Abstract
Study Objectives:
The objective of this study was to investigate if combined measures of activation in the thalamus and working memory capacity could guide the diagnosis of Kleine-Levin Syndrome (KLS). A second objective was to obtain more insight into the neurobiological causes of KLS.
Design:
Matched group and consecutive recruitment.
Setting:
University hospital neurology department and imaging center.
Patients or Participants:
Eighteen patients with KLS diagnosed according to the International Classification of Sleep Disorders and 26 healthy controls were included.
Interventions:
N/A.
Measurements and Results:
Working memory capacity was assessed by the listening span task. A version of this task (reading span) was presented to the participants during functional magnetic resonance imaging (fMRI). Activation in the thalamus was measured in a region of interest analysis. A combination of the working memory capacity and the thalamic activation measures resulted in 80% prediction accuracy, 81% sensitivity, and 78% specificity regarding the ability to separate KLS patients from healthy controls. The controls had an inverse relation between working memory capacity and thalamic activation; higher performing participants had lower thalamic activation (r = -0.41). KLS patients showed the opposite relationship; higher performing participants had a tendency to higher thalamic activation (r = -0.35).
Conclusions:
This study shows that functional neuroimaging of the thalamus combined with neuropsychological assessment of working memory function provides a means to guide diagnosis of Kleine-Levin Syndrome. Results in this study also indicate that imaging of brain function and evaluation of cognitive capacity can give insights into the neurobiological mechanisms of Kleine-Levin Syndrome.
Citation:
Engström M; Karlsson T; Landtblom AM. Thalamic activation in the Kleine-Levin Syndrome. SLEEP 2014;37(2):379-386.
Keywords: Functional magnetic resonance imaging (fMRI), working memory, thalamus, hypersomnia, narcolepsy
INTRODUCTION
Diagnosis of the sleep disorder Kleine-Levin Syndrome (KLS) remains a challenge. This is so because the typical symptoms of hypersomnia and additional cognitive, behavioral, and perceptual disturbances also occur in other disorders.1,2 The diagnostic difficulties are reinforced by the long interval (sometimes several months) between the characteristic sleep episodes. Early diagnosis of KLS is crucial since KLS commonly debuts in the early teens, and the symptoms have severe impact on development, school performance, and socialization.
To date KLS diagnostics is symptom-based, and no objective diagnostic criteria have been defined as of yet. Quite a large number of reported cases indicate absence of structural brain abnormalities in patients with KLS.3 Nor have there been any clear signs of aberrations in electroencephalogram (EEG) activity, except for a nonspecific slowing of background EEG activity during sleep episodes. In addition, hormone and orexin levels are usually normal.1,2 Between the typical sleep episodes most patients function normally. Nevertheless, we have observed persistent working memory difficulties that also are manifested after relapse.4,5
As stated above, conventional structural neuroimaging does not provide means for KLS diagnosis. However, several functional neuroimaging studies during the last decade have suggested possible diagnostic measures. Our group was early to report frontotemporal hypoperfusion in KLS,6 a finding that has been replicated in subsequent single photon emission computed tomography (SPECT) studies.7,8 Hypoperfusion of the thalamus during sleep episodes has also been reported.7–9 The cardinal finding from our group was inter-ictal hyperactivation in the left thalamus assessed by functional magnetic resonance imaging (fMRI) during a working memory task.5 This finding was later reproduced in a larger group of patients and also in patients who returned for a second fMRI.10 Even though the finding of fMRI hyperactivation in the left thalamus during working memory has been stable and reproducible on the group level, we found sparse evidence of thalamic hyperactivation on individual fMRI.
Based on previous findings, working memory dysfunction and thalamic hyperactivation are key features of KLS that persist between sleep episodes. Therefore we investigated the relation between individual working memory capacity and measures of individual activation levels in the thalamus during an fMRI working memory task. The primary aim was to explore if these measures could separate KLS patients from healthy controls and thus investigate the possibilities to use these measures to guide KLS diagnosis. A second aim was to obtain more insight into the neurobiological causes of KLS. We hypothesized that if the thalamic hyperactivation in KLS is a result of neural inefficiency, we would observe an increasingly higher activity in those subjects who perform worse on the working memory task. On the other hand, if this hyper-activation is a result of a compensatory mechanism, high-performing KLS patients would have higher thalamic activity than low-performing KLS patients.
METHODS
Subjects
Eighteen patients with KLS diagnosed according to the International Classification of Sleep Disorders (American Academy of Sleep Medicine, 2005) were included. The mean age was 25.9 years (SD = 11.4). Ten patients were females; 8 were males. All patients were examined during an asymptomatic state. At the time of examination, 11 patients had active disease, 4 patients had their last episode 3-6 months prior to fMRI, and 3 patients had relapsed. Statistical data regarding age at onset, and duration and frequency of sleep episodes are presented in Table 1. One patient was treated with serotonin reuptake inhibitor. The remaining 17 patients had no pharmacological treatment for KLS at the time of examination.
Table 1.
Descriptive statistics of KLS patients regarding age at onset, sleep episodes, and education
Functional MRI and working memory results for 8 of these patients were reported in our previous paper.5 In addition, all 18 KLS patients were included in a comprehensive brain activity and connectivity study aiming to investigate the relation between brain and effort in health and disease.11 Six patients performed the examination twice. It was 2-4 years between the first and the second examination.
In addition, 26 healthy controls (mean age = 24.1, SD = 5.3, females/males = 14/12) were recruited to participate in the study. The controls had no signs of sleep disorder or other neurological, psychiatric, or cognitive deficits that could interfere with the study results, as assessed by a clinical interview before inclusion.
Behavioral Working Memory Assessment
Approximately 1 h before fMRI scanning, the subjects performed the listening span working memory task.12 During the listening span task, the subjects listened to sentences read out loud one at a time by the investigator and were asked to tell if the sentences were semantically correct or not. The working memory task was to remember the last word of each sentence. After 1 to 5 sequentially presented sentences, the subjects were asked to repeat each target word in correct order. Details of the listening span task are described in more detail in Engström et al.5 On the same occasion, the subjects were familiarized with a computerized version of the task (the reading span task), which was to be performed during the fMRI session. In addition, the subjects were administered other neuropsychiatric tasks that will be reported elsewhere.
fMRI Data Acquisition
Image data were acquired on a Philips Achieva (Best, the Netherlands) 1.5 T clinical scanner using the standard head coil. For fMRI, a blood oxygen level dependent (BOLD) sensitive sequence with following scanning parameters was used: echo time, TE = 40 ms, repetition time TR = 2700 ms, flip angle = 90°. Thirty-two transversal slices were acquired in interleaved fashion. The voxel sizes were 3 × 3 × 3 mm3. The number of dynamics for the working memory task was 302.
Working Memory Task during fMRI
The reading span working memory task that was administered during fMRI scanning was an fMRI-adapted version of the listening span task described in the section on Behavioral Working Memory Assessment. During fMRI, the sentences were presented visually for 5 s each. After each set of sentences, 5 words were presented for 5 s each, and the subjects were asked to indicate if these words were target words or new words (lures). This procedure was repeated for 1-4 sentences in the same sequential order for all subjects. The subjects were instructed to answer as quickly and accurately as they could. Task duration was approximately 14 minutes. The working memory task administered during fMRI is described in more detail in our previous study.5
The working memory task was presented using MR compatible video goggles (Resonance Technology Inc, Northridge, CA, USA) and Superlab software (Cedrus Corporation, San Pedro, CA, USA). The subjects made their responses using the Lumi Touch (Photon Control Inc. Burnaby, BC, Canada) button box.
fMRI Image Analysis
Image analysis was performed using SPM5 software (Wellcome Department of Imaging Neuroscience, University College, London, UK). Images in each fMRI scan were realigned to correct for movement during scanning and normalized to Montreal Neurological Institute (MNI) template using nonlinear affine registration. The normalized images were resliced to 2 × 2 × 2 mm3 to fit to the coordinates of the standard template, and thereafter the images were smoothed with 8 mm full width half maximum (FWHM) Gaussian kernel for noise reduction and to ameliorate differences in intersubject anatomy.
The image analysis was performed using the general linear model (GLM). Contrast images containing information of the standard task difficulty contrast were calculated for all subjects. That is to say, a contrast vector of [0, -3, -1, 1, 3] was used in order to investigate brain activation of increasing difficulty of the task. The first regressor represented the periods when the sentences were presented and the remaining regressors represented word recognition after 1, 2, 3, and 4 consecutive sentences assuming a linear BOLD response to increasing task difficulty.
At the second level analysis, the contrast images of each subject were entered into a 2-sample t-test to obtain the differences in thalamic activation between KLS and controls. The brain activation in the KLS group was exclusively masked by the activation of the control group. A generous mask with uncorrected P = 0.05 threshold was used in order to obtain clusters in the thalamus that was exclusively activated by KLS patients and not by controls. To separate activation in the thalamus from the whole-brain working memory activation, we applied an image mask of the bilateral thalamus, which was created using the Wake Forrest University (WFU) PickAtlas tool.13 The resulting images were preliminary thresholded at P = 0.001. Results are presented as significant if the p-values of the activation cluster or peak were < 0.05, Family Wise Error (FWE) corrected for multiple comparisons.
To obtain an estimate of the BOLD response in the left thalamus during the most difficult level of the working memory task, i.e., word recognition after 4 sentences, we calculated the mean contrast values in a region of interest. These contrast values represent the β coefficients in the regression model (GLM) and are approximations of the magnitudes of the BOLD response. Unthresholded images (threshold T-value = 1) were used to calculate the contrast estimates. The region of interest was a sphere of radius 5 mm centered at the activation peak [-6, -6, 6; MNI coordinates] obtained in the left thalamus of KLS patients in our previous study.5 The contrast estimates were normalized between 0 (the lowest value of both groups) and 100 (the highest value of both groups).
Statistics
Statistics were calculated using the IBM SPSS Statistics v 20 software (IBM, Armonk, NY, USA) and GraphPad Prism v. 5.0d (GraphPad Software Inc., La Jolla, CA, USA). Behavioral results from the 4 different levels of the working memory task administered during fMRI were analyzed with nonparametric Mann Whitney test, since the results were not normally distributed according to the D'Agostino and Pearson omnibus normality test. As the purpose was to investigate if previous findings could be replicated, we used one-tailed t-tests to compare KLS patients and healthy controls.
Correlation between working memory performance scores from the test administered before fMRI and working memory performance scores during the most difficult level of the fMRI-task (Level 4) was calculated with the Spearman test. Correlation between pre-fMRI measures of working memory capacity and the contrast estimates representing thalamic activation was calculated with the Pearson correlation coefficient, since these values were normally distributed.
Receiver operating characteristics (ROC) was calculated for working memory performance scores and thalamic activation separately. In addition, conjoint ROC was calculated from a combination of the 2 measures, calculated as index values (I):
 |
where Th is the normalized contrast estimate from the thalamus and WM is the normalized working memory score. To estimate prediction accuracy, sensitivity, and specificity of thalamic activation and working memory capacity as diagnostic measures we made a binary logistic regression using 3 models: working memory capacity and thalamic activation modeled separately, and a model that combined the two measures. Sensitivity (Se) was calculated according to:
 |
where Ntp denotes the number of true positives and Nfn denotes the number of false negatives. Specificity (Sp) was calculated according to:
 |
where Ntn denotes the number of true negatives and Nfp denotes the number of false positives.
RESULTS
Behavioral Results
Confirming our previous results, KLS patients performed worse than controls during the working memory task administered before fMRI (P = 0.0003). The mean values were 15.2 (SD = 4.9) and 20.4 (SD = 3.7) for KLS patients and controls, respectively. In order to investigate the distribution of the working memory performance scores, we plotted the scores of each individual in each group (KLS and controls, respectively) around the mean value of the KLS patients, which was considered as a reference value. As shown in Figure 1A, the majority of the healthy subjects (88%) scored higher than the reference value. However, also 50% of the KLS patients scored higher than this value.
Figure 1.
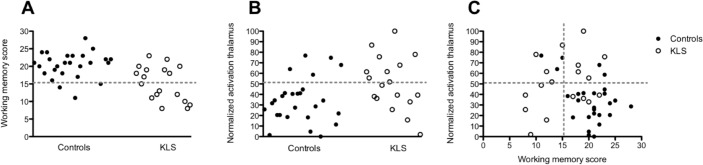
(A) The number of correct responses during the pre-fMRI working memory task. The dashed line indicates the reference value (KLS mean value) = 15.2. (B) Thalamic activation during the most difficult level of the working memory task (Level 4). The dashed line represents the reference value (KLS mean value) = 50.9. (C) Working memory scores plotted against thalamic activation.
The fMRI working memory task was designed so that all subjects should perform better than chance (> 50% correct answers) also at the most difficult level (Level 4 = word recognition after 4 presented sentences). In the present study, all subjects performed better than 70% at Level 4. There was, however, a significant difference in performance between KLS patients and controls at Level 4 of the scanner version of the working memory task (P = 0.0007). A graph showing working memory performance during fMRI is available in supplemental Figure S1. Performance on the working memory task, which was administered before fMRI, and Level 4 of the adapted working memory task, which was administered during fMRI, correlated significantly (Spearman r = 0.47, two-tailed P-value = 0.002).
Brain Imaging Results
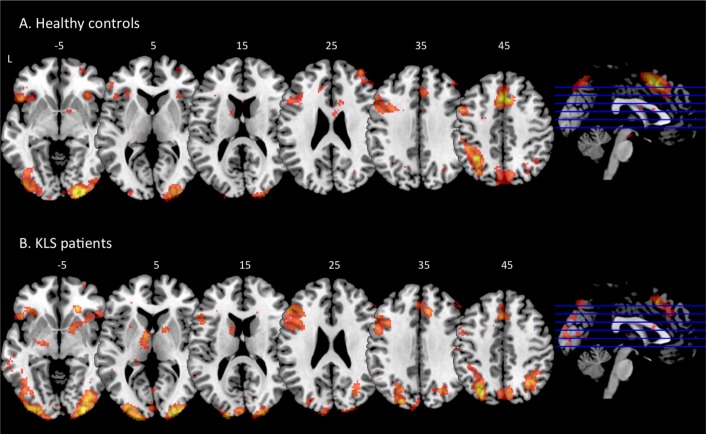
As shown in Figure 2, KLS patients had similar brain activation as healthy controls in most cortical areas. However, as reported previously, KLS patients had reduced activation in the medial frontal and anterior cingulate cortices (cluster P < 0.001) and larger activation clusters in the left dorsolateral prefrontal cortex (P = 0.005) and the left inferior frontal cortex (P = 0.017) as compared to the healthy controls. In this study the KLS patients also had increased activation in the left precuneus (P < 0.001), the left cuneus (P = 0.001), and the right superior parietal cortex (P = 0.022). The KLS patients also had significantly more activation in the right putamen (P = 0.007).
Figure 2.
Brain activation during the working memory task in healthy controls (A) and KLS patients (B). The sagittal images show the levels of the displayed axial images in inferior-superior direction. The numbers above each axial slice represents the z coordinate in Montreal Neurological Institute (MNI) space. L = left hemisphere.
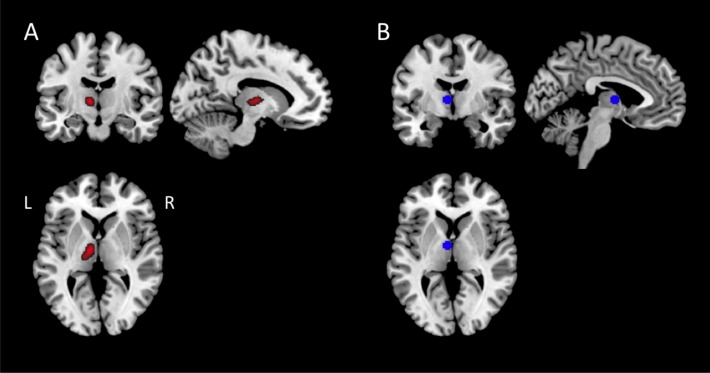
In Figure 2, it is clearly visible that the KLS patients had hyperactivation in the left thalamus, which confirms our previous results from a smaller material.5 The difference in thalamic activation between KLS and controls was highly significant: peak P = 0.008, Family Wise Error corrected for multiple comparisons at the region of interest analysis and cluster P < 0.001 at the whole-brain level of analysis. The peak activation for the KLS group was found in [-10 -4 6], which represents the ventral anterior nucleus of the thalamus. This activation peak was very close the peak observed in the previous study [-6 -6 6]. However, the center of the activated cluster was located in medial thalamus (Figure 3A), whereas the peak activation in the current and previous studies was located in the anterior thalamus (Figure 3B).
Figure 3.
(A) Brain activation in the left thalamus region of interest present in KLS patients but not in the controls. (B) The image shows the area from which the thalamic activation was calculated. The region included the peak activation of the present results.
To compare the magnitude of thalamic activation between KLS and controls during working memory performance, we calculated the mean values of the contrast estimates in a region of interest based on previous results.5 There was a statistically significant difference (P = 0.01) in the magnitude of thalamic activation between KLS (normalized mean value = 50.9) and controls (normalized mean value = 34.2). In keeping with the analysis of working memory performance scores (see Behavioral Results Section), the mean value of the KLS patients was chosen as reference value. In Figure 1B the individual measures of activation in the left thalamus are visualized for KLS and controls, respectively. It is shown that most healthy controls (81%) had lower thalamic activation than the reference value, and 5 controls (19%) had higher activation. The individual measures of thalamic activation in KLS patients were evenly distributed around the mean value.
Changes over Time
Six KLS patients performed fMRI twice. Figure 4 shows that the brain activation was more extended at the first examination. The statistical analysis showed that there were larger activation clusters in the anterior cingulate cortex (cluster P < 0.001), the left anterior insular cortex (P = 0.006), the left precuneus (P = 0.012), and the left occipital cortex (P = 0.018) during the first examination compared to the second. The region of interest analysis also showed that there was more extended activation in the left (MNI coordinates = [-14 -12 4], peak P = 0.010) and the right (MNI coordinates = [24 -30 4], peak P = 0.025) thalamus at the first examination. In Figure 4 it is seen that there was coinciding activation in the anterior thalamus as well as in the left dorsolateral prefrontal, the medial frontal, and the parietal cortices at both examinations.
Figure 4.
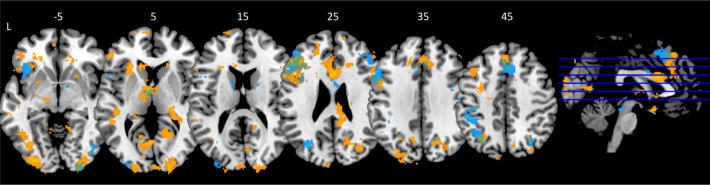
Brain activation during the working memory task in KLS patients at the first (yellow) and second (blue) examination. Coinciding activation clusters are displayed in green. The sagittal image shows the levels of the displayed axial images in inferior-superior direction. The numbers above each axial slice represents the z coordinate in Montreal Neurological Institute (MNI) space. L = left hemisphere. For visualization purpose the activation images are thresholded at P = 0.005.
Capacity-Activity Correlation
When we plotted the measures of brain activation in the left thalamus against performance scores of the working memory task administered before fMRI, we observed 2 different patterns (Figure 1C). Healthy controls who scored low on the working memory task had relatively high thalamic activation, whereas healthy controls who scored high had relatively low thalamic activation. The correlation between the contrast estimates in the thalamus and the working memory performance scores was significant in the control group (r = -0.41, P = 0.04). This result shows an inverse linear relationship between thalamic activation and working memory capacity in healthy controls. On the other hand, the relation between thalamic activity and working memory capacity was the opposite in the KLS group. High-performing KLS patients had high thalamic activity whereas low-performing patients had low activity (r = 0.35). This relation is clearly visible in Figure 1C; however, the correlation was not statistically significant (P = 0.15).
In the lower right quadrant of Figure 1C it can be seen that the majority (84%) of subjects with high working memory scores and low thalamic activation were healthy controls. Only KLS patients were found in the lower left quadrant that shows results for low working memory capacity and low thalamic activity.
Diagnostic Measures
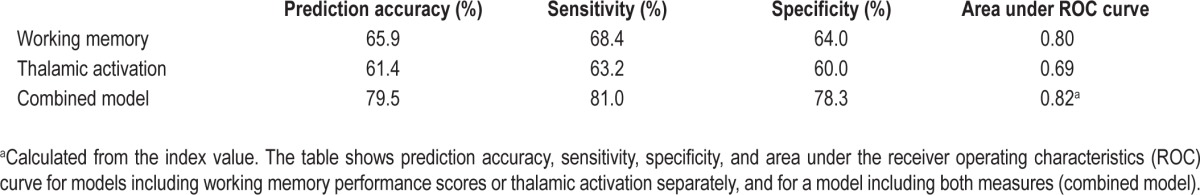
Both working memory capacity assessment and measures of thalamic activation performed better than random as diagnostic measures for KLS. The area under the ROC curve was larger for working memory capacity than for thalamic activation (Table 2, supplemental Figure S2). Combining both measures (the index value) increased the area under the ROC curve somewhat, indicating better diagnostic predictability of the combined measure. This indication was confirmed by the logistic regression analysis. The prediction accuracy for the combined model was 80% compared to 66% and 61% for working memory capacity and thalamic activation, respectively. The same trend was observed for the measures of sensitivity and specificity (Table 2).
Table 2.
Evaluation of working memory capacity and thalamic activation as diagnostic measures for KLS
DISCUSSION
This study highlights that combined measures of working memory capacity and working memory-induced activation in the thalamus should be further investigated for their ability to guide KLS diagnosis. Findings in this study indicate that the thalamic hyperactivity observed during working memory performance in KLS might be a compensatory mechanism in those subjects with high working memory capacity.
Working Memory and KLS
In this study on a larger group of KLS patients, we could reproduce the results obtained in a subset of these patients in a previous study. That is to say, we could reproduce findings of thalamic hyperactivity during working memory as well as reduced activation in medial frontal and anterior cingulate cortices and increased activation in the left inferior frontal and dorsolateral prefrontal cortices. This result is consistent with the current view where dysfunction of complex thalamo-cortical circuits is hypothesized to be involved in KLS pathophysiology. Thalamic dysfunction is probably related to hypersomnia symptoms—a conclusion supported by observed hypoperfusion of the thalamus during sleep episodes (see Arnulf et al.14 for a review). In this study, the KLS patients were examined between sleep episodes and after remission. We observed abnormal function in the thalamus and the frontal cortex, as well as impaired working memory capacity. This implies that the patients do not fully recover between periods.
A subset of 6 KLS patients was examined twice, with two to four years between the first and second examination. The fMRI results show that there was more extended activation in the anterior cingulate cortex and the left insular cortex, the precuneus, and the occipital lobe, during the first examination as compared to the second examination. In addition, the activation was spread over larger areas in the thalamus at the first examination. These differences could be caused by changes in brain activation over time due to the disease course, inter-session changes due to learning, and/or MR scanner related variability. Changes due to the disease course are plausible due to the long time interval between scanning sessions. If this is the case, reduction in thalamic activation points to brain activation normalization. On the other hand, a long interval between scanning sessions increases the risk of scanner related variability. In addition, a small learning effect cannot be neglected, since practice has been shown to reduce brain activation.15 Nevertheless, it is generally agreed that fMRI group data is highly reproducible across scanning sessions. Recent data also indicate good reliability for repeated measurements on single subjects using working memory tasks.16,17 In conclusion, since the group of KLS patients examined twice is small, it is difficult to make strong conclusions about the observed differences between the first and second fMRI session.
KLS Diagnostic Measures
Of the two factors investigated in the present study, assessment of working memory capacity seemed to have somewhat higher prediction accuracy, sensitivity, and specificity than thalamic activation (Table 2). The area under the ROC curve was also higher for working memory capacity assessment. However, as a complementary diagnostic measure, working memory assessments cannot exclusively predict KLS, since several other disorders with similar symptoms as KLS, such as schizoaffective disorder,18 bipolar disorder,19 and narcolepsy20 also manifest working memory dysfunction: that is to say, working memory dysfunction is not selective for KLS, but is a concomitant feature of many disorders of the brain. Hyperactivation of the thalamus during working memory performance has also been reported in bipolar disorder,21,22 and thalamic dysfunction of other kind has been reported in bipolar disorder and schizophrenia.23,24
Prediction accuracy, sensitivity, and specificity were substantially increased (> 10%) when combining working memory performance scores and thalamic activation. This finding indicates that these two measures provide complementary information with respect to KLS diagnostics. As seen in Figure 1C, there is no simple relationship between working memory performance scores and thalamic activation that could be applied for both KLS patients and healthy controls. In fact, the two groups showed opposite capacity-to-activation relations. The healthy subjects showed an inverse relationship between working memory capacity and activation in the thalamus. Thus, healthy subjects with low working memory capacity had comparable higher thalamic activation than healthy subjects with high working memory capacity. This finding is in concordance with other studies that have observed higher activation levels in regions involved in executive function in subjects with lower cognitive capacity.25–27 Because subjects with higher cognitive capacity in general have lower levels of brain activation in areas that are involved with executive function, this neurocognitive behavior is often referred to as neural efficiency.28 Contrary to the findings in healthy subjects, KLS patients with high working memory capacity had comparable high thalamic activation. This might indicate that increased activation in the thalamus in KLS patients is a result of a compensatory mechanism, an issue that will be further discussed below. These findings of opposite capacity-to-activation relations in healthy subjects and KLS patients explain the better diagnostic predictability of combined measures of working memory capacity and activation in the thalamus.
Neurobiological Causes of KLS
Hyperactivation of the thalamus during working memory performance in KLS patients has been the most characteristic fMRI finding since our first study on eight KLS patients,5 whose results were recently replicated in a larger patient group and in patients who returned for a second fMRI10; their results were also replicated in the present study. This finding of thalamic abnormality is supported by the observations of thalamic hypoperfusion in KLS patients during sleep episodes.7–9 These observations point to the possibility that abnormal function of the thalamus is a key to the neuropathology of KLS. In a previous study,10 we showed that the concentration of N-acetylaspartate (NAA) in the thalamus of KLS patients was inversely proportional to the thalamic activation level during working memory performance. Thus, KLS patients with high thalamic activation had low concentration of NAA, which is a biomarker of neuronal loss or neuronal malfunction. On the other hand, we did not find any significant difference in NAA concentration between the group of KLS patients and the group of healthy controls. This means that there were no obvious signs of NAA-related neuronal malfunction characteristic for all investigated KLS patients, but only in those patients with high thalamic activation during working memory performance.
Since previous studies5,10 showed that KLS patients on a group level have lower working memory capacity and higher thalamic activation between sleep episodes, our primary hypothesis was that we would observe an inverse relation between these two measures as a sign of neural inefficiency in KLS. However, this hypothesis had to be rejected since there were signs of the opposite relationship, as high-performing KLS patients also had high thalamic activation. This finding favors the alternative hypothesis that the thalamic hyperactivation in KLS is a compensatory mechanism.
The results in the present study suggest that the key neuropathological site of KLS might not be found in the thalamus itself but in other nodes of the ascending arousal system that regulates sleep and wakefulness. Nevertheless, the significantly abnormal activation pattern in the thalamus of KLS patients is still valid as a possible surrogate biomarker for diagnosis of KLS.
Limitations and Future Directions
This study has some limitations, which we plan to consider in future studies. One limitation is that we did not adjust for the time when the patients had their last episode in the data analysis. Some patients were newly diagnosed with KLS and other patients had experienced their last episode before the fMRI examination. Additionally, some patients were examined just after and some patients just before a sleep episode. These circumstances may have influenced the results of individual patients.
In this study, working memory capacity and brain activation in KLS patients were compared with healthy subjects. We could show that measures of working memory function could discriminate between KLS patients and healthy controls. Future studies will address if KLS patients also could be discriminated from patients with other hypersomnic disorders, such as narcolepsy. Results from such studies will hopefully provide a proof of concept of image-guided diagnosis of hypersomnic disorders.
CONCLUSIONS
This study shows that functional neuroimaging of the thalamus in combination with neuropsychological assessment of working memory function provide means to guide diagnosis of KLS. Results in this study also indicate that imaging of brain function and evaluation of cognitive capacity can give insights into the neurobiological mechanisms of KLS.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was financed by KLS foundation, Linköping University and Linköping University Hospital local funds. The work was performed at the Center for Medical Image Science and Visualization (CMIV), Linköping University, Linköping, Sweden. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the KLS foundation, Linköping University, and Linköping University Hospital local funds for financing this study, and Patrick Vigren for clinical assessment of the KLS patients.
SUPPLEMENTAL MATERIAL
Working memory assessment during fMRI. The graph shows the mean values (proportion of correct answers) and standard errors of mean (SEM) of the 4 different levels from the fMRI working memory task (Levels 1-4) for KLS patients and controls, respectively.
Receiver operating characteristics (ROC) curves representing pre-fMRI working memory performance scores, P = 0.0009 (A), thalamic activation during the most difficult level of the working memory task (Level 4), P = 0.03 (B), and a combined measure (index) of working memory capacity and thalamic activation, P = 0.0003 (C).
REFERENCES
- 1.Arnulf I, Zeitzer JM, File J, Farber N, Mignot E. Kleine-Levin syndrome: a systematic review of 186 cases in the literature. Brain. 2005;128:2763–76. doi: 10.1093/brain/awh620. [DOI] [PubMed] [Google Scholar]
- 2.Arnulf I, Lin L, Gadoth N, et al. Kleine-Levin syndrome: a systematic study of 108 patients. Ann Neurol. 2008;63:482–92. doi: 10.1002/ana.21333. [DOI] [PubMed] [Google Scholar]
- 3.Engström M. Neuroimaging of sleep and sleep disorders. In: Thorpy M, Nofzinger E, Maquet P, editors. Neuroimaging in Kleine-Levin Syndrome. Cambridge University Press; 2013. [Google Scholar]
- 4.Landtblom AM, Dige N, Schwerdt K, Säfström P, Granerus G. Short-term memory dysfunction in Kleine-Levin syndrome. Acta Neurol Scand. 2003;108:363–7. doi: 10.1034/j.1600-0404.2003.00171.x. [DOI] [PubMed] [Google Scholar]
- 5.Engström M, Vigren P, Karlsson T, Landtblom AM. Working memory in 8 Kleine-Levin Syndrome patients: An fMRI study. Sleep. 2009;32:681–8. doi: 10.1093/sleep/32.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landtblom AM, Dige N, Schwerdt K, Säfström P, Granerus G. A case of Kleine-Levin syndrome examined with SPECT and neuropsychological testing. Acta Neurol Scand. 2002;105:318–21. doi: 10.1034/j.1600-0404.2002.1c162.x. [DOI] [PubMed] [Google Scholar]
- 7.Huang YS, Guilleminault C, Kao PF, Liu FY. SPECT findings in Kleine-Levin syndrome. Sleep. 2005;28:955–60. doi: 10.1093/sleep/28.8.955. [DOI] [PubMed] [Google Scholar]
- 8.Hong SB, Joo EY, Tae WS, Lee J, Han SJ, Lee HW. Episodic diencephalic hypoperfusion in Kleine-Levin syndrome. Sleep. 2006;29:1091–3. doi: 10.1093/sleep/29.8.1091. [DOI] [PubMed] [Google Scholar]
- 9.Billings ME, Watson NF, Keogh BP. Dynamic fMRI changes in Kleine-Levin syndrome. Sleep Med. 2011;12:531–2. doi: 10.1016/j.sleep.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Vigren P, Tisell A, Engström M, et al. Low thalamic NAA-concentration corresponds to strong neural activation in working memory in Kleine-Levin Syndrome. PlosOne. 2013;8:e56279. doi: 10.1371/journal.pone.0056279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engström M, Landtblom AM, Karlsson T. Brain and effort: brain activation and effort-related working memory in healthy participants and patients with working memory deficits. Front Hum Neurosci. 2013;7:1–17. doi: 10.3389/fnhum.2013.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daneman M, Carpenter PA. Individual differences in working memory and reading. J Verb Learn Verb Behav. 1980;19:450–66. [Google Scholar]
- 13.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 14.Arnulf I, Rico T, Mignot E. Diagnosis, disease course, and management of patients with Kleine-Levin syndrome. Lancet Neurol. 2012;11:918–28. doi: 10.1016/S1474-4422(12)70187-4. [DOI] [PubMed] [Google Scholar]
- 15.Cheina JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Cogn Brain Res. 2005;25:607–23. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Plichta MM, Schwarz AJ, Grimm O, et al. L Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage. 2012;60:1746–58. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
- 17.Wei X, Yoo SS, Dickey CC, Zou KH, Guttmann CR, Panych LP. Functional MRI of auditory verbal working memory: long-term reproducibility analysis. NeuroImage. 2004;21:1000–8. doi: 10.1016/j.neuroimage.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 18.Gooding DC, Tallent KA. Spatial working memory performance in patients with schizoaffective psychosis versus schizophrenia: a tale of two disorders? Schiz Res. 2002;53:209–18. doi: 10.1016/s0920-9964(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 19.Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Dis. 2006;8:103–16. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- 20.Moraes M, Rossini S, Reimao R. Executive attention and working memory in narcoleptic outpatients. Arq Neuro-Psiq. 2012;70:325–40. doi: 10.1590/s0004-282x2012005000007. [DOI] [PubMed] [Google Scholar]
- 21.Cerullo MA, Adler CM, Delbello MP, Strakowski SM. The functional neuroanatomy of bipolar disorder. Int Rev Psych. 2009;21:314–22. doi: 10.1080/09540260902962107. [DOI] [PubMed] [Google Scholar]
- 22.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Dis. 2004;6:540–9. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 23.Sui J, Pearlson G, Caprihan A, et al. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA plus joint ICA model. NeuroImage. 2011;57:839–55. doi: 10.1016/j.neuroimage.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleck DE, Eliassen JC, Durling M, et al. Functional MRI of sustained attention in bipolar mania. Mol Psych. 2012;17:325–36. doi: 10.1038/mp.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haier RJ, Siegel BV, Nuechterlein KH, et al. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–217. [Google Scholar]
- 26.Gobel EW, Parrish TB, Reber PJ. Neural correlates of skill acquisition: decreased cortical activity during a serial interception sequence learning task. NeuroImage. 2011;58:1150–7. doi: 10.1016/j.neuroimage.2011.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prat CS, Just MA. Exploring the neural dynamics underpinning individual differences in sentence comprehension. Cereb Cortex. 2011;21:1747–60. doi: 10.1093/cercor/bhq241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci Biobehav Rev. 2009;33:1004–23. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Working memory assessment during fMRI. The graph shows the mean values (proportion of correct answers) and standard errors of mean (SEM) of the 4 different levels from the fMRI working memory task (Levels 1-4) for KLS patients and controls, respectively.
Receiver operating characteristics (ROC) curves representing pre-fMRI working memory performance scores, P = 0.0009 (A), thalamic activation during the most difficult level of the working memory task (Level 4), P = 0.03 (B), and a combined measure (index) of working memory capacity and thalamic activation, P = 0.0003 (C).