Abstract
Study Objectives:
To document the monthly changes in sleep/insomnia status over a 12-month period; to determine the optimal time intervals to reliably capture new incident cases and recurrent episodes of insomnia and the likelihood of its persistence over time.
Design:
Participants were 100 adults (mean age = 49.9 years; 66% women) randomly selected from a larger population-based sample enrolled in a longitudinal study of the natural history of insomnia. They completed 12 monthly telephone interviews assessing insomnia, use of sleep aids, stressful life events, and physical and mental health problems in the previous month. A total of 1,125 interviews of a potential 1,200 were completed. Based on data collected at each assessment, participants were classified into one of three subgroups: good sleepers, insomnia symptoms, and insomnia syndrome.
Results:
At baseline, 42 participants were classified as good sleepers, 34 met criteria for insomnia symptoms, and 24 for an insomnia syndrome. There were significant fluctuations of insomnia over time, with 66% of the participants changing sleep status at least once over the 12 monthly assessments (51.5% for good sleepers, 59.5% for insomnia syndrome, and 93.4% for insomnia symptoms). Changes of status were more frequent among individuals with insomnia symptoms at baseline (mean = 3.46, SD = 2.36) than among those initially classified as good sleepers (mean = 2.12, SD = 2.70). Among the subgroup with insomnia symptoms at baseline, 88.3% reported improved sleep (i.e., became good sleepers) at least once over the 12 monthly assessments compared to 27.7% whose sleep worsened (i.e., met criteria for an insomnia syndrome) during the same period. Among individuals classified as good sleepers at baseline, risks of developing insomnia symptoms and syndrome over the subsequent months were, respectively, 48.6% and 14.5%. Monthly assessment over an interval of 6 months was found most reliable to estimate incidence rates, while an interval of 3 months proved the most reliable for defining chronic insomnia.
Conclusions:
Monthly assessment of insomnia and sleep patterns revealed significant variability over the course of a 12-month period. These findings highlight the importance for future epidemiological studies of conducting repeated assessment at shorter than the typical yearly interval in order to reliably capture the natural course of insomnia over time.
Citation:
Morin CM; LeBlanc M; Ivers H; Bélanger L; Mérette C; Savard J; Jarrin DC. Monthly fluctuations of insomnia symptoms in a population-based sample. SLEEP 2014;37(2):319-326.
Keywords: Insomnia, incidence, persistence, epidemiology, course, natural history
INTRODUCTION
Insomnia is a prevalent public health problem associated with significant psychological (e.g., risk of depression), medical (e.g., risk of hypertension), and economic burden (e.g., absenteeism).1–3 Cross-sectional studies have reported variable prevalence estimates of both insomnia syndrome (6% to 13%) and insomnia symptoms (20% to 60%).4–7 Longitudinal studies have also produced variable incidence rates of new onset insomnia varying between 3% and 20% over intervals ranging from 1 to 7.5 years.8–14 Other prospective studies have also reported highly variable persistence rates ranging from 20% to 69% for periods of 1 to 11 years13,15–17 and remission rates ranging from 8% to 79%.11,15,18–22
Despite their contribution to improving the knowledge base about the epidemiology of insomnia, several methodological limitations make it difficult to synthesize results and draw meaningful comparisons across these studies. Among these are differences in the samples (e.g., clinical vs. general population), criteria for defining insomnia cases (e.g., symptoms vs. syndrome), varying follow-up intervals, as well as lack of distinguishing individuals with or without prior history of insomnia for incident cases. Typically, intervals between assessments are one year apart, but they can vary and range anywhere between 4 months to as long as 20 years.4,19 Most studies have used few time-points, often conducting a single follow-up after baseline assessment.9,10,20,23 The few investigations that included more than two assessments4,12,15 often used long intervals that ranged from 6 months to 5 years between any two assessments.4,12 With long intervals between assessments, it is not possible to determine whether insomnia actually persisted continuously or was interspersed with periods of remission.
Longitudinal studies with few time-point assessments often use different recall periods to evaluate the presence of sleep difficulties, periods that can vary between the past two weeks to the past year.21,22 Nevertheless, a majority of studies used the previous month as a reference period in order to minimize recall bias and to be consistent with diagnostic criteria for chronic insomnia in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR)24 and the International Classification of Diseases, Tenth Revision (ICD-10).25 Interestingly, there are no data showing that assessment of insomnia with reference to the past month accurately represents sleep patterns for the previous year. It is plausible that sleep patterns during that reference period may not be representative of the entire interval elapsed between baseline and follow-up assessments, particularly if intervals are far apart. To date, no study has prospectively documented the course of insomnia at a more microscopic level (i.e., with short intervals between assessments). Thus, it remains unclear to what extent insomnia represents a chronic and stable condition, as opposed to a waxing and waning condition that includes recurring and transient episodes. This information would be useful to better characterize the natural course of insomnia, document burden of illness, and determine the most appropriate time to intervene.
The objective of the present study was to document the monthly fluctuations of sleep patterns and insomnia over a one-year period in a population-based sample. We were interested in examining what is the optimal time window or interval in the context of epidemiological research to reliably capture new incident cases of insomnia and recurrent episodes of insomnia and to make a reliable prediction about its persistence over time.
METHODS
Study Context and Participants
Data from this study are derived from a larger epidemiological study conducted in Canada. The study began with a population-based telephone survey to document the prevalence of insomnia and determinants of treatment-seeking behaviors.5,26 Sample selection involved random-digit dialing and use of Kish method.27 At the end of the telephone interview, respondents were invited to participate in the longitudinal phase of the study, which involved completion of several postal evaluations over 5 years. The objective of this longitudinal arm of the study was to describe the natural course of insomnia (e.g., incidence, persistence, remission rates) and identify risk factors associated with insomnia. The study also involved completion of 12 monthly telephone interviews over a one-year period (from October 2008 to October 2009) for a subsample of 100 individuals randomly selected among the French-speaking participants of the province of Québec. The present study reports the results obtained from this subsample.
Of the 10,002 participants who completed the initial telephone survey, 3,067 took part in the longitudinal arm of the study, including 1,921 French-speaking residents of the province of Québec. Among that subgroup, 115 individuals were randomly selected and solicited by telephone to participate in this substudy and complete 12 monthly interviews. Six declined to participate, and 9 were excluded after repeated attempts to reach them, yielding a final sample of 100 participants. Participants received a compensation of $100 for completing the 12 interviews.
Classification of Participants
After each of the 12 monthly assessments, participants were classified into 1 of 3 subgroups. Consistent with our previous studies,12,15 sleep status was defined by an algorithm using a combination of insomnia diagnostic criteria from the DSM-IVTR and the ICD-10, and the use of sleep-promoting medication. The data used to classify participants were derived from the Insomnia Severity Index28,29 and Pittsburgh Sleep Quality Index30 (described in the next section).
Insomnia Syndrome
Participants classified in this group met all diagnostic criteria for insomnia. They were dissatisfied (score of 3 or 4 on a 0-4 scale) with their sleep patterns and had symptoms of initial, middle, or late insomnia ≥ 3 nights per week. Initial insomnia was defined by sleep onset latency > 30 min; middle insomnia was defined by a period of wakefulness after sleep onset lasting > 30 min; and late insomnia was defined by a final awakening occurring > 30 min before the desired wake time. Participants also reported significant distress or daytime impairments related to sleep difficulties (score of 3 or 4 on a scale of 0-4). The one-month duration criterion was not used to classify participants in this subgroup because the assessments were repeated monthly. Lastly, participants were automatically categorized as having an insomnia syndrome if they used prescribed sleep promoting medication ≥ 3 nights per week for at least the past month.
Insomnia Symptoms
Participants classified in the insomnia symptoms subgroup reported initial, middle, or late insomnia ≥ 3 nights per week without fulfilling all diagnostic criteria for an insomnia syndrome (i.e., they reported being satisfied with their sleep or reported no or minimal distress or daytime consequences). Also included in this group were participants dissatisfied with their sleep but without symptoms of initial, middle, or late insomnia. Participants using prescribed medications < 3 nights per week or over-the-counter (OTC) medication for sleep at least one night per week were also classified in this group.
Good Sleepers
Participants in this subgroup were satisfied with their sleep (i.e., score of 0-2 on a scale of 0-4), did not report symptoms of insomnia, and did not use prescribed or OTC medication to promote sleep.
Telephone Interview
Telephone interviews were conducted by 5 trained and supervised undergraduate psychology students. Most participants had the same interviewer for the 12 interviews. The first interview started with a brief description of the study objective and, after obtaining verbal consent, the interviewer proceeded with questions on sleep and insomnia symptoms, utilization of sleep promoting products, occurrence of stressful life events, and physical and mental health problems over the previous month. The 12 interviews were identical; average interview duration was 10 minutes.
Sleep/Insomnia
The first part of the interview covered sleep difficulties in the preceding month, including the type, the severity, the duration, and the impact of insomnia symptoms. Standard questions from the Insomnia Severity Index (ISI) were asked.28,29 Participants rated the severity of their difficulty falling asleep, staying asleep or waking up too early on a 5-point scale from “0” (no difficulty) to “4” (very severe difficulty). These questions were followed by estimates of weekly frequency of (a) sleep onset latency > 30 min, (b) time awake after sleep onset > 30 min, (c) final morning awakening occurring > 30 min before the planned time, and (d) total sleep time < 6 hours. Participants rated their sleep quality on a 4-point scale from“0” (very refreshing) to “3” (not refreshing) and the weekly frequency of experiencing non-refreshing sleep. Sleep satisfaction was evaluated using a 5-point scale from“0” (very satisfied) to “4” (very dissatisfied). Finally, participants “dissatisfied” or “very dissatisfied” with their sleep and or those confirming sleep problems (yes/ no), reported the frequency, the duration, the consequences (i.e., impairment of daytime functioning, occupational or social activities, fatigue, mood disturbance), the noticeability to others, and the level of concern about their sleep problems using a 5-point rating scale from“0” (not at all) to “4” (very much).
Products Used for Sleep Problems
Participants were asked if they had used prescription medication, OTC medication, natural products, or alcohol to promote sleep in the preceding month. If participants confirmed using any of these products, they were then asked to estimate the weekly use of these products.
Depressive Symptoms
To investigate the presence and severity of depressive symptoms, 2 questions drawn from the Patient Health Questionnaire-9 (PHQ-9)31 were administered. Participants estimated how often they had been bothered by (1) little interest or pleasure in doing things and (2) feeling down, depressed, or hopeless over the last 2 weeks on a 4-point scale from“0” (not at all) to “3” (almost every day). The mean score of these 2 questions were used to quantify the severity of depressive symptoms.
Anxiety Symptoms
Participants estimated how often over the last 2 weeks (1) they had felt nervous, anxious or on edge and (2) whether they had difficulty controlling their worries on a 4-point scale from“0” (not at all) to “3” (almost every day). The mean score of those 2 items was used to quantify the severity of anxiety symptoms.
Data Analysis
Data manipulation and analyses were completed using SAS 9.3. Data distributions were checked for normality and outliers. Missing values were not imputed. Number of sleep status changes was computed for each participant and group comparisons between subgroups at baseline were examined using a one-way ANOVA, and post hoc comparisons were computed using Bonferroni α corrections. All hypothesis testing was based on a two-tailed α level of 5%.
Conditional probabilities were computed to estimate (1) the probability of a participant changing their sleep status anytime over the 12 monthly assessments relative to their baseline status and (2) the probability of a participant having insomnia at a given month to report insomnia again after 1 to 6 months, in order to establish the minimal duration for insomnia to become “chronic” (i.e., having a 100% probability to report insomnia in the next month).
To examine the extent to which a single annual assessment might have underestimated sleep changes, 2 series of computations were completed. First, consistent with most epidemiological studies, rates of sleep changes comparing baseline (T1) and one-year follow-up (T12) were computed; these rates represent our comparison point. Second, cumulative rates of sleep changes were computed over various time intervals, such as 1, 3, 6, 9, and 12 months. Four sleep changes (or sleep trajectories) were documented: (1) incidence of new cases of insomnia symptoms or syndrome for those who were good sleepers at baseline (i.e., a change from good sleeper status at baseline to a symptoms or syndrome status at any future time point, which varied as a function of the time interval being studied), (2) incidence and recurrence of insomnia symptoms or syndrome episodes (e.g., a change from good sleeper status at any given time point to a symptoms or syndrome status at any future time point), (3) persistence of insomnia symptoms or syndrome (e.g., no change in symptoms or syndrome status from one assessment at any time point to any future time point), and (4) remission of insomnia (i.e., a change from symptoms or syndrome status at any time point to good sleeper status at any future time point). Marginal means and standard errors for incidence, persistence, and remission rates were computed using logistic generalized estimating equations (GEE, using SAS GENMOD procedure). GEE models were preferred for their capacity to control for random (participant) variance (since the same participant may be included in a specific analysis depending of the availability of multiple occasions of sleep changes), their robustness to variance-covariance misspecification, and their emphasis on producing marginal (population based) means instead of conditional (subject-based) means.
All analyses were weighted according to a normalized weight derived from the joint distribution of gender, age group (18 to 49 years, 50 years and over), and sleep status. The joint distributions in the sample (N = 100) and in the larger study (N = 3,067) were compared to compute sampling weights.
RESULTS
Sample
Participants were 100 adults (66% women) between 23 and 85 years of age, (mean = 49.9; SD = 13.9). A majority of the participants completed at least a high-school degree (82%), were married or living with a partner (64%), working full-time (50%), and had a day work schedule (63%; Table 1). At baseline, 42 participants were classified as good sleepers, 34 presented insomnia symptoms, and 24 presented an insomnia syndrome.
Table 1.
Description of the sample at baseline
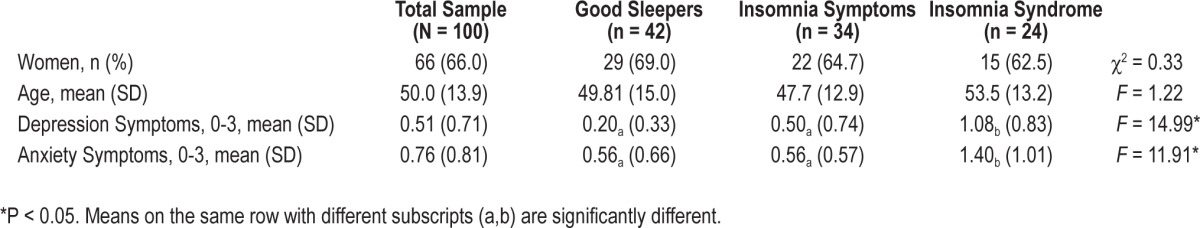
Of the projected 1,200 interviews (i.e., 100 participants × 12 interviews), 1,125 (93.7%) were completed as planned, resulting in 1,121 classifiable sleep status. The average number of interviews completed per participant was 11.2 (SD = 1.3, range = 5 to 12), with 54 participants completing all 12 interviews. Participants with one or more missing interviews were not significantly different from those who completed all interviews on gender, marital status, income, or sleep status at baseline; however, they were significantly younger than participants who completed all interviews (mean = 46 vs. 53 years old), t98 = 2.71, P = 0.008.
Based on the weighted data, 42.9% of the sample (N = 100) met criteria for insomnia symptoms at least one month during the study (but never met criteria for an insomnia syndrome), 9.0% met criteria for an insomnia syndrome at least once (but never for insomnia symptoms), 26.7% reported insomnia symptoms and syndrome on at least one occasion during the study, and 21.4% never reported any insomnia symptoms or syndrome during the 12 assessments.
Changes of Sleep Status over Time
Of the total sample, 66% changed sleep status at least once over the 12 monthly assessments, leaving 34% who exhibited a stable sleep status over that same period. A change of status was most common among those who presented insomnia symptoms at baseline (31/34; weighted % = 93.4%), followed by those with an insomnia syndrome (14/24; weighted % = 59.5%), and good sleepers (21/42; weighted % = 51.5%; Table 2). Thus, sleep was more stable among good sleepers and participants with insomnia syndrome than among participants with insomnia symptoms, X(2, N = 100) = 17.15, P < 0.001. Adjusting for the variable number of interviews per participant, participants changed sleep status an average of 2.73 times over the 12 monthly assessments. Participants with insomnia symptoms at baseline changed sleep status significantly more frequently (Mweighted = 3.46) than good sleepers (Mweighted = 2.12; F2,97 = 3.05, P = 0.05), while participants with an insomnia syndrome (Mweighted = 2.76) did not differ significantly from either good sleepers or participants with insomnia symptoms.
Table 2.
Proportion of participants (weighted data) with at least one change toward a different sleep status according to baseline sleep status
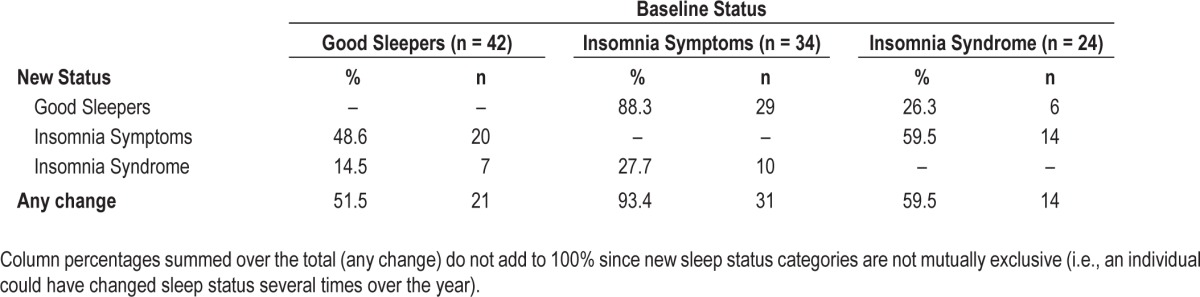
Table 2 presents data on the proportion of participants with at least one change to a different sleep status relative to their baseline status and the direction of the change (i.e., worsened or improved) during the course of the year. Among good sleepers at baseline, 48.6% (weighted %) subsequently developed insomnia symptoms and 14.5% developed an insomnia syndrome at least once over the year. Among participants with insomnia symptoms at baseline, 88.3% experienced improved sleep (i.e., became good sleepers) and 27.7% reported worsening of sleep (i.e., met criteria for an insomnia syndrome) at least once over the year. By comparison, 59.5% of the participants with insomnia syndrome at baseline partially improved their sleep (i.e., insomnia symptoms) and 26.3% experienced a complete remission (i.e., became good sleepers) at least once over the year.
Conditional probabilities were computed to determine the minimum duration an insomnia syndrome should persist before it could be defined as a chronic condition. Specifically, the probability to continue meeting criteria for an insomnia syndrome after 1, 2, 3, 4, 5, or 6 consecutive months was computed (Figure 1). Since the distribution of probabilities was non-normal (i.e., skewness and kurtosis over 2), the median conditional probability is reported instead of the arithmetic mean. After an initial diagnosis of insomnia syndrome, the median probability to report the same diagnosis in the following month [i.e., the conditional probability p(sy | sy)], was 50% on average. After two consecutive months with an insomnia syndrome, the probability to meet criteria for insomnia syndrome at the third month [i.e., p(sy sy | sy)], increased to 87.5%. The median probabilities after 3, 4, 5, or 6 consecutive months with insomnia diagnoses were all 100%.
Figure 1.
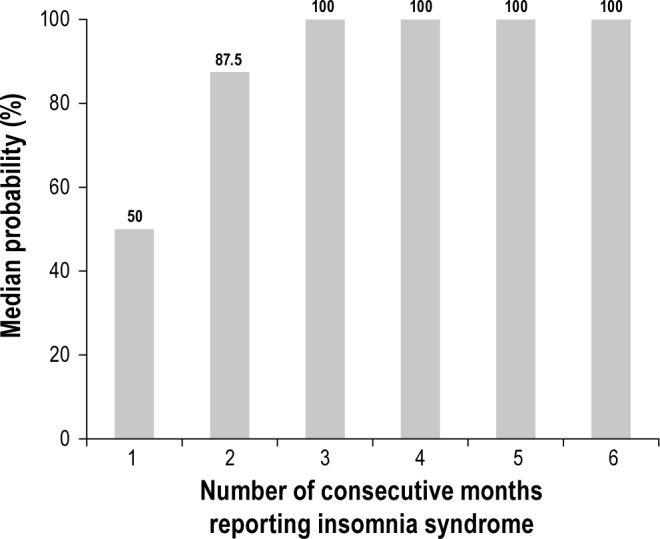
Median conditional probabilities to report insomnia syndrome based on the number of consecutive months with the diagnosis.
A series of GEE analyses (similar to logistic regressions for dependent observations) were conducted to estimate the magnitude of the differences in incidence, persistence, and remission rates between the commonly used “baseline and 12-month follow-up” method compared to various (smaller) time intervals. First, weighted cumulative rates for incidence of new cases, incidence and recurrence of insomnia episodes, persistence of insomnia episodes, and remission of insomnia episodes were examined as a function of time intervals that ranged from 1, 3, 6, 9, and 12 months (Figures 2–5).
Figure 2.

Cumulative incidence rates of new cases of insomnia symptoms or syndrome (weighted mean ± standard error) according to various duration intervals and a monthly sampling frequency. The last column computes the weighted incidence rate based on a yearly sampling frequency (comparing 1- vs. 12-month sleep to identify incidence cases). All numbers are based on N = 42 good sleepers at T1 but the 1 vs. 12-month estimate was based on 34 participants (i.e., those who completed the interview at both 1- and 12-month assessments). GS, good sleepers; SX, insomnia symptoms; SY, insomnia syndrome.
Figure 3.

Cumulative incidence rates of episodes (new or recurrence) of insomnia symptoms or syndrome (weighted mean ± standard error) according to various duration intervals and a monthly sampling frequency. The last column computes the weighted episode incidence rate based on a yearly sampling frequency (comparing 1- vs. 12-month sleep to identify incidence cases—which are by default all new cases). GS, good sleepers; SX, insomnia symptoms; SY, insomnia syndrome.
Figure 4.

Cumulative persistence rates of episodes of insomnia symptoms or syndrome (weighted mean ± standard error) according to various duration intervals and a monthly sampling frequency. The last column computes the weighted persistence rate based on a yearly sampling frequency (comparing 1- vs. 12-month sleep to identify persistent cases). GS, good sleepers; SX, insomnia symptoms; SY, insomnia syndrome.
Figure 5.
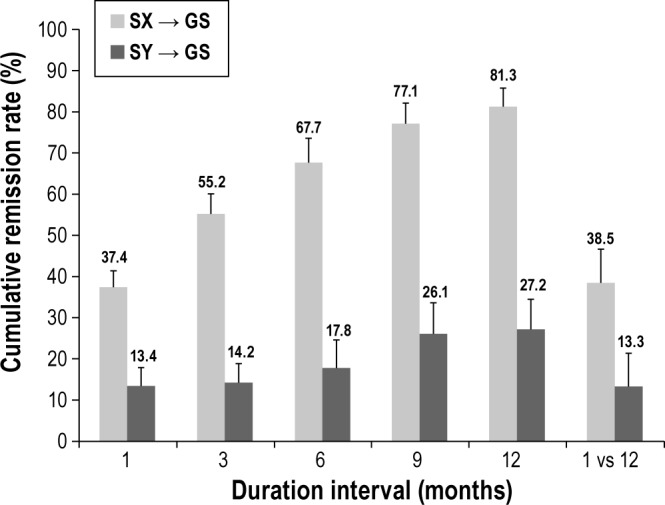
Cumulative remission rates of episodes of insomnia symptoms or syndrome (weighted mean ± standard error) according to various duration intervals and a monthly sampling frequency. The last column computes the weighted remission rate based on a yearly sampling frequency (comparing 1- vs. 12-month sleep to identify remission cases). GS, good sleepers; SX, insomnia symptoms; SY, insomnia syndrome.
Second, incidence, persistence, and remission rates at each time point (i.e., 1, 3, 6, and 9 months) were compared to the rates obtained for the commonly used “yearly” interval (comparing baseline, i.e., month 1 vs. month 12 sleep status). For these analyses, caution is warranted for the 12-month interval data, as there were substantially fewer observations available to describe these trajectories (i.e., incidence rates at 1 month were based on about 500 observations, while only 21 observations were available for incidence estimates at 12 months).
Weighted cumulative incidence rates (Figure 2) of new cases of insomnia symptoms (computed from participants categorized as good sleepers at baseline) were 11.4% after 1 month, 35.6% after 3 months, and increased linearly to 48.6% after 12 months. For insomnia syndrome, weighted cumulative incidence rates were 3.5% at a 1-month interval, 9.1% at a 3-month interval, and 14.5% at 6-, 9-, and 12-month intervals; no new case of insomnia syndrome emerged between 6- and 12-month follow-ups. When sleep status was compared only between baseline and 12-month follow-up, weighted incidence rates for new insomnia symptoms cases (i.e., good sleepers at baseline and insomnia symptoms at 12 months) were much lower (3.8%) than cumulative estimates and no new insomnia syndrome cases were observed.
Weighted cumulative incident or recurrent cases (Figure 3) of episodes of insomnia symptoms (computed from participants categorized as good sleepers at one time-point) were 18.2% after 1 month, 30.0% after 3 months, with continued increases up to 57.1% after 12 months (see footnote A). For episodes of insomnia syndrome, weighted cumulative incidence rates were 3.3% at a 1-month interval, 5.9% at a 3-month interval, and reached a plateau at 10.6% after 6 months and 11.9% after 12 months. When sleep status was compared between baseline and 12-month follow-up, weighted incidence rates for incident or recurrent episode of insomnia symptoms were much lower (3.8%) than cumulative rates, and no new insomnia syndrome cases were observed at that time.
Among participants with insomnia symptoms at any time-point, weighted persistence rates (Figure 4) were 48.6% after 1 month, 57.4% after 3 months, and increased to 63.4% and 62.8% after 9 and 12 months, respectively. Among those with an insomnia syndrome at any time point, persistence rates were 47.0% after 1 month, 56.7% after 3 months, and increased to 59.1% and 58.4% after 9 and 12 months, respectively. Comparisons of sleep status derived from baseline and 12-month follow-up assessment only yielded weighted persistence rates (i.e., insomnia at baseline and at 12-month follow up) that were in the same range (according to standard errors) for both symptoms (53.1%) and syndrome (65.4%) as those derived from the other method.
Rates of remission episodes (Figure 5) among participants with insomnia symptoms at any time-point were 37.4% after 1 month, and increased linearly from 55.2% after 3 months to 81.3% after 12 months. Thus, after 12 months, 81.3% of the participants who had insomnia symptoms at any assessment point were in remission (i.e., became good sleepers) at least once during that same interval. Among participants with an insomnia syndrome at any time point, remission rates were 13.4% after 1 month, 14.2% after 3 months, and increased to 27.2% after 12 months. When sleep status was compared between baseline and 12-month follow-up only, weighted remissions rates (i.e., insomnia at baseline and good sleepers at 12-month follow up) were much lower for both symptoms (38.5%; similar to 1-month remission rates according to standard errors) and syndrome (13.3%; similar to 1-, 3-, and 6-month remission rates according to standard errors) than rates derived from the other method.
DISCUSSION
The main findings of this study indicate that there are frequent changes in the sleep/insomnia status of a community-based sample over a 12-month period. The variability is particularly pronounced among individuals with insomnia (both symptoms and syndrome) at baseline, whereas good sleepers showed a more stable course of their sleep status. These results underscore the importance for prospective epidemiological studies to conduct repeated assessments at shorter than the typical yearly interval in order to reliably capture the natural course of insomnia over time.
Although the epidemiology of insomnia has received considerable attention in the last decade, few studies have used a prospective design with more than two repeated assessments. This longitudinal study is unique in its microscopic investigation of insomnia with the use of 12 repeated assessments at monthly intervals. Compared to studies that used only two assessments and longer intervals between assessments,14,16,22 the present study yielded higher estimates of incident cases for both symptoms (49%) and syndromes (15%). Conversely, rates of persistent insomnia over several consecutive monthly intervals were lower than previously reported in studies using fewer assessment points.9–10,20 Although it may be no surprise that repeated, monthly assessments capture more new cases relative to a yearly assessment with the “last month” as reference period, this finding raises several important questions. How many consecutive monthly assessments are needed to reliably capture true incident cases of chronic insomnia? What is the most “cost-efficient time window” to provide reliable estimate of incidence, persistence, and remission? How long should insomnia persist to be considered chronic?
Based on our results, the largest number of new insomnia cases (35.6% for symptoms and 9.1% for syndrome) emerged during the first three months of the study; an additional 10% developed symptoms at the 9- and 12-month follow-ups, but there was no new case of insomnia syndrome after the 6-month assessment. Data derived from the 9- and 12-month intervals should be interpreted cautiously given the smaller number of observations available for these longer intervals. Overall, these findings suggest that repeated, monthly assessments over 3-(to capture symptoms) or 6-month intervals (to capture a full syndrome) with a recall period of one month might provide the most accurate data about incidence rates while also being most cost-effective in terms of human resources needed and burden on participants.
The results about the minimum duration required to determine chronicity of insomnia are quite informative and consistent with recent changes in DSM5 diagnostic criteria to define a chronic insomnia disorder.32 The present data showed that the presence of an insomnia syndrome for one month is not a reliable indicator of chronic insomnia, given that there is a 50% probability that a participant will not meet criteria for an insomnia syndrome the following month. However, when the condition is present for three consecutive months, there is a 100% probability that the condition will persist at the next monthly assessment. Thus, the present findings provide evidence supporting the recent DSM-5 increase from one to three months to define chronic insomnia.
With regard to time course and prognosis, results of the insomnia symptoms subgroup are of particular interest given their more variable course over time. Only 6.6% of this subgroup remained stable for all 12 assessments, suggesting that reports of insomnia symptoms may often be a fairly transient condition. While the majority of these individuals tended to remit rather than worsen over time, the subgroup of individuals (27.7%) who worsened from symptoms to a full insomnia syndrome is of particular interest given their poorer prognosis. Additional information to better characterize this subgroup of individuals at risk for persistent insomnia would be helpful to guide early interventions or even prevention programs at the population level.
There are several limitations that must be kept in mind when interpreting the present findings. First, a sample size of 100 is quite small to make reliable estimates about incidence and persistence of insomnia. In addition, it is plausible that conducting monthly assessments of sleep/insomnia status may produce some reactivity to the measurement process and alter the way an individual perceives his sleep and respond to a survey. Also, while a monthly recall period seemed reasonable for the purpose of the present study, the optimal recall period is likely to vary as a function of the specific purpose of assessment. For instance, the use of daily sleep diaries over a 2-week period is the recommended standard for assessing outcome in insomnia trials,33 but it could be that a shorter or longer recall period would be more appropriate for longitudinal studies and perhaps even preferable to a 1-month recall period as in the present study. One way to check on the most appropriate interval for a study as this one would have been to ask participants to estimate their sleep/insomnia retrospectively for the previous 3, 6, or even 12 months, in addition to their monthly recalls. By asking for two ratings (one for the previous month and one for the longer period elapsed until that assessment), this would have allowed for testing accuracy of sleep/insomnia ratings across different reporting periods and directly check for memory and recall bias.34 Finally, the analyses of this paper are fairly descriptive and additional analyses with a larger sample could yield some very useful information about trajectory of changes and several moderating variables (e.g., life events, stress, change of health status, treatment initiation) that could potentially alter the course of insomnia over time. Such analyses could identify potentially modifiable behaviors that confer risk to the incidence and maintenance of insomnia.35–37
Additional prospective studies with repeated assessments would be helpful to achieve a better understanding of the natural history of insomnia and factors moderating its trajectories over time. Such longitudinal studies should also use standard definitions of insomnia cases, separating symptoms from syndromes, and use operational criteria to assess frequency, intensity, and duration of insomnia complaints. It is only by standardizing these methodological features that we can move the field forward and improve our understanding of the true prevalence, course, and morbidity of insomnia.
FOOTNOTE
A. To illustrate computation of these estimates, the 30% was based on a total of 176 data points derived from 69 participants with data available for that many 3-month intervals. Those 176 intervals are non-overlapping (each referring to distinct assessments; for example, month 1 to 4 for the first interval, month 4 to 7 for the second interval, etc.) and they included at least one occasion where the participant met criteria for a good sleep status and this status was not terminal (i.e., it was not the final status of the interval). Thirty percent of these 176 observations involved a monthly change from good sleep to insomnia symptoms, which could be a first new episode or a recurrence of an insomnia episode.
DISCLOSURE STATEMENT
This was not an industry supported study. Research supported by Canadian Institutes of Health Research grant (#42504). Dr. Morin has served as consultant for Merck, Valeant, and Novartis and received grant support from Merck and Novartis. All remaining authors have no financial conflicts of interest to disclose.
REFERENCES
- 1.Baglioni C, Riemann Is chronic insomnia a precursor to major depression? Epidemiological and biological findings. Curr Psychiatry Rep. 2012;14:511–8. doi: 10.1007/s11920-012-0308-5. [DOI] [PubMed] [Google Scholar]
- 2.Suka M, Yoshida K, Sugimori H. Persistent insomnia is a predictor of hypertension in Japanese male workers. J Occup Health. 2003;45:344–50. doi: 10.1539/joh.45.344. [DOI] [PubMed] [Google Scholar]
- 3.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- 4.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morin CM, LeBlanc M, Daley M, Grégoire JP, Mérette C. Epidemiology of insomnia: Prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7:123–30. doi: 10.1016/j.sleep.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 7.Ohayon MM, Reynolds CF., 3rd Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD) Sleep Med. 2009;10:952–60. doi: 10.1016/j.sleep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–18. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 9.Foley DJ, Monjan AA, Izmirlian G, Hays JC, Blazer DG. Incidence and remission of insomnia among elderly adults in a biracial cohort. Sleep. 1999;22(Suppl 2):S373–8. [PubMed] [Google Scholar]
- 10.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 11.Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men-A 10-year prospective population based study. Sleep. 2001;24:425–30. doi: 10.1093/sleep/24.4.425. [DOI] [PubMed] [Google Scholar]
- 12.LeBlanc M, Mérette C, Savard J, Ivers H, Baillargeon L, Morin CM. Incidence and risk factors of insomnia in a population-based sample. Sleep. 2009;32:1027–37. doi: 10.1093/sleep/32.8.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: A longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 14.Singareddy R, Vgontzas AN, Fernandez-Mendoza J, et al. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13:346–53. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 16.Sivertsen B, Salo P, Mykletun A. The bidirectional association between depression and insomnia: the HUNT Study. Psychosom Med. 2012;74:758. doi: 10.1097/PSY.0b013e3182648619. [DOI] [PubMed] [Google Scholar]
- 17.Vollrath M, Wicki W, Angst J. The Zurich Study VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239:113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 18.Ellis JG, Perlis ML, Neale LF, Espie CA, Bastien CH. The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46:1278–85. doi: 10.1016/j.jpsychires.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Hohagen F, Rink K, Kappler C, et al. Prevalence and treatment of insomnia in general practice. A longitudinal study. Eur Arch Psychiatry Clin Neurosci. 1993;242:329–36. doi: 10.1007/BF02190245. [DOI] [PubMed] [Google Scholar]
- 20.Jansson-Frojmark M, Linton SJ. The course of insomnia over one year: a longitudinal study in the general population in Sweden. Sleep. 2008;31:881–6. doi: 10.1093/sleep/31.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RE, Shema SJ, Kaplan GA. Prospective data on sleep complaints and associated risk factors in an older cohort. Psychosom Med. 1999;61:188–96. doi: 10.1097/00006842-199903000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Lam SP, Li SX, et al. Long-term outcomes and predictors of chronic insomnia: a prospective study in Hong Kong Chinese adults. Sleep Med. 2012;13:455–62. doi: 10.1016/j.sleep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Mendelson WB. Long-term follow-up of chronic insomnia. Sleep. 1995;18:698–701. doi: 10.1093/sleep/18.8.698. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 25.World Health Organization (WHO) The ICD-10 classification of mental and behavioral disorder: diagnostic criteria for research (10th ed.) Geneva, Switzerland: 1992. [Google Scholar]
- 26.Morin CM, LeBlanc M, Bélanger L, Ivers H, Mérette C. Epidemiology of insomnia in the adult Canadian population. Can J Psychiatry. 2011;56:540–8. doi: 10.1177/070674371105600905. [DOI] [PubMed] [Google Scholar]
- 27.Kish L. Survey Sampling. New York: John Wiley and Sons Inc; 1965. [Google Scholar]
- 28.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;23:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-V) Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 33.Carney CE, Buysse DJ, Ancoli-Israel S, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35:287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broderick JE, Junghaenel DU, Schneider S, Pilosi JJ, Stone AA. Pittsburgh and Epworth sleep scale items: accuracy of ratings across different reporting periods. Behav Sleep Med. 2013;11:173–88. doi: 10.1080/15402002.2012.654549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Åkerstedt T, Kecklund G, Axelsson J. Impaired sleep after bedtime stress and worries. Biol Psychol. 2007;76:170–3. doi: 10.1016/j.biopsycho.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Sadeh A, Keinan G, Daon K. Effects of stress on sleep: the moderating role of coping style. Health Psychol. 2004;23:542–5. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- 37.Morin CM, Rodrigues S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]


