared to be more impaired across the 6.5-h exposure duration during both day and night during the 555-nm exposure but not during the 460-nm exposure, which sustained per min reaction times at low levels throughout.
The time-of-day variation in the spectral response of alertness was further examined relative to the endogenous circadian modulation of psychomotor task performance. Mean reaction times from the first 24 h of constant routine and the two spectral conditions during monochromatic light exposure were expressed relative to scheduled wake time and averaged across individuals. The resultant mean profiles were fitted with a dual harmonic cosinor model to assess the circadian component in wake dependent changes in PVT reaction time under 460 nm, 555 nm, and dim light control conditions (Figure 4A). Under daytime and nighttime 555-nm exposure, reaction times were slower at the onset of light exposure than reaction times during the constant routine at the corresponding time 48 h earlier (Figure 4B). In contrast, daytime and nighttime exposure to 460-nm monochromatic light improved reaction times compared to corresponding reaction times during the constant routine (Figure 4B).
Figure 4.
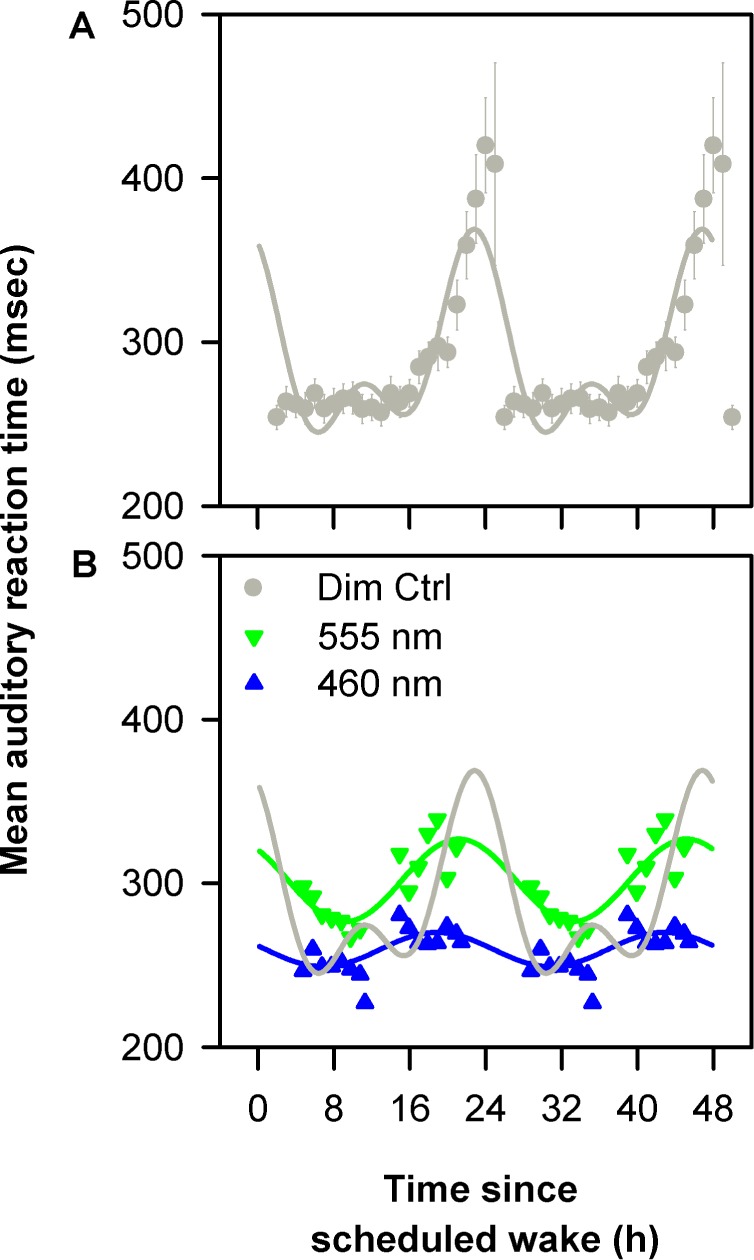
Effects of spectral modulation on circadian sleep drive. Mean (± standard error of the mean) reaction times from 10-min psychomotor vigilance task (PVT) sessions from the first 24 h of constant routine (Dim Ctrl) were expressed relative to scheduled wake time, double plotted and fitted with a cosinor model to assess the circadian component (r2 = 0.63; P < 0.0001) (A). The mean reaction times from 10-min PVT sessions during 6.5-h monochromatic light exposure were expressed relative to scheduled wake time and fitted with cosinor models based on spectral condition (460 nm: r2 = 0.57, P < 0.0001; 555 nm r2 = 0.71; P < 0.0001) (B). Daytime and nighttime exposure to 460-nm monochromatic light decreased reaction times compared to corresponding reaction times during the constant routine (B). In addition, 460-nm monochromatic light exposure was associated with a lower amplitude in reaction time rhythm as compared to 555-nm light and constant routine (B).
Waking EEG
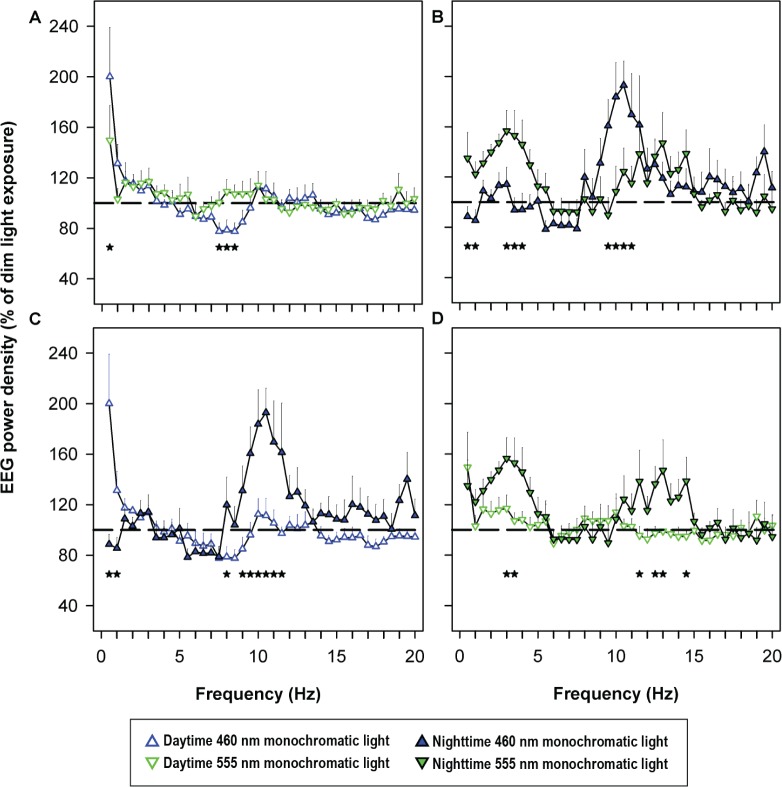
Exposure to 460-nm light during daytime and nighttime resulted in frequency-specific changes in the waking EEG, as compared with exposure to 555 nm. Daytime 460-nm light significantly reduced power density in the theta/low-frequency alpha range (7.5-8.5 Hz) as compared to 555-nm light (Figure 5A). Nighttime exposure to 460-nm light reduced delta power density (0.5 to 4.0 Hz), and increased power density in the high-frequency alpha range (9.5-11.0-Hz) as compared to 555 nm exposure (Figure 5B). Comparisons between daytime and nighttime 460-nm conditions demonstrated increased power density within the delta range (0.5-1.0 Hz) and decreased alpha power density (8.5-11.5 Hz) in the daytime condition as compared to the nighttime (Figure 5C). Comparisons between daytime and nighttime 555 nm conditions demonstrated decreased delta power density (3.0-3.5 Hz) and decreased high-frequency alpha and beta power density (11.5-14.5 Hz) in the daytime condition as compared to the nighttime (Figure 5D).
Figure 5.
Electroencephalogram (EEG) spectra during daytime and nighttime monochromatic light exposure. Log-transformed average power density during 6.5-h monochromatic light exposure was expressed relative to power density during the same clock time 48 h earlier under constant routine (100%, dotted line). Average power density in each 0.5 Hz frequency bin was compared between 460 nm and 555 nm exposure during the daytime (A) and nighttime (B) individually and between daytime and nighttime 460 nm (C) and daytime and nighttime 555 nm (D) conditions. Relative percentage power density data were log-transformed and subjected to two-way mixed model analysis with restricted maximum likelihood estimation method with spectral condition and frequency as fixed effects. Data were further subjected to Dunnett post hoc multiple comparison test when comparing between two groups. Data are shown as mean ± standard error of the mean. Significant differences (P < 0.05) in average power density in each 0.5- Hz frequency bin is expressed as *. Differences between daytime 460 nm and 555 nm exposure were found in the 7.5-8.5 Hz bins (A, *) and in the 0.5-4.0, 9.5-11.0 Hz bins during nighttime (B, *). There were significant differences between daytime and nighttime 460 nm in the 0.5-1.0, 8.0-11.5 Hz bins (C, *) and significant differences between daytime and nighttime 555 nm in the 3-3.5, 11.5-14.5 Hz bins (D, *). Panel B was published previously in reference 7.
Using the selective frequency ranges that demonstrated significant differences between 460-nm and 555-nm conditions during daytime (7.5-8.5 Hz) and nighttime (0.5-4.0 and 9.5-11.0 Hz) exposures, we examined the exposure duration-dependent changes in waking EEG during light exposure. Daytime 460 nm consistently maintained theta power density at lower levels as compared to 555-nm monochromatic exposure (P < 0.05; Figure 6A). Nighttime 460-nm light consistently suppressed delta activity across the exposure duration as compared to 555 nm exposure (P < 0.001; Figure 6B). The high-frequency alpha activity was higher under 460-nm nighttime light exposure as compared to the 555-nm light condition (P < 0.001; Figure 6C). There was no effect of exposure duration or the interaction between exposure duration and spectral condition on daytime or nighttime EEG temporal profiles.
Figure 6.
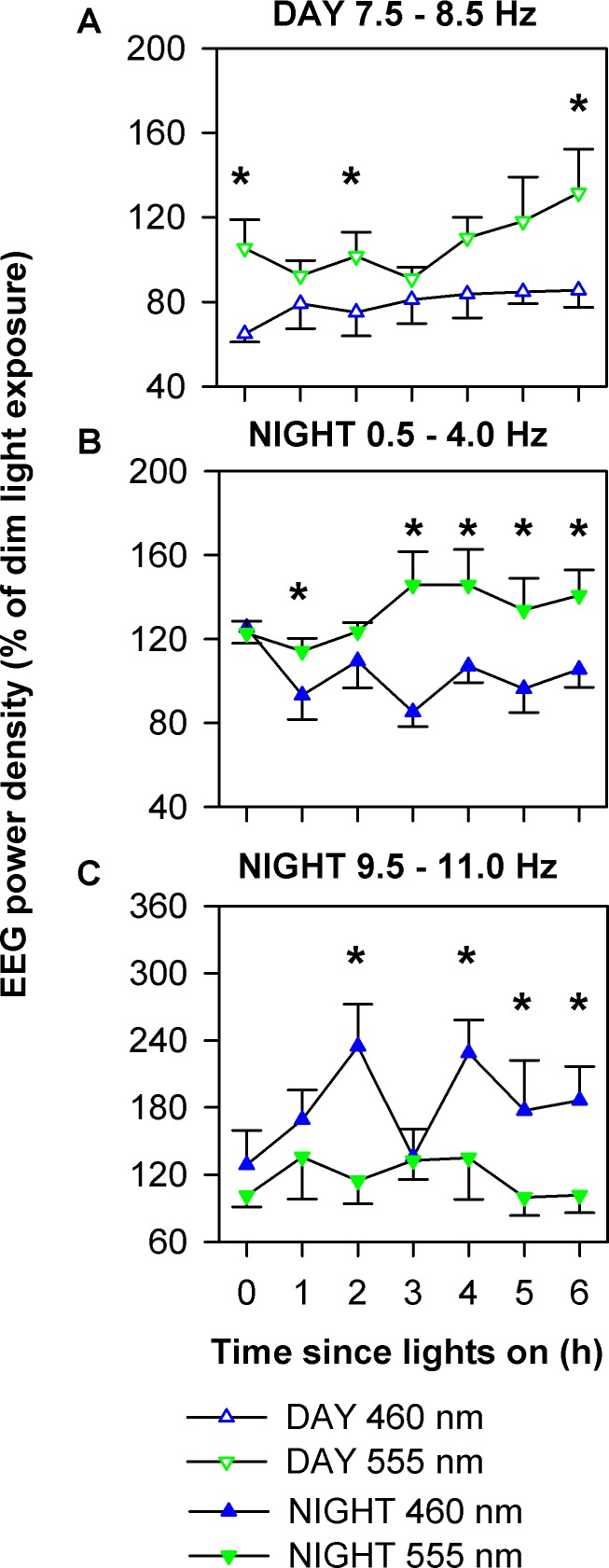
Temporal dynamics in electroencephalogram (EEG) power density during daytime and nighttime monochromatic light exposure. Using the specific frequency ranges that were significantly different between 460 nm and 555 nm (Figure 5), average power density from each 3-min Karolinska Drowsiness Test session were log-transformed and expressed relative to the average of the total 6.5-h duration during the corresponding time from the constant routine on the day prior to the light exposure. Data were subjected to two-way mixed-model analysis with restricted maximum likelihood estimation method with spectral condition and exposure duration as fixed effects. Data were further subjected to Dunnett post hoc multiple comparison test when comparing between two groups. Data are shown as mean ± standard error of the mean. Significant differences in power density between conditions at specific times of the light exposure session are shown as *. During the daytime, EEG power density in the 7.5-8.5-Hz range under the 460-nm condition was consistently lower than under the 555-nm condition (A) (P < 0.05). During the nighttime, EEG power density in the 0.5-4.0 Hz range under the 460 nm condition was consistently lower than under the 555 nm condition (B) (P < 0.001) whereas EEG activity in the 9.5-11.0 Hz range changed under the 460-nm condition during light exposure but remained consistently higher than under 555 nm exposure (C) (P < 0.001).
DISCUSSION
Exposure to 460-nm monochromatic light for 6.5 h significantly improved auditory performance and EEG correlates of alertness during both the day and night as compared to 555-nm monochromatic light. Exposure to 460-nm light at night improved performance to a level approaching that achieved under daytime 460-nm exposure. The nighttime alerting effects were associated with an increase in high-frequency alpha EEG activity, considered a specific marker of the circadian drive for alertness, whereas daytime short-wavelength light exposure was not. Daytime and nighttime short-wavelength light exposure was associated with suppression of delta and thetalow-alpha activity, which are known to be influenced by both circadian and homeostatic factors. These data suggest that, although short-wavelength light is able to improve alertness during both the day and the night, the mechanism by which the alerting effects are achieved may differ by time of day.
Previous studies have shown that white light exposure can improve alertness during both the day and night. White light exposure at night also acutely reduces subjective sleepiness and EEG correlates of sleepiness and improves psychomotor task performance in a dose-dependent manner.2 It has been hypothesized that melatonin suppression is the mechanism underlying light-induced increase in alertness at night.6,7,31–33 The current results confirm that 6.5-h exposures to short-wavelength light can improve alertness during the night, but also reveal that such exposure can improve alertness during the day, when melatonin levels are undetectable. The daytime improvement in alertness in response to short-wavelength light exposure is in agreement with previous studies showing similar improvement in alertness in response to bright white light exposure at ∼1000 lux18 or ∼5000 lux20 as compared to dim (< 10 lux) white light during the day. Brain imaging studies have demonstrated that blue-light exposure causes greater activation of neural centers regulating cognition including the brainstem, thalamus, hypothalamus, hippocampus, and amygdala as compared to green-light during the day.8,34 These studies have shown that the thalamus is most consistently activated in response to light exposure during a cognitive task. Direct ipRGC projections to the thalamic regions have been characterized in rodents and similar projections may mediate the direct alerting responses of light exposure in humans.12,35 Therefore, a diffuse multiregional central activation likely mediates overt changes in cognition and behavior in response to light exposure. Such observations provide supporting evidence of the mechanism by which blue light during the day elicits acute and sustained alerting effects as observed in the current study using psychomotor performance tests and EEG-based measures of neural activation, even in the absence of melatonin suppression.
The sustained improvement in alertness in response to monochromatic 460-nm light during the day and night is further supported in the per min examination of PVT reaction times. As expected, PVT reaction times gradually increased with test duration under both 460- and 555-nm exposure during the day, although the rate of deterioration in reaction time was significantly lower under 460-nm exposure as compared to 555-nm exposure (P < 0.05). At night, under both the 460- and 555-nm conditions, reaction times improved during the first few minutes of the 10-min test. Notably, the rate of deterioration during the last 7 to 8 min of the test was lower under 460-nm exposure as compared to 555-nm exposure, although the difference did not reach significance (P = 0.06). The failure of the 555-nm exposure to sustain performance at night suggests a suboptimal response, whereby cone photoreceptors may induce an initial alerting response but cannot sustain it over time.17 In contrast, the alerting response was maintained relatively consistently during 460-nm exposure, suggesting a tonic sustained response, presumably via melanospin activation in the ipRGCs.17
Exposure to short-wavelength light decreased EEG delta and theta activity during both the day and night, which are both markers of homeostatic sleep propensity.36,37 In contrast, EEG high-frequency alpha activity, which is a specific marker of the circadian drive for alertness and inversely correlates with plasma melatonin levels,36,37 only increased in response to short-wavelength exposure during the night and was unaffected by daytime 460-nm light exposure. The absence of an increasing effect on high-frequency alpha activity during the daytime is likely due to a ceiling effect because high-frequency alpha activity reaches its highest levels at this circadian phase30 and exposure to short-wavelength light cannot induce a further increase in high-frequency alpha activity. These data suggest that 460-nm monochromatic light improves alertness by reducing the effects of homeostatic sleep drive both during the day and night, but nighttime improvements in alertness are further facilitated by an additional increase in the circadian drive for alertness.
In summary, short-wavelength sensitivity of the acute alerting effects of light indicates that the visual photopic system is not the primary photoreceptor system mediating these responses to light either during the day or during the night. The frequency-specific changes in the waking EEG indicate that short-wavelength light is a powerful stimulant that attenuates the negative effects of both homeostatic sleep pressure and the circadian drive for sleep on alertness, particularly at night. Importantly, short-wavelength light restores nocturnal alertness levels to near-daytime levels and suggest that exposure to short-wavelength light can prevent the performance impairment associated with nocturnal work and maintain alertness and performance at levels similar to those observed during the day. Whether these effects persist with short-wavelength enriched polychromatic exposures suitable for real-world application remains to be tested at night, although data from preliminary studies suggest that the use of short-wavelength-enriched white light during the day in office settings improves subjective ratings of alertness, daytime sleepiness, fatigue, and work performance as compared to ordinary office lighting.38
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Rahman has received research funding from Government of Ontario, Pharmacia Canada Inc., Genesis Research Foundation, OBGYN Graduate Scholarship in Science and Technology at the University of Toronto, Faculty of Medicine and the Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award from Canadian Institutes of Health Research; Dr. Rahman holds a patent for Prevention of Circadian Rhythm Disruption by Using Optical Filters; owns equity in Melcort and ZircLight, Inc., and is a co-investigator on studies sponsored by Biological Illuminations, LLC. Dr. Flynn-Evans is a co-investigator on studies sponsored by Vanda Pharmaceuticals and Respironics, Inc., and is a member of the clinical advisory board at Isis Parenting. Dr. Aeschbach has served as member of the Scientific Advisory Board of Zeo, Inc. Dr. Brainard was the principal investigator on a research grant to Thomas Jefferson University from North American Philips; The Light Research program directed by Dr. Brainard at Thomas Jefferson University has received unrestricted monetary gift from Philips Lighting of Netherlands and equipment gift from the Lighting Sciences Group and Lutron, Inc.; Dr. Brainard holds patents for Photoreceptor System for Melatonin Regulation and Photo-therapy and Method for Modifying or Resetting the Circadian Cycle Using Short Wavelength Light. Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for Astra Zeneca, Bombardier, Inc., Boston Celtics, Celadon Trucking Services, Cephalon, Inc. (acquired by Teva Pharmaceutical Industries Ltd. October 2011), Garda Slochana Inspectorate, Gerson Lehrman Group for Novartis, Koninklijke Philips Electronics, NV. (acquired Respironics, Inc. March 2008); Minnesota Timberwolves, Novartis, Portland Trail Blazers, Sleep Multimedia, Inc., Vanda Pharmaceuticals, lnc., and Zeo, Inc. Dr. Czeisler owns an equity interest in Lifetrac, lnc., Somnus Therapeutics, Inc., Vanda Pharmaceuticals, Inc., and Zeo, Inc. He received royalties from CNN, the Massachusetts Medical Society/New England Journal of Medicine, McGraw Hill, Penguin Press/Houghton Mifflin Harcourt, and Philips Respironics, Inc. He has also received research support from Cephalon, ResMed and Philips Respironics. Dr. Czeisler has received lecture fees from AWHONN (Association of Women's Health, Obstetric and Neonatal Nurses), Harvard School of Public Health, Hokkaido University Graduate School of Medicine, Japan Aerospace Exploration Agency (JAXA), LOTTE Health Products, Mount Sinai School of Medicine, National Sleep Foundation, North East Sleep Society, Rockpointe (for Cephalon, Inc.), Sleep Research Society, Society of Thoracic Surgeons, Stress Research Institute, University of Stockholm, University of Chicago, University of Colorado, the World Federation of Sleep Research, and Sleep Medicine Societies and WME Entertainment LLC. Dr. Czeisler has also received research prizes with monetary awards from the American Academy of Sleep Medicine and clinical trial research contracts from Cephalon, Inc. His research laboratory at the Brigham and Women's Hospital has received unrestricted research and education funds and/or support for research expenses from Committee for Interns and Residents, the CIR Policy, and Education Initiative. The Harvard Medical School Division of Sleep Medicine (HMS/DSM), which Dr. Czeisler directs, has received unrestricted research and educational gifts and endowment funds from Boehringer Ingelheim Pharmaceuticals, Inc., Cephalon, Inc., George H. Kidder, Esq., Gerald McGinnis, GlaxoSmithKline, Herbert Lee, Hypnion, Jazz Pharmaceuticals, Jordan's Furniture, Merck & Co., Inc., Peter C. Farrell, PhD., Pfizer, ResMed, Respironics, Inc., Sanofi-Aventis, Inc., Sealy, Inc., Sepracor, Inc., Simmons, Sleep Health Centers LLC, Spring Aire, Takeda Pharmaceuticals, and Tempur-Pedic. The HMS/DSM has received gifts from many outside organizations and individuals including Cephalon, Inc., Committee for Interns and Residents, Eisai, Inc., Farrell Family Foundation, Fisher & Paykel Healthcare Corporation, Gerald McGinnis, Jazz Pharmaceuticals, Jordan's Furniture, Lilly USA, LLC, Neurocare Center for Sleep, NeuroScience, Novartis Consumer Health, Philips-Respironics, Inc., Praxair US Homecare, Purdue Pharma, ResMed Foundation, Safeway, Sanofi-Aventis, Inc., Select Comfort Corporation, Sleep HealthCenters LLC, Somaxon Pharmaceuticals, Transcept Pharmaceuticals, United Healthcare, Vanda Pharmaceuticals, Inc., Wake Up Narcolepsy, Inc., Watermark Medical, Weight Watchers International, YMCA of the USA, and Zeo, Inc. The HMS/DSM Sleep and Health Education Program has received Educational Grant funding from Cephalon, Inc., Takeda Pharmaceuticals, Sanofi-Aventis, Inc., and Sepracor, Inc. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. Dr. Lockley has received consulting fees from Naturebright, Sound Oasis, Thomas Jefferson University, and Wyle Integrated Science and Engineering (NASA); unrestricted lighting equipment gifts from Biological Illuminations LLC, Bionetics Corporation, and Philips Lighting; unrestricted monetary gift to support research from Swinburne University of Technology, Australia; fellowship gift from Optalert, Pty, Melbourne, Australia; Dr. Lockley holds equity in iSLEEP, Pty, Melbourne, Australia; advance author payment and royalties from Oxford University Press, and payment for editing a textbook section from Elsevier; honoraria and/or travel and accommodation support for invited seminars, conference presentations or teaching from 8th International Conference on Managing Fatigue; Harvard University; Lighting Science Group Corp; Ontario Association of Fire Chiefs; Rio Tinto; Woolcock Institute of Medical Research; Wyle Integrated Science and Engineering. He has received investigator-initiated research grants from Biological Illuminations LLC, Philips Lighting and Philips-Respironics Inc. and sponsor-initiated research contracts with Vanda Pharmaceuticals. He holds a process patent for the use of short-wavelength light for resetting the human circadian pacemaker and improving alertness and performance which is assigned to the Brigham and Women's Hospital per Hospital policy. No income has been received. Dr. Lockley also served as a paid expert on behalf of four public bodies in arbitration hearings related to sleep, circadian rhythms and work hours.
ACKNOWLEDGMENTS
The authors thank Elizabeth Lydon and Peter Dearborn, research staff, and research participants at the Division of Sleep Medicine, the Sleep & EEG Core at the Brigham and Women's Hospital for help with analysis of the EEG data; the technical, dietary, nursing and medical staff at the Center for Clinical Investigation at the Brigham and Women's Hospital, Jonathan Williams, MD for medical supervision; Ralph Todesco (BWH), John Hanifin (Thomas Jefferson University), Ron Kovak and Jon Cooke (PTI, Inc.) for technical support for the generation of monochromatic light.
Work was performed at Brigham and Women's Hospital, Boston, MA. This work was supported by the National Space Biomedical Research Institute (HPF01301) and the National Institute of Mental Health (R01MH45130). SAR, EEF, GCB, CAC, and SWL are supported in part by the National Space Biomedical Research Institute through NASA NCC 9-58. The project described was supported by Brigham and Women's Hospital General Clinical Research Center grant M01 RR02635. The project described was supported by Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women's Hospital from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
REFERENCES
- 1.Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 3.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–5. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- 6.Cajochen C, Munch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–6. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 7.Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–8. [PubMed] [Google Scholar]
- 8.Vandewalle G, Gais S, Schabus M, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007;17:2788–95. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- 9.Czeisler CA, Shanahan TL, Klerman EB, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 10.Klerman EB, Shanahan TL, Brotman DJ, et al. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J Biol Rhythms. 2002;17:548–55. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- 11.Zaidi FH, Hull JT, Peirson SN, et al. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–8. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–70. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panda S, Sato TK, Castrucci AM, et al. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–5. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 15.Güler AD, Ecker JL, Lall GS, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–5. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altimus CM, Guler AD, Alam NM, et al. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nature Neurosci. 2010;13:1107–12. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gooley JJ, Rajaratnam SMW, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra3. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phipps-Nelson J, Redman JR, Dijk DJ, Rajaratnam SM. Daytime exposure to bright light, as compared to dim light, decreases sleepiness and improves psychomotor vigilance performance. Sleep. 2003;26:695–700. doi: 10.1093/sleep/26.6.695. [DOI] [PubMed] [Google Scholar]
- 19.Vandewalle G, Balteau E, Phillips C, et al. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–21. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–R20. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lehrl S, Gerstmeyer K, Jacob JH, et al. Blue light improves cognitive performance. J Neural Transm. 2007;114:457–60. doi: 10.1007/s00702-006-0621-4. [DOI] [PubMed] [Google Scholar]
- 22.Sletten TL, Revell VL, Middleton B, Lederle KA, Skene DJ. Age-related changes in acute and phase-advancing responses to monochromatic light. J Biol Rhythms. 2009;24:73–84. doi: 10.1177/0748730408328973. [DOI] [PubMed] [Google Scholar]
- 23.Revell VL, Arendt J, Fogg LF, Skene DJ. Alerting effects of light are sensitive to very short wavelengths. Neurosci Lett. 2006;399:96–100. doi: 10.1016/j.neulet.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Goulet G, Mongrain V, Desrosiers C, Paquet J, Dumont M. Daily light exposure in morning-type and evening-type individuals. J Biol Rhythms. 2007;22:151–8. doi: 10.1177/0748730406297780. [DOI] [PubMed] [Google Scholar]
- 25.Staples VS, Archer SN, Arber S, Skene DJ. Daily light exposure profiles in older non-resident extreme morning and evening types. J Sleep Res. 2009;18:466–71. doi: 10.1111/j.1365-2869.2009.00762.x. [DOI] [PubMed] [Google Scholar]
- 26.Scheuermaier K, Laffan AM, Duffy JF. Light exposure patterns in healthy older and young adults. J Biol Rhythms. 2010;25:113–22. doi: 10.1177/0748730410361916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mrosovsky N, Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol Int. 2003;20:989–99. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 28.Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 29.Gillberg M, Kecklund G, Åkerstedt T. Relations between performance and subjective ratings of sleepiness during a night awake. Sleep. 1994;17:236–41. doi: 10.1093/sleep/17.3.236. [DOI] [PubMed] [Google Scholar]
- 30.Nelson W, Tong YL, Lee J, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–23. [PubMed] [Google Scholar]
- 31.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–7. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 32.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Gotz T, Cajochen C. Non-visual effects of light on melatonin, alertness and cognitive performance: can blue-enriched light keep us alert? PLoS ONE. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perrin F, Peigneux P, Fuchs S, et al. Nonvisual responses to light exposure in the human brain during the circadian night. Curr Biol. 2004;14:1842–6. doi: 10.1016/j.cub.2004.09.082. [DOI] [PubMed] [Google Scholar]
- 34.Vandewalle G, Schmidt C, Albouy G, et al. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 36.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: Evidence for frequency-specific circadian and homeostatic influences. Neurosci Lett. 1997;239:121–4. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- 37.Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- 38.Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness, performance and sleep quality. Scand J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]