Abstract
Study Objectives:
There is uncertainty over which characteristics increase obstructive sleep apnea syndrome (OSAS) severity in children. In candidates for adenotonsillectomy (AT), we evaluated the relationship of OSAS severity and age, sex, race, body mass index (BMI), environmental tobacco smoke (ETS), prematurity, socioeconomic variables, and comorbidities.
Design:
Cross-sectional screening and baseline data were analyzed from the Childhood Adenotonsillectomy Trial, a randomized, controlled, multicenter study evaluating AT versus medical management. Regression analysis assessed the relationship between the apnea hypopnea index (AHI) and risk factors obtained by direct measurement or questionnaire.
Setting:
Clinical referral setting.
Participants:
Children, ages 5 to 9.9 y with OSAS.
Measurements and Results:
Of the 1,244 children undergoing screening polysomnography, 464 (37%) were eligible (2 ≤ AHI < 30 or 1 ≤ obstructive apnea index [OAI] < 20 and without severe oxygen desaturation) and randomized; 129 (10%) were eligible but were not randomized; 608 (49%) had AHI/OAI levels below entry criteria; and 43 (3%) had levels of OSAS that exceeded entry criteria. Among the randomized children, univariate analyses showed significant associations of AHI with race, BMI z score, environmental tobacco smoke (ETS), family income, and referral source, but not with other variables. After adjusting for potential confounders, African American race (P = 0.003) and ETS (P = 0.026) were each associated with an approximately 20% increase in AHI. After adjusting for these factors, obesity and other factors were not significant.
Conclusions:
Apnea hypopnea index level was significantly associated with race and environmental tobacco smoke, highlighting the potential effect of environmental factors, and possibly genetic factors, on pediatric obstructive sleep apnea syndrome severity. Efforts to reduce environmental tobacco smoke exposure may help reduce obstructive sleep apnea syndrome severity.
Clinical Trial Registration:
Clinicaltrials.gov (#NCT00560859).
Citation:
Weinstock TG; Rosen CL; Marcus CL; Garetz S; Mitchell RB; Amin R; Paruthi S; Katz E; Arens R; Weng J; Ross K; Chervin RD; Ellenberg S; Wang R; Redline S. Predictors of obstructive sleep apnea severity in adenotonsillectomy candidates. SLEEP 2014;37(2):261-269.
Keywords: Adenotonsillectomy, apnea hypopnea index, disparities, obstructive sleep apnea syndrome, polysomnography, tobacco
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a disorder in which the soft tissues of the upper airway collapse during sleep, resulting in recurrent episodes of hypopneas and apneas, arousal, and intermittent hypoxemia, and is associated with adverse behavioral, cognitive, quality of life, and health outcomes. More than 10% of children are reported to have habitual snoring, a symptom of OSAS,1 whereas estimates of objectively measured OSAS criteria range from 0.1% to 4.7%.1,2 In two community-based nonclinical samples, OSAS prevalence was increased among racial/ ethnic minorities, children with socioeconomic disadvantage, and those born prematurely.1,3 It is unknown whether these risk factors generalize to otherwise healthy children seen for snoring and adenotonsillar hypertrophy from clinical settings from across the US.
To develop better strategies for screening and managing OSAS, it is important to identify risk factors for the presence and severity in OSAS in a clinical practice setting. We analyzed screening and baseline data from the Childhood Adenotonsillectomy Trial, a randomized, controlled multicenter study of health outcomes in children with mild to moderate OSAS, randomized to early adenotonsillectomy (AT) or to watchful waiting with supportive care, to identify demographic and health characteristics associated with the presence and severity of OSAS in snoring children considered to be candidates for AT. We hypothesized that OSAS severity would be associated with African American (AfA) race, low socioeconomic status, prematurity, obesity, and asthma. We also hypothesized that a history of atopy and environmental tobacco smoke (ETS), which may increase nasopharyngeal swelling or inflammation, also would be associated with OSAS severity. Because AfA race may be a surrogate for environmental or health factors, we hypothesized that any relationship between AfA race and OSAS would be attenuated after considering potential mediators such as obesity and ETS.
METHODS
Study Design and Sample
The design of the Childhood Adenotonsillectomy Trial has been described before.4 In brief, the targeted sample was children ages 5.0-9.9 y with mild to moderate OSAS, defined by parental report of the child's snoring and a standardized, centrally scored polysomnogram showing an obstructive apnea index (OAI, number of obstructive apneas per hour of sleep) ≥ 1 or apnea hypopnea index (AHI) ≥ 2.5 (Children with an AHI between 1 and 2, who may be classified with OSAS, were excluded to increase specificity for OSAS; in this analysis, they are considered to have “minimal OSAS.”) Children with severe OSAS defined as having an OAI > 20, AHI > 30, or percentage sleep time at an oxyhemoglobin saturation of < 90% for > 2% of total sleep time were ineligible for randomization. Other inclusion criteria included tonsillar size ≥ 1 based on a standardized scale of 0-4 and deemed to be a candidate for AT by otolaryngology (ear, nose, and throat [ENT]) evaluation. Exclusion criteria included comorbidities, medication use for attention deficit hyperactivity disorder, and a BMI z-score ≥ 3.4 Subjects were recruited from pediatric sleep centers/sleep laboratories, pediatric ENT clinics, general pediatric clinics, and the general community from seven clinical centers.4 Ethics approval was obtained by each institution, children provided assent, and parents provided written informed consent.
Study Procedures
Study personnel screened the clinical records of children referred to clinics and sleep laboratories to identify potentially eligible children with snoring and/or adenotonsillar hypertrophy. After informed consent was obtained, the child underwent further evaluation that included assessment of polysomnography (PSG) eligibility through the central scoring of a standardized polysomnogram and an ENT examination to confirm appropriateness for AT. Caregivers completed questionnaires addressing the child's medical, social, and family histories and the child underwent standardized assessments of anthropometric characteristics, neurocognitive and behavioral functions, and other measures.
Definitions
Categorical variables were defined using standardized questions. ETS was considered positive if the primary caregiver reported current smoking one or more cigarette per day6; maternal prenatal smoking was considered positive if the biological mother reported smoking one or more cigarettes per day while pregnant with the child; prematurity was based on a report of birth at least 4 weeks early; history of asthma, hay fever, or atopy (identified as reported eczema) were considered positive if the caregiver reported that the child had this condition diagnosed or treated by a physician. A history of allergies was considered positive based on parent report of “any allergies.” Socioeconomic status was defined on the basis of a reported family income < or ≥ $30,000 per year. Family history of OSAS was identified if the caregiver reported that OSAS had been diagnosed in the child's parent or sibling. Weight was assessed from measured weight and height values, expressed as body mass index (BMI), age, and sex adjusted percentiles and z-scores (http://www.cdc.gov/growthcharts/) as well as categorical variables (obesity: BMI > 95th percentile for age and sex; overweight BMI > 85% percentile. Birth weight was assessed as a continuous variable from recalled weight at birth, with low birth weight defined as < 2,500 g. Race was categorized as Caucasian, black/African American (AfA), and other. Season of recruitment was defined as December-July (lower allergen exposure) versus August-November.
Statistical Analysis
We analyzed data from two samples: (1) all subjects undergoing screening PSG; and (2) subjects who met study eligibility criteria and were randomized. Screened subjects were categorized into four groups according to OSAS severity and enrollment status: (1) excluded because of a low OAI and AHI (OAI < 1 or AHI < 2 ; primary snorers/minimal OSAS); (2) eligible by PSG but who were not randomized due to caregiver decisions not to proceed in the study; (3) eligible and enrolled; (4) excluded due to severe OSAS (AHI ≥ 30, OAI ≥ 20 or > 2% total sleep time with a saturation of peripheral oxygen [SpO2] < 90%). We compared those with the most severe OSAS (group 4) to all others (groups 1, 2, and 3); primary snorers/minimal OSAS (group 1) to those with mild to severe OSAS (groups 2, 3, and 4); and those who were eligible and randomized (group 3) to those eligible and not randomized (group 2). These comparisons allowed us to assess two sets of objectives: variation of risk factors across a wide range of OSAS severity, and variation of risk factors in children whose parents elected to continue in the study contrasted to those who did not continue. Group differences were compared using t-tests or linear regression for continuous variables and chi-square tests, the Cochran-Armitage trend test, or logistic or multinomial logistic regression for categorical variables. For descriptive analyses among randomized subjects, OSAS severity groups were defined to identify approximately equal quartiles in the AHI distribution (AHI < 3; AHI 3 to 4.9; AHI ≥ 5 to 9.9; AHI ≥ 10). Among the randomized children (who had more extensive data), we also evaluated the association between OSAS severity and subject characteristics. In multivariate regression models, the outcome was log-transformed AHI level. We used a fivefold cross-validation method to select variables to be included in the final multivariate model7 in addition to age, sex, race, obesity, and recruitment site, which were always included in the base model. We explored additional multivariate models by changing the variables included in the base model or by including variables of interest as covariates to assess their effects on model fit. We assessed whether the effect of race on AHI severity was mediated by obesity, ETS, family income, history of asthma, or birth weight, individually and in combination, using published methods.8 Tests were two-sided and significance tests were not adjusted for multiple comparisons. Analyses were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC) and R version 2.13.0 or higher.
RESULTS
A total of 1,244 children underwent screening PSG. Of these, 464 (37%) had an AHI/OAI level in the eligibility range and were enrolled. An additional 129 children (10%) were eligible by PSG but not enrolled due to changes in caregiver interest. Almost one-half (n = 608; 49%) of the snoring children considered to be AT candidates and screened with PSG were not eligible due to AHI/OAI levels below the study's threshold, whereas 43 children (3%) were ineligible due to PSG characteristics indicating severe OSAS (Figure 1).
Figure 1.
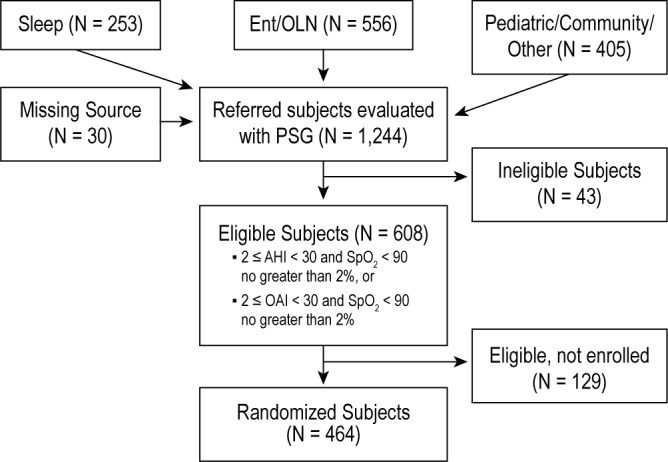
Consort diagram indicating flow of participants in the Childhood Adenotonsillectomy Trial. CHAT study.
Summary statistics, by study eligibility and enrollment status, for children who underwent screening PSG are shown in Table 1. The distributions of age and sex were comparable across groups. Among children who met PSG eligibility, those who were randomized compared to those not randomized had comparable levels of BMI but were more likely to be AfA. A greater proportion of children identified from ENT clinics compared to sleep centers were randomized, whereas fewer children from pediatric/community sources compared to ENT or sleep centers were randomized.
Table 1.
Subject characteristics by polysomnography eligibility and randomization status
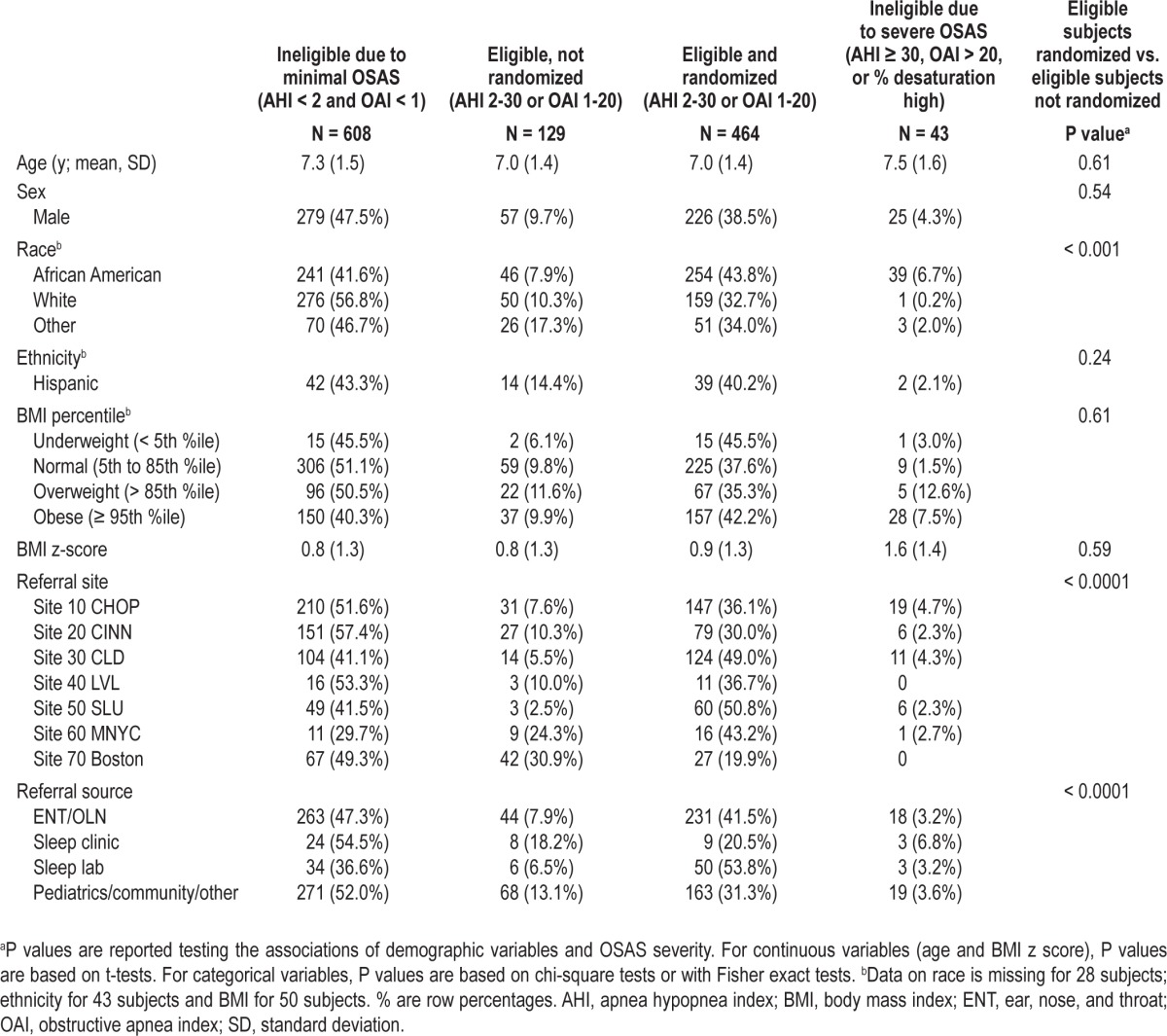
Table 2 illustrates the distributions of subject characteristics across the spectrum of OSAS severity among all screened children by comparing the most severe OSAS group (AHI > 30 or OAI > 30) to all others, and the primary snoring/ minimal OSAS group (AHI < 2 and OAI < 1) to children with mild to severe OSAS (AHI ≥ 2 or OAI ≥ 1). Children with the most severe OSAS were more likely to be AfA and obese than children with less severe OSAS; i.e., of the 43 children who were ineligible due to severe OSAS, 39 were AfA and two were Hispanic. The group with severe OSAS did not differ by age, sex, referral site, or recruitment source compared to those with primary snoring or less severe OSAS. Similarly, when comparing the primary snorers/minimal OSAS to those with mild or more severe OSAS, those with OSAS were more likely to be AfA and obese. A higher percentage of children from the pediatric/community sites were determined to be primary snorers or have minimal OSAS compared to children referred from sleep laboratories.
Table 2.
Characteristics according to obstructive sleep apnea syndrome severity, all screened children
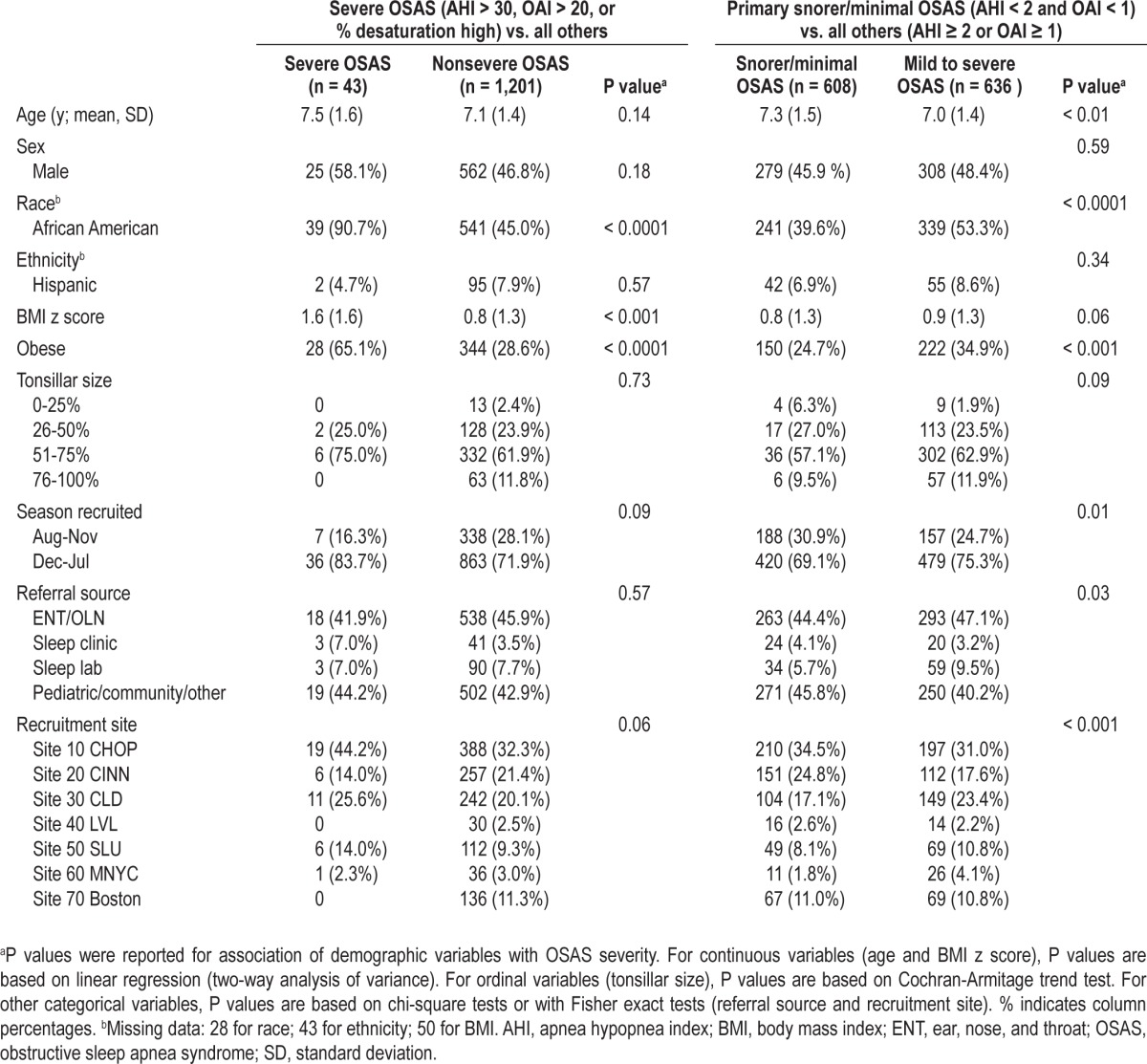
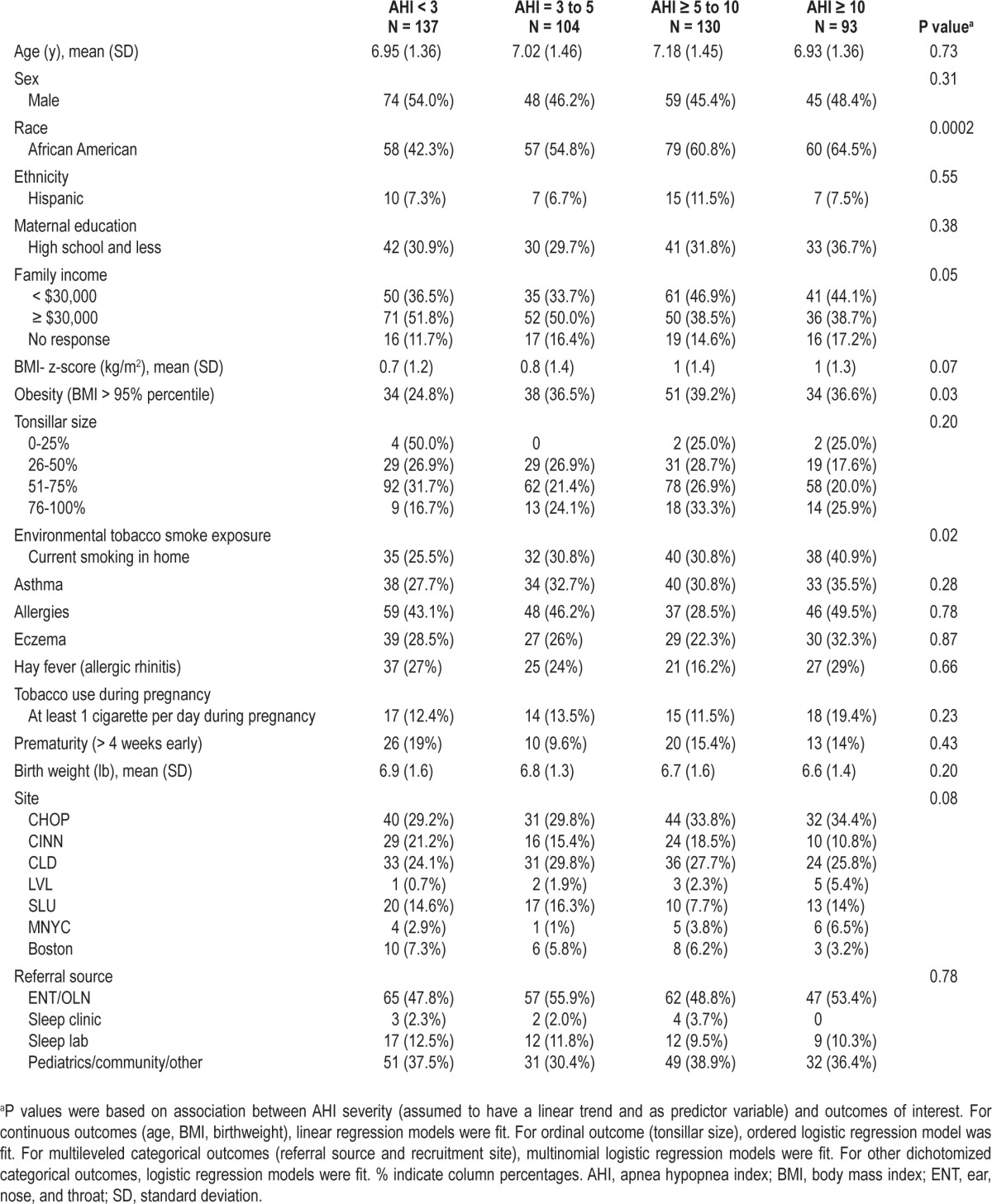
Table 3 shows the summary statistics for the more detailed clinical characteristics available for eligible children who were randomized. In univariate analyses, increasing levels of OSAS severity were associated with AfA race, family income, obesity, and ETS. OSAS severity was not associated with age, sex, tonsillar size, asthma, eczema, hay fever, prematurity, family history of OSAS, in utero tobacco exposure, or recruitment site.
Table 3.
Randomized participants' characteristics by AHI level
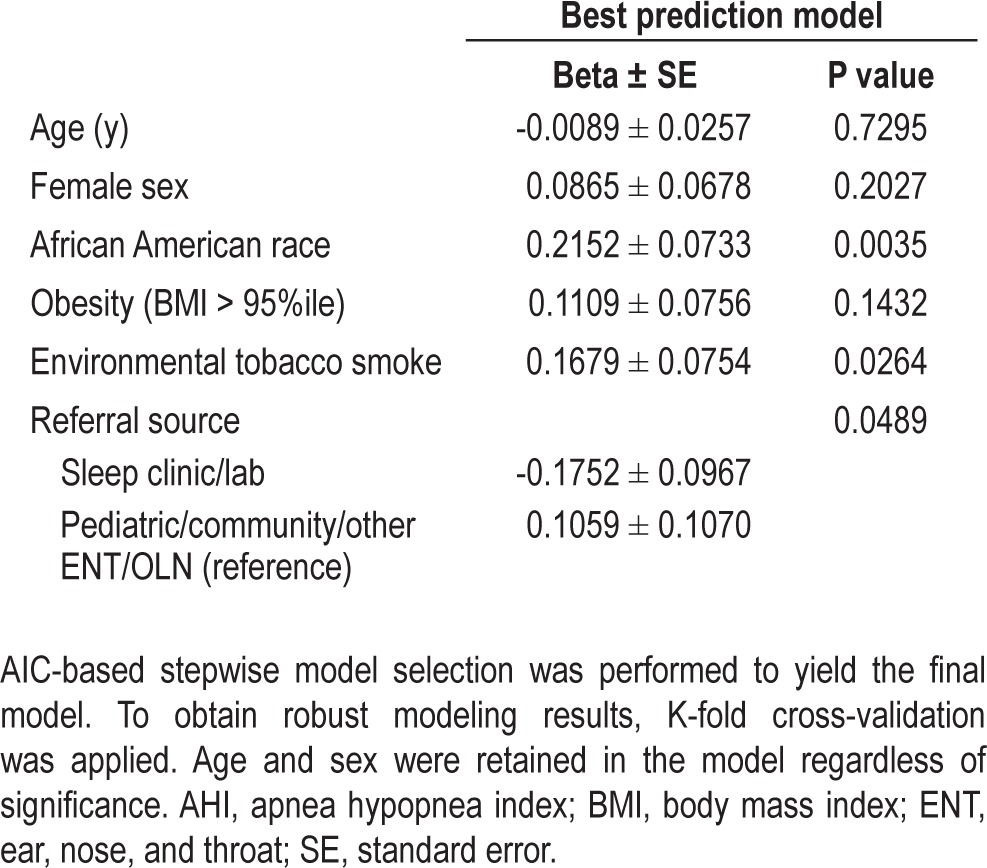
Multivariate analyses showed that AfA race was significantly associated with the AHI level after considering potential confounders such as maternal education and family income (neither of which was significant in race-adjusted models) as well as age, sex, obesity, referral source, ETS, prenatal smoking, prematurity, birth weight, allergies, hay fever, and asthma. The results of the final multivariable model relating the log-transformed AHI level and risk factors in the group of randomized children are depicted in Table 4. This model estimated that AfA children had an average increase in log AHI (events/h) that was 0.2 higher (P = 0.004) compared with other children, which corresponds to a 20% increase in AHI. The magnitude of the race effect was similar in alternative models that adjusted for prematurity, ETS exposure, maternal education, and family income. After adjusting for race, ETS (P = 0.026) and referral source (P = 0.049) were also significantly associated with log AHI. Exposure to ETS was associated with a 0.17 increase in log AHI compared to no exposure, which corresponds to an almost 20% increase in AHI.
Table 4.
Results of multivariable regression model; randomized participants (N = 408) Outcome: Log (AHI)
We further investigated whether obesity was a potential mediator for the association between race and AHI. Figure 2 presents the regression coefficients and their significance levels from various statistical models to illustrate the changes in relevant effects before and after adjusting for other variables. The odds of obesity increased 1.7-fold (95% confidence interval [CI]: 1.1-2.5) for AfA race using univariate logistic regression (Figure 2A). Obesity was also associated with a 1.2-fold increase in AHI level in univariate analysis (β = 0.16, P = 0.03). After controlling for race, the magnitude of this effect declined to 1.1-fold (β = 0.14, P = 0.058). In contrast, adjustment for obesity did not affect the association of AfA race and AHI (β 0.25 and 0.24, respectively). Therefore, obesity did not appear to mediate the relationship between AfA race and AHI levels.
Figure 2.
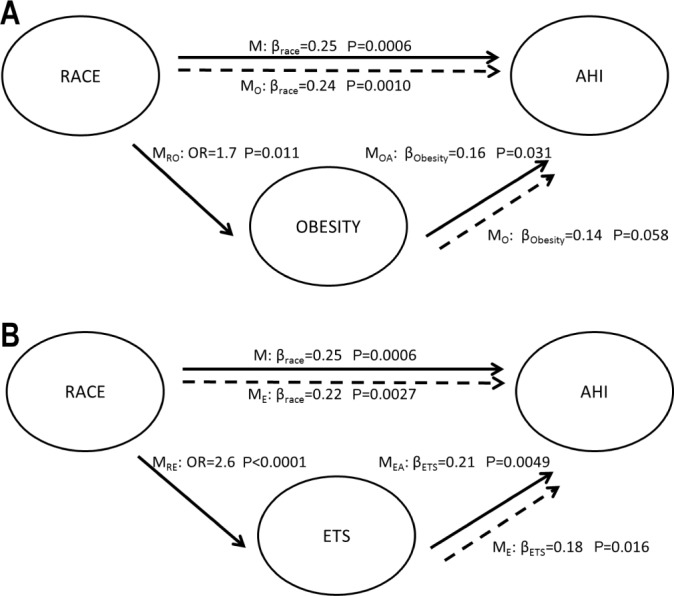
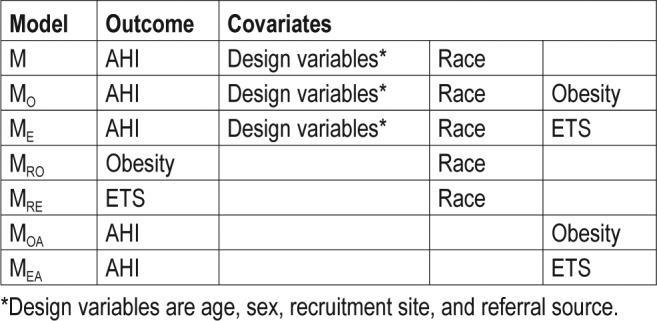
Similar analyses were performed to assess whether ETS was a potential mediator. The odds of ETS increased 2.6-fold (95% CI: 1.7-4.0) for AfA race in univariate analysis (P < 0.0001). ETS also was significantly associated with AHI, both in univariate (β = 0.21, P = 0.005) and in multivariate analysis controlling for AfA race (β = 0.18, P = 0.016). However, the associations of AfA race and AHI levels were similar whether or not adjusting for ETS (Figure 2B), suggesting that ETS was not a mediator for the relationship of AfA race and AHI levels.
We also evaluated the association between race and other potential mediators that could partially explain OSAS severity. Although family income, maternal education, asthma, and birth weight were related to race, mediation analysis demonstrated that these factors did not mediate the relationships between race and AHI.
DISCUSSION
In a large sample of children considered to be AT candidates, we considered the potential influences of a large number of patient characteristics in predicting OSAS severity. As reported before in two community samples,1,3 we found that the strongest predictor for OSAS severity was AfA race. The association between OSAS severity and AfA race could not be explained by differences in the available measures of socioeconomic status, obesity, or reports of inflammatory respiratory tract comorbidities such as asthma or hay fever. In addition, we observed a novel association between ETS and OSAS severity. No significant association between OSAS severity and sex or age was observed within the study's age range. In this sample selected to have tonsillar hyper-trophy, no significant association was observed between category of tonsillar size (from examination) and OSAS severity.
Race and Socioeconomic Factors
Almost all children with polysomnographic values indicating a level of OSAS that exceeded study severity thresholds were AfA. Even within the randomized group and after accounting for potential confounders, AfAs had an average AHI value 20% higher than that of other children. A statistical mediation analysis did not identify any socioeconomic variable, co-morbid condition, or design variable that explained this association.
Several prior studies have examined the relationship of OSAS and race in adults and children in community settings.1,3,9–11 Two nonclinic studies3,1 each demonstrated higher AHI levels in AfA compared with white children. One study suggested that the association between race and OSAS is stronger in younger than in older individuals.9 Thus, there may be genetic factors that influence susceptibility to OSAS, perhaps through influences on body fat distribution, inflammation, ventilatory control, or craniofacial structure. The latter is supported by a candidate gene association study providing preliminary evidence of variants in inflammatory and ventilatory control genes in OSAS.12
Race is confounded with socioeconomic status, and it is likely that an association with race also may reflect environmental exposures that preferentially affect poor families. In a study that addressed the combined influence of race and neighborhood disadvantage on OSAS, it was estimated that approximately 50% of the race effect could be explained by neighborhood-level variables.10 A recent Canadian study also showed an association between neighborhood disadvantage and OSAS.13 Although the putative triggers for OSAS have not been identified, exposures to indoor and outdoor irritants and allergens may exacerbate nasopharyngeal inflammation and predispose to airway collapse during sleep. In the current analysis, family-level measures of socioeconomic status were not associated with OSAS. In contrast, ETS was significantly associated with OSAS severity. However, adjusting for ETS did not appreciably attenuate the association between race and OSAS severity, suggesting that other unmeasured factors are likely important.
Obesity
Obesity is a well-established risk factor for adult OSA,14–16 and has been implicated in pediatric OSAS.17–19 Obesity may reduce airway patency through pharyngeal fat deposition, increased levels of circulating inflammatory mediators, or altered ventilation and oxygen reserves. Although we observed a significant association between levels of obesity with OSAS severity in unadjusted analyses, multivariable analysis demonstrated little effect of obesity after adjusting for race in the overall sample. Because most of our patient sample was AfA, it is possible that obesity may play a larger role in white patient samples, as has been reported before.20 To explore this, we examined the association between obesity and log AHI in race-stratified analyses and observed a significant positive association between obesity and log AHI in the non-AfA group (beta coefficient = 0.26, P = 0.030); in contrast, in the AfA group, no association was observed (beta coefficient = 0.04; P = 0.699). These exploratory analyses suggest that risk factors for OSAS may differ across population groups. Future research is needed to address which risk factors other than obesity (such as nasal allergy, tonsillar mass or ventilatory control) influence OSAS susceptibility.21–24
Environmental Tobacco Smoke
We observed a significant relationship between OSA severity and ETS that was independent of race. ETS has been shown to be associated with sleep disturbances in adults,25 pregnant women,26 and in young children.27 ETS also has been associated with snoring and OSAS in adults28–30 and with snoring in children.24 One study using serum cotinine to quantify ETS showed a dose-dependent association with snoring and reported apneas in asthmatic children.31 Our findings support the importance of ETS as a risk factor for objectively measured OSAS in children. Mechanisms may include chronic irritant exposure causing upper airway inflammation. It is possible that prenatal tobacco exposure (which is highly correlated with post-natal exposure) may influence neurotransmitter levels involved in ventilatory control.32 Although we tested for an association between in utero smoking and OSAS, misclassification of this exposure due to inaccurate maternal reporting may have diminished any real association.
Wheeze, Asthma, Atopy
Asthma, wheeze, atopy, and OSAS may coaggregate.24 Atopy may cause upper airway nasopharyngeal inflammation as well as bronchial hyperresponsiveness,33–36 and possibly exacerbate OSAS by triggering gastroesophageal reflux.37 Prior research has shown an association between childhood OSAS with asthma control11 and improvement of asthma symptoms in patients with OSAS who are treated with CPAP.38,39 Although we did not demonstrate an association between asthma, hay fever, or eczema and OSAS using questionnaire-based assessments, it is possible that quantitative measures of allergic and respiratory disease are needed to identify the relevant exposures. Additionally, we may have been limited in our ability to detect associations given the exclusion of individuals with severe asthma from the study participation.
Inferences Related to Recruitment and Study Enrollment
In this study, nearly half of snoring children considered to be AT candidates had very low AHI levels. The lack of PSG evidence for OSAS among children referred for suspected OSAS is consistent with prior literature indicating weak associations between symptoms and signs of OSAS and polysomnographic abnormality.40
Commonly, there are concerns about recruitment of minority populations into clinical research. Among our eligible pool of subjects, AfA families were more likely to enroll in this clinical trial than were individuals of other races. This may be because AfAs have less strong treatment preferences or perhaps perceive greater research participation benefits than other races. It is plausible that white children with severe OSAS were underrepresented due to strong parental treatment preferences. However, almost all children with severe OSAS excluded during PSG screening were AfA. The current findings also concur with data showing more severe OSAS in AfA children recruited for observational research from the community.1
The observed differences in enrollment by geography and referral site underscore the importance of the local factors and culture influencing recruitment to clinical trials.
Strengths and Limitations
Strengths of this study include its large sample size; inclusion of children from geographically diverse sites; and use of standardized, centrally scored sleep studies. Statistical mediation analyses systematically evaluated the interrelationships among a number of possible risk factors. Data were available for children screened as well as those who continued on to randomization, allowing for greater generalizability and a wider assessment of OSAS severity. The study also has several limitations. Although a comprehensive set of risk factors was assessed, many risk factors were identified by questionnaire only, and may have resulted in nondifferential misclassification resulting in underestimation of the strength of association. Quantitative evaluation of ETS with biological measures, such as cotinine level, is needed to better understanding the dose-response association between this exposure and OSAS severity.
Little information was available on children who declined screening PSG, preventing further evaluation of the entire targeted sample.
Summary
This study identifies AfA race and ETS as risk factors for OSAS severity in children. These findings suggest the presence of environmental and possibly genetic factors that potentially interact in the pathogenesis of pediatric sleep apnea. These findings suggest the potential utility of developing OSAS screening approaches that target high risk groups, such as AfA children and children exposed to ETS. Additional research is needed to define these specific factors, including other environmental risk factors that may predispose AfA children to OSAS. Further efforts to reduce ETS, and possibly other sources of inhaled irritants, may be important to diminish OSAS severity.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Chervin has received research grants from the NIH and the University of Michigan; grant support from Philips Respironics and Fisher Paykel through the University of Michigan for educational program in sleep biomedical innovation; served on the Board of Directors for American Academy of Sleep Medicine, American Sleep Medicine Foundation, American Board of Sleep Medicine, and International Pediatric Sleep Association; served on the advisory board as a volunteer for Sweet Dreamzzz (notfor-profit community organization); has consulted for Proctor & Gamble through a contract established with the University of Michigan; has consulted for Zansors (not compensated); Zansors, licensed questionnaire (developed by author) from University of Michigan; UpToDate, section editor; Cambridge University Press, book editor; and is Named in patents, held by the University of Michigan, for sleep disorder-related signal analysis and treatments. Dr. Redline's institution has received research support from ResMed Foundation and ResMed and equipment from ResMed and Philips Respironics. Dr. Ellen-berg has been involved in an advisory position for Pfizer, Bristol-Myers Squibb, Genentech, Millenium, Johnson & Johnson, Merck, Onyx, Genzyme, and Theravance; has lectured for Amgen; and has consulted for Tarsa, Millenium, Merck, and Johnson & Johnson. Dr. Marcus had the use of research equipment loaned to her by Philips Respironics and Ventus for research unrelated to this study. Dr. Paruthi was principal investigator on a study sponsored by UCB and has participated in speaking engagements for the American Academy of Sleep Medicine. Dr. Rosen has received honoraria from ACCP, ATS, and AASM for presentations unrelated to this manuscript. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The CHAT gratefully acknowledges the superb support of all study coordinators and the generous participation of the families enrolled in the study. We also are grateful for the helpful guidance during the study of the CHAT Data and Safety Monitoring Board: Lynn Taussig, MD (Chair); Thomas Anders, MD; Julie Buring, ScD; Karina Davidson, PhD; Estelle Gauda, MD; Steven Piantadosi, MD, PhD; Bennett Shaywitz, MD; Benjamin Wilfond, MD; Tucker Woodson, MD; Robert Zeiger, MD.
Grant support: NIH grants: HL083075, HL083129, UL1-RR-024134,UL1 RR024989. For the Childhood Adenotonsillectomy Trial (CHAT): Boston Children's Hospital, Harvard University, Boston MA (Eliot Katz, MD; Janice Ware, PhD; Dwight Jones, MD); Brigham and Women's Hospital and Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA (Susan Redline, MD MPH; Susan Surovec); Cardinal Glennon Children's Hospital, Saint Louis University, St. Louis, MO (Ron Mitchell, MD; Shalini Paruthi, MD; Karen Snyder, MS); Children's Hospital of Philadelphia (Carole Marcus, MBBCh; Nina H. Thomas, PhD; Lisa Elden, MD); Cincinnati Children's Medical Center, University of Cincinnati, Cincinnati, OH (Raouf Amin, MD; Dean Beebe, PhD; Paul Willging, MD); Montefiore Children's Hospital, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY (Raanan Arens, MD; Hiren Muzumdar, MD; Shelby Harris, PsyD CBSM); Rainbow Babies and Children's Hospital, Case Western Reserve University School of Medicine, Cleveland, OH (Carol Rosen, MD; H. Gerry Taylor, PhD; Robert Sprecher, MD); University of Kentucky, Louisville, Kentucky (David Gozal, MD); University of Michigan, Ann Arbor, MI (Ronald Chervin, MD; Susan Garetz, MD, Bruno Giordani, PhD; Tim Hoban, MD); University of Pennsylvania, Philadelphia, PA (Susan Ellenberg PhD; Renee H. Moore, PhD; Kim Lacy, RN, BSN; Melissa Fernando).
Author Contributorship: Dr. Weinstock drafted the initial manuscript, assisted with analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. Dr. Rosen conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Marcus conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Garetz conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Mitchell conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Amin assisted with the design of the study, assisted with data collection tools, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Paruthi conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Katz conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Arens assisted with data collection and critically reviewed the manuscript, approving the final manuscript as submitted. Mr. Weng assisted with data collection and analysis. Dr. Ross assisted with data collection and critically reviewed the manuscript, approving the final manuscript as submitted. Dr. Chervin conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Ellenberg assisted with data collection, analysis, reviewed and approved the final manuscript as submitted. Dr. Wang assisted with data collection, analysis, reviewed and approved the final manuscript as submitted. Dr. Redline conceptualized and designed the study, designed data collection tools, assisted with analysis, reviewed and revised the manuscript and approved the final manuscript as submitted.
ABBREVIATIONS
- AfA
African American
- AHI
apnea hypopnea index
- AI
arousal index
- AT
adenotonsillectomy
- BMI
body mass index
- CHAT
Childhood Adenotonsillectomy Trial
- ETS
environmental tobacco smoke
- OAI
obstructive apnea index
- OSAS
obstructive sleep apnea syndrome
- PSG
polysomnography
REFERENCES
- 1.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-rear-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 2.Brunetti L, Rana S, Lospalluti ML, et al. Prevalence of obstructive sleep apnea syndrome in a cohort of 1,207 children of Southern Italy. Chest. 2001;120:1930–5. doi: 10.1378/chest.120.6.1930. [DOI] [PubMed] [Google Scholar]
- 3.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children. Associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–32. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 4.Redline S, Amin R, Beebe D, et al. Rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34:1509–17. doi: 10.5665/sleep.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redline S, Budhiraja R, Kapur V, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. 2007;3:169–200. [PubMed] [Google Scholar]
- 6.Ferris BG. Recommended respiratory disease questionnaires for use with adults and children in epidemiological research: epidemiology standardization project. Am Rev Resp Dis. 1978;118:1–53. [PubMed] [Google Scholar]
- 7.Hastie T, Tibshirani R, Friedman J. Springer; 2009. The elements of statistical learning: data mining, inference, and prediction. Chapter 7. [Google Scholar]
- 8.MacKinnon DP. Introduction to statistical mediation analysis. New York: Taylor & Francis; 2008. [Google Scholar]
- 9.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 10.Spilsbury JC, Storfer-Isser A, Kirchner HL, et al. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149:342–7. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 11.Ross KR, Storfer-Isser A, Hart MA, et al. Sleep-disordered breathing is associated with asthma severity in children. J Pediatr. 2012;160:736–42. doi: 10.1016/j.jpeds.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin EK, Patel SR, Goodloe RJ, et al. A candidate gene study of obstructive sleep apnea in European Americans and African Americans. Am J Respir Crit Care Med. 2010;182:947–53. doi: 10.1164/rccm.201002-0192OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouillette RT, Horwood L, Constantin E, Brown K, Ross NA. Childhood sleep apnea and neighborhood disadvantage. J Pediatr. 2011;158:789–95 e1. doi: 10.1016/j.jpeds.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 14.Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26:703–9. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
- 15.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 16.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: The Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–13. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 17.Hannon TS, Rofey DL, Ryan CM, Clapper DA, Chakravorty S, Arslanian SA. Relationships among obstructive sleep apnea, anthropometric measures, and neurocognitive functioning in adolescents with severe obesity. J Pediatr. 2012;160:732–5. doi: 10.1016/j.jpeds.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Forbes EE, Ryan ND, Rofey D, Hannon TS, Dahl RE. Rapid eye movement sleep in relation to overweight in children and adolescents. Arch Gen Psychiatry. 2008;65:924–32. doi: 10.1001/archpsyc.65.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannon TS, Lee S, Chakravorty S, Lin Y, Arslanian SA. Sleep-disordered breathing in obese adolescents is associated with visceral adiposity and markers of insulin resistance. Int J Pediatr Obes. 2011;6:157–60. doi: 10.3109/17477166.2010.482156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonuck KA, Chervin RD, Cole TJ, et al. Prevalence and persistence of sleep disordered breathing symptoms in young children: a 6-year population-based cohort study. Sleep. 2011;34:875–84. doi: 10.5665/SLEEP.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery-Downs HE, Jones VF, Molfese VJ, Gozal D. Snoring in preschoolers: associations with sleepiness, ethnicity, and learning. Clin Pediatr (Phila) 2003;42:719–26. doi: 10.1177/000992280304200808. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G, Spickett J, Rumchev K, Lee AH, Stick S. Snoring in primary school children and domestic environment: a Perth school based study. Respir Res. 2004;5:19. doi: 10.1186/1465-9921-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalra M, Lemasters G, Bernstein D, et al. Atopy as a risk factor for habitual snoring at age 1 year. Chest. 2006;129:942–6. doi: 10.1378/chest.129.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall NS, Almqvist C, Grunstein RR, Marks GB. Predictors for snoring in children with rhinitis at age 5. Pediatr Pulmonol. 2007;42:584–91. doi: 10.1002/ppul.20606. [DOI] [PubMed] [Google Scholar]
- 25.Nakata A, Takahashi M, Haratani T, et al. Association of active and passive smoking with sleep disturbances and short sleep duration among Japanese working population. Int J Behav Med. 2008;15:81–91. doi: 10.1080/10705500801929577. [DOI] [PubMed] [Google Scholar]
- 26.Ohida T, Kaneita Y, Osaki Y, et al. Is passive smoking associated with sleep disturbance among pregnant women? Sleep. 2007;30:1155–61. doi: 10.1093/sleep/30.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson A, Ludvigsson J, Hermansson G. Adverse health effects related to tobacco smoke exposure in a cohort of three-year olds. Acta Paediatr. 2008;97:354–7. doi: 10.1111/j.1651-2227.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- 28.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154:2219–24. [PubMed] [Google Scholar]
- 29.Zhang L, Samet J, Caffo B, Punjabi NM. Cigarette smoking and nocturnal sleep architecture. Am J Epidemiol. 2006;164:529–37. doi: 10.1093/aje/kwj231. [DOI] [PubMed] [Google Scholar]
- 30.Phillips BA, Danner FJ. Cigarette smoking and sleep disturbance. Arch Intern Med. 1995;155:734–7. [PubMed] [Google Scholar]
- 31.Yolton K, Xu Y, Khoury J, et al. Associations between secondhand smoke exposure and sleep patterns in children. Pediatrics. 2010;125:e261–8. doi: 10.1542/peds.2009-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuetze P, Eiden RD. The association between maternal smoking and secondhand exposure and autonomic functioning at 2-4 weeks of age. Infant Behav Dev. 2006;29:32–43. doi: 10.1016/j.infbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138:838–44. doi: 10.1067/mpd.2001.114474. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Mendoza-Sassi RA, Cesar JA, Chadha NK. Intranasal corticosteroids for nasal airway obstruction in children with moderate to severe adenoidal hypertrophy. Cochrane Database Syst Rev. 2008:CD006286. doi: 10.1002/14651858.CD006286.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122:e149–55. doi: 10.1542/peds.2007-3398. [DOI] [PubMed] [Google Scholar]
- 36.Kheirandish-Gozal L, Serpero LD, Dayyat E, et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J. 2009;33:1077–84. doi: 10.1183/09031936.00130608. [DOI] [PubMed] [Google Scholar]
- 37.Thakkar K, Boatright RO, Gilger MA, El-Serag HB. Gastroesophageal reflux and asthma in children: a systematic review. Pediatrics. 2010;125:e925–30. doi: 10.1542/peds.2009-2382. [DOI] [PubMed] [Google Scholar]
- 38.Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99:529–34. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. Europ Respir J. 2007;29:307–11. doi: 10.1183/09031936.00059706. [DOI] [PubMed] [Google Scholar]
- 40.Carroll JL, McColley SA, Marcus CL, Curtis S, Loughlin GM. Inability of clinical history to distinguish primary snoring from obstructive sleep apnea syndrome in children. Chest. 1995;108:610–8. doi: 10.1378/chest.108.3.610. [DOI] [PubMed] [Google Scholar]


