Abstract
Study Objectives:
Evaluate long-term effects of group interventions on sleep and pain outcomes in a primary care population of older adults with osteoarthritis pain and sleep disturbance.
Design:
Double-blind, cluster-randomized controlled trial with 18-mo follow-up.
Setting:
Group Health and University of Washington, Seattle, WA, from 2009 to 2011.
Participants:
Three hundred sixty-seven adults age 60 y and older, with osteoarthritis pain and insomnia symptoms.
Interventions:
Six weekly sessions of group cognitive behavioral therapy for insomnia and pain (CBT-PI), pain alone (CBT-P), and education-only control (EOC) delivered in patients' primary care clinics.
Measurements and Results:
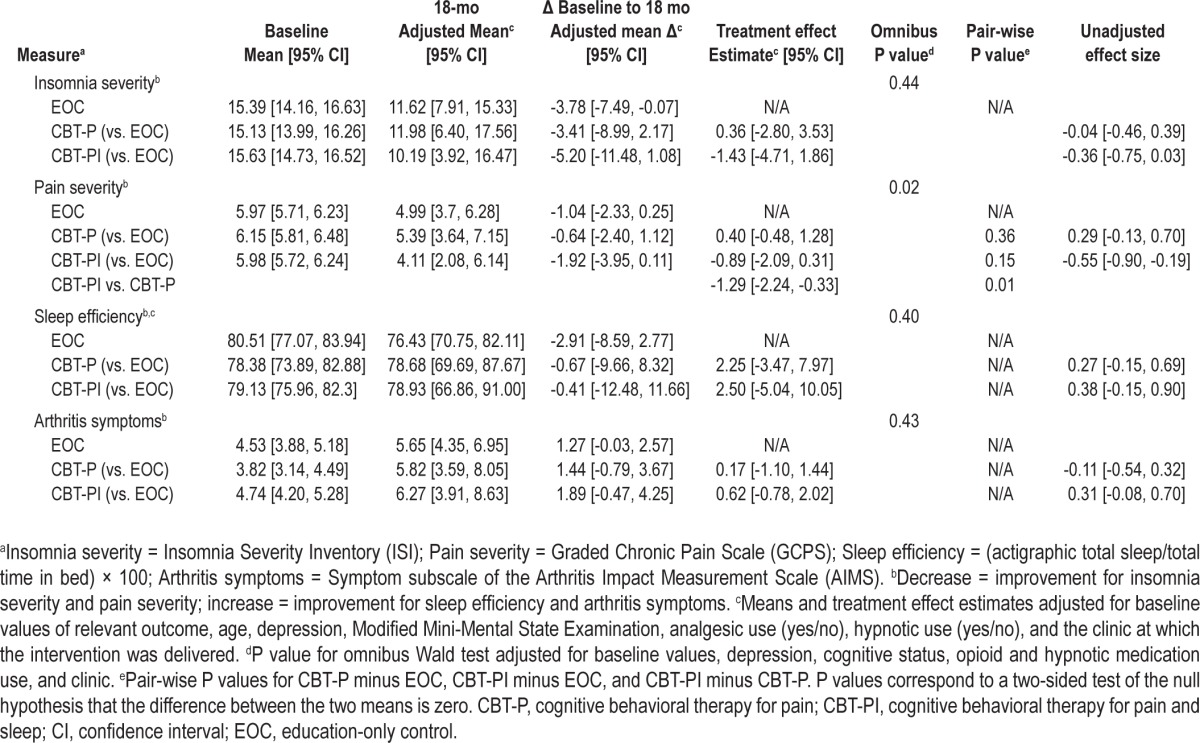
There were no significant differences between treatment groups in sleep outcomes at 18 mo. This is a change from published significant 9-mo follow-up results for insomnia severity (Insomnia Severity Index) and sleep efficiency. There were no significant treatment differences in pain at either follow-up. Post hoc analyses of participants with greater insomnia and pain severity at baseline (n = 98) showed significant (P = 0.01) 18-mo reductions in pain comparing CBT-PI versus CBT-P (adjusted mean difference [AMD] = -1.29 [95% confidence interval (CI): -2.24,-0.33]). Moderate, albeit nonsignificant, CBT-PI versus EOC treatment effects for insomnia severity (AMD = -1.43 [95% CI: -4.71, 1.86]) and sleep efficiency (AMD = 2.50 [95% CI: -5.04, 10.05]) were also observed. Possible trial design and methodological considerations that may have affected results are discussed.
Conclusions:
Results suggest patients with higher levels of comorbid pain and insomnia may be most likely to experience sustained benefit from cognitive behavioral therapy interventions over time, and inclusion of insomnia treatment may yield more clinically meaningful improvements than cognitive behavioral therapy for pain alone.
Trial Registration:
clinicaltrials.gov identifier: NCT01142349.
Citation:
McCurry SM; Shortreed SM; Von Korff M; Balderson BH; Baker LD; Rybarczyk BD; Vitiello MV. Who benefits from CBT for insomnia in primary care? Important patient selection and trial design lessons from longitudinal results of the Lifestyles trial. SLEEP 2014;37(2):299-308.
Keywords: Aging, cognitive behavioral therapy, insomnia, osteoarthritis, pain
INTRODUCTION
Prevalence studies indicate high rates of comorbid chronic pain and insomnia in the aging population that might benefit from treatment. Among older adults, osteoarthritis (OA), which affects 50% of persons age 65 y or older, is one of the most common comorbidities associated with poor sleep.1 In the United States, 60% of arthritis sufferers report pain during the night,2 and pain secondary to arthritis is one of the most common factors predicting sleep disturbance in the population at large.3,4 Both insomnia and pain adversely affect physical function, mood, and cognition, and their combined healthcare costs place a substantial economic burden on patients and society.5,6
Cognitive behavioral therapy for insomnia (CBT-I) is a well-established, evidence-based treatment.7–10 Research suggests that positive effects of CBT-I on sleep quality are robust over time, although follow-up assessment beyond 1 y is rare with one notable exception.11 CBT-I has been found to be efficacious in populations with a variety of comorbid medical conditions, including persons with chronic pain.12–16
There is a growing body of literature indicating that poor sleep is associated with increased pain thresholds and next-day pain reports,17–20 suggesting that integrating sleep/pain interventions could enhance treatment effects for both outcomes.21 Several trials evaluating CBT-I in pain populations have reported positive sleep outcomes, but they have shown mixed benefits on pain.22–25 Unfortunately, these studies have suffered from a variety of methodological concerns that make it difficult to draw conclusions from the results, including recruitment of convenience clinic samples, variable pain diagnoses, exclusion of common age-related medical morbidities, relatively small sample sizes, inadequate controls, lack of active pain treatment comparisons, and short longitudinal follow-up periods.
We recently reported the short-term (post-intervention and 9-mo) results from a large randomized controlled trial (called Lifestyles) of older adults with comorbid OA pain and insomnia.26 The Lifestyles study had notable strengths compared to earlier trials, including a large population-based sample (n = 367), broad eligibility criteria, treatment delivery within a primary care setting, very low study attrition, ongoing monitoring of treatment fidelity, and a highly credible attention control group that was well accepted by study participants. Over the 9-mo assessment period, a combination cognitive behavioral treatment for pain and insomnia was associated with more favorable outcomes for insomnia severity than CBT for pain alone or an education control.26 There were, however, no differences in pain severity across the three treatment arms. In the current paper, we report long-term sleep and pain outcomes from the Lifestyles trial at 18 mo after enrollment in this sample of older adults with comorbid insomnia and OA pain. We also present analyses for a subset of patients with more severe baseline pain and insomnia symptoms, and discuss the implications of study findings for targeting delivery of CBT interventions in population-based samples and healthcare systems.
METHODS
The Lifestyles trial was a double-blind, controlled, cluster-randomized trial of a 6-w-long cognitive behavioral pain coping skills intervention (CBT-P), cognitive behavioral therapy for pain and insomnia (CBT-PI), and an education-only control (EOC). Details describing Lifestyles enrollment and study design rationale have been published elsewhere,27,28 as have the results from short-term (posttreatment and 9-mo) follow-up.26 Here we provide a brief overview of the trial design. Participants were blinded to which study arms contained active treatments by being told that they would be assigned to one of three groups, all of which taught skills to manage pain, sleep problems, and stress but differed in emphasis and had never been compared to one another. Assessors were blind to which of the intervention arms participants were assigned. The study was approved by Group Health, an integrated practice healthcare management organization in Western Washington State, and University of Washington institutional review boards.
Participants
Members of Group Health age 60 y or older who had received health care for OA in the prior 3 y were screened for chronic pain and insomnia severity via mailed survey.27 Persons with both clinically significant pain and insomnia were eligible for enrollment. Significant arthritis pain was defined by Grade II, III, or IV pain on the Graded Chronic Pain Scale (GCPS).29 Significant insomnia was defined by self-reported sleep difficulties (trouble falling asleep, difficulty staying asleep, waking up too early, or waking up unrefreshed) 3 or more nights per week during the past month with at least one daytime sleep related problem, consistent with established research diagnostic criteria.30 Three hundred sixty-seven participants were enrolled in the trial.28
Randomization and Interventions
Eligible participants were assigned to CBT-P, CBT-PI, or EOC through a clustered randomization procedure.28 Clusters were participant groups that received one of the three interventions. Interventions were delivered in a classroom setting at the participants' Group Health primary care clinic. Each class consisted of six weekly 90-min sessions. CBT-P involved pain education, physical activation, goal setting, relaxation, activity pacing, guided imagery, and cognitive restructuring. CBT-PI included standard components of CBT for insomnia (sleep hygiene education, stimulus control, sleep restriction, and daily sleep monitoring) added to the CBT-P intervention. The EOC intervention contained educational content related to pain and sleep management, but classes were facilitated in a nondirective, self-help format that included no homework assignments, no guided practice or instruction in CBT principles, and no daily behavioral self-monitoring. Classes were co-led by a pair of female mental health professionals (Masters-level family counselor and PhD psychologist) experienced in working with older adults. Details of the Lifestyles interventions are published elsewhere.26,28
Data Collection
Baseline, 2-mo (posttreatment), 9-mo, and 18-mo follow-up assessments were each carried out at two visits to participants' homes 1 w apart. Actigraphy and sleep diary data were collected during the intervening weeks.
Measures
Primary Outcomes
Insomnia Severity: Score on the Insomnia Severity Index (ISI)31 a seven-item questionnaire assessing global insomnia severity (possible range 0-28; higher is worse).
Pain Severity: Seven GCPS29 items assessing arthritis pain intensity (average pain, worst pain, pain right now), and interference with usual, work, recreational, social, and family activities (possible range 0-10; higher is worse).
Clinically significant improvement in both primary outcomes was defined as a 30% or more reduction from baseline.32
Secondary Outcomes
Sleep Efficiency: Average time asleep as a percent of time in bed, measured using wrist actigraphy (Actiwatch-2; Respironics, Inc., Bend, OR) for 1 w at each assessment (possible range 0-100; higher is better). The night (in-bed) period was defined as “lights out” at bedtime until the final morning rising. Bed and rising times were derived from a daily sleep log kept by participants. Participants were required to have a minimum of 3 nights' actigraphy data to be included in sleep efficiency analyses (range 3-7 days, mean = 6.88 and 6.86 days, respectively, at baseline and 18-mo assessments).
Arthritis Symptoms: A three-item arthritis symptom subscale from the Arthritis Impact Measurement Scales Version 2, Short Form, Revised (AIMS)33–35 (possible range 1-10, higher is better).
Baseline Covariates
Depression: The Geriatric Depression Scale (GDS),36 a 30-item questionnaire assessing depressive symptoms in older persons.
Mental Status: The Modified Mini-Mental State Examination (3MS),37 a 100-point cognitive screen based on an expanded version of Folstein's Mini-Mental State Examination.
Analgesic or Hypnotic Use: Participant self-report of current medication use to relieve pain and/or improve sleep.
Statistical Analysis
We used analysis of variance (ANOVA) and chi-square tests to compare baseline patient characteristics across the intervention arms (Table 1). Baseline information was collected on 366 persons, and primary outcome information was collected on 320 participants (87.2%) at the 18-mo assessment. Forty-seven participants were missing insomnia severity 18-mo outcomes, 46 were missing pain severity, and 45 were missing arthritis symptom scale data. Sixty individuals were missing actigraphy information because in addition to participant dropout, some actigraphy data were lost due to recording failure; this equipment failure was completely at random.
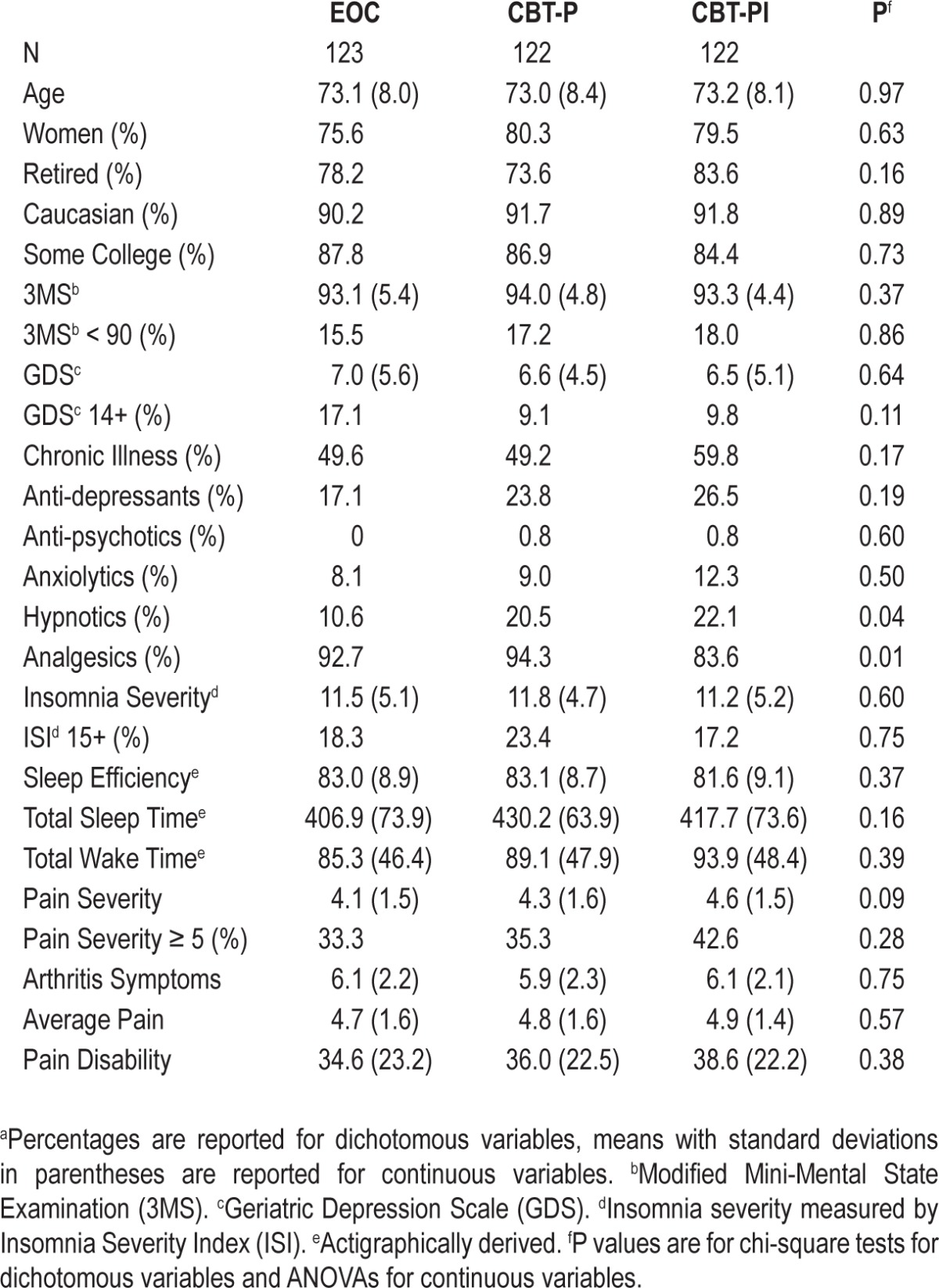
Table 1.
Baseline values of demographic, health, sleep and pain measures for the education-only control (EOC), cognitive behavioral therapy for pain (CBT-P) and cognitive behavioral therapy for pain and insomnia (CBT-PI) groups.a
Multiple imputation methods using 10 imputations were used to accommodate missing information in statistical analyses. Imputation models were estimated using demographics and observed pain, insomnia, and physical function information collected at all study visits. Missing information due to dropout, missed visits, missed items during assessment, and missing actigraphy data were imputed using Fully Conditional Specification imputation models38,39 implemented using Stata's 11.1 ICE package.40
We used a modified intention-to-treat41,42 analysis including all individuals who attended the first group session regardless of the number of sessions they completed over the 6-w intervention. For each outcome, we initially tested the null hypotheses of no difference across the three intervention arms at a significance level of 0.05. Post hoc pair-wise tests and corresponding confidence intervals (CIs) are reported only for outcomes for which this omnibus test was rejected.
Intervention effects for primary and secondary outcomes were estimated from a linear regression model using 18-mo follow-up data estimated using generalized estimating equations with an independence working correlation matrix.43 Treatment effect estimates were computed as the average treatment effect over the 10 imputations and standard errors were combined across the 10 imputations, taking into account the uncertainty in the imputation process using standard formulae.44,45 The omnibus hypothesis of no difference across the three intervention arms was tested using the modified Wald test,46,47 using the appropriate degrees of freedom and taking into account the imputation process,44 and within each imputation estimating the covariance matrix using the sandwich estimator to account for any within-group correlation. Additionally, a small sample adjustment48 was used because standard error estimates with fewer than 40 groups are biased downward.49,50
Linear regression models were adjusted for baseline values of the relevant outcome, age, depression, 3MS, analgesic use (yes/no), hypnotic use (yes/no), and the clinic at which the intervention was delivered.28 We calculated unadjusted effect sizes and appropriate 95% CIs accounting for correlation between participants in the same class.51 In addition to performing analyses in the entire sample, planned analyses were performed in a subgroup of patients with severe pain at baseline, defined as a pain severity score of 5 or greater. Ad hoc analyses on an additional subgroup of individuals with both severe pain (GCPS 5 or greater) and severe insomnia symptoms (ISI 11 or greater) were also performed. Analyses were performed using Stata© 11.1.
Detectable Effect Sizes
Power for primary and secondary outcomes analyses was calculated assuming an intraclass correlation of 0.022, estimated from prior pain severity data. Because we lacked comparable data for other outcomes, we assumed an equal intraclass correlation (0.022). Considering intraclass correlation and an 85% retention rate, the effective sample size was 87 in each treatment arm.41,52 In the conservative case that an intervention effect is observed in only one of the two arms, the Wald test is equivalent to a two-sample test comparing means. We based detectable effect sizes on a two-sample z-test comparing means with 80% power and a two-sided test with a significance level of 0.05. The estimated detectable standardized effect size for the Lifestyles' 18-mo follow-up was approximately 0.42.
RESULTS
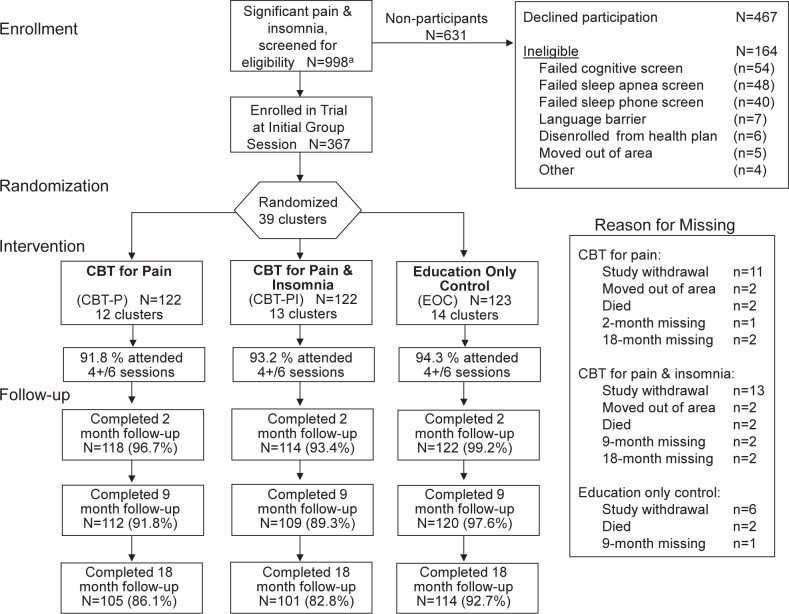
The Lifestyles trial included 367 participants assigned to three experimental arms (Figure 1). Treatment arms did not differ significantly by age, sex, ethnicity, education, or by primary or secondary outcome measures at baseline (Table 1). Differences were observed in the proportion of participants using analgesics and hypnotics; for this reason we adjusted for baseline use of these medications in all regression models. The observed 18-mo retention rates were 83%, 86%, and 93% for CBT-PI, CPT-P, and EOC, respectively. The unadjusted intra-class correlations of primary and secondary outcomes were substantially larger than projected based on preliminary data: 0.14 for insomnia severity, 0.11 for pain severity, 0.11 for sleep efficiency, and 0.15 for arthritis symptoms.
Figure 1.
Consort flow diagram for enrollment of potentially eligible participants. Imputed 18-mo analyses for all three treatment arms based on n = 367. CBT, cognitive behavioral therapy.
Unadjusted baseline, adjusted 18-mo means, adjusted mean 18-mo change from baseline, and adjusted mean differences between treatment groups are presented in Table 2 along with 95% CIs. The modified Wald test showed no significant differences in insomnia severity, pain severity, sleep efficiency, or arthritis symptoms across the three intervention arms. A planned subgroup analysis was performed on 136 participants with baseline pain severity scores of at least 5.0. Similar statistically non-significant adjusted mean differences between treatment groups were observed in this analysis (Table 2).
Table 2.
Modified intent-to-treat analysis for primary and secondary sleep and pain outcomes for the entire sample and for the planned subgroup analysis of participants with severe pain at baseline. Multiple imputation was used (10 imputations) to accommodate missing data at baseline and 18 month follow-up
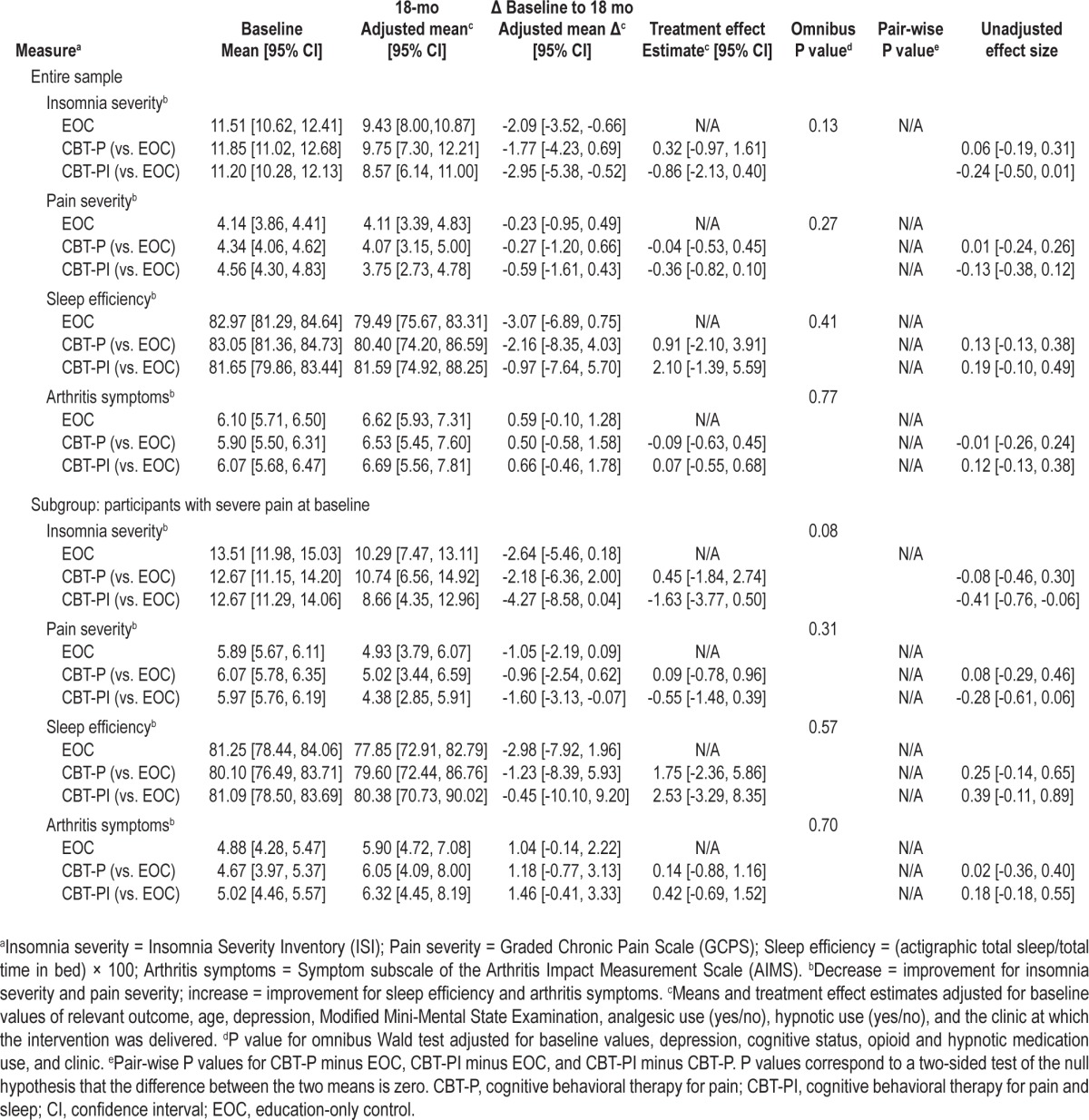
Although benefits for insomnia observed over 9 mo26 were reduced at 18 mo and did not achieve statistical significance for any group comparison, results indicated that both insomnia and pain severity estimates of adjusted mean differences between CBT-PI participants and EOC participants were greater in the planned subgroup analysis of persons with severe baseline pain than in the overall sample comparisons (see Table 2). For example, at 18 mo the adjusted mean difference (comparing CBT-PI and EOC) for insomnia severity was -0.86 (95% CI -2.13, 0.40) in the full group, versus an adjusted mean difference of -1.63 (-3.77, 0.50) in the subgroup of participants with high pain severity scores at baseline.
The results of an ad hoc subgroup analysis of participants who had baseline elevated levels of both insomnia and pain severity (ISI 11+, GCPS 5+; n = 98) are reported in Table 3. Although the Wald test results were consistent with the overall sample for insomnia severity, sleep efficiency, and arthritis symptoms, the Wald test for pain severity rejected the null hypothesis of no difference between the adjusted mean differences in the three treatment arms (P = 0.02). This result was driven by the adjusted mean difference between the CBT-PI and CBT-P groups, which was estimated to be -1.29 (-2.24, -0.33; P = 0.01). The adjusted mean difference comparing the CBT-PI and EOC group was -0.89 (-2.09, 0.31; P = 0.15) and comparing the CBT-P and EOC group it was 0.40 (-0.48, 1.28; P = 0.36).
Table 3.
Modified intent-to-treat analysis for the ad hoc subgroup analysis of participants with severe pain and insomnia symptoms at baseline. Multiple imputation was used (10 imputations) to accommodate missing data at baseline and 18-mo follow-up
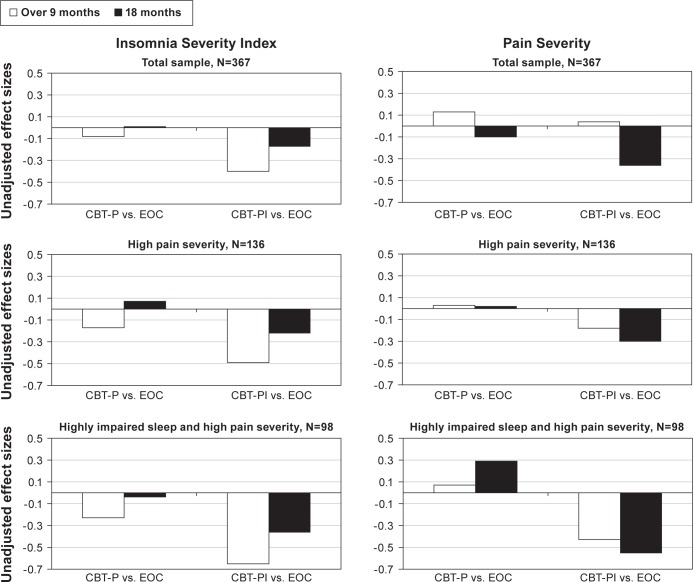
Unadjusted effect sizes for the two primary outcomes are presented in Figure 2 for the total Lifestyles sample (n = 367), the planned elevated pain severity sample (n = 136), and the post hoc elevated insomnia and pain severity sample (n = 98). The figure shows results over 9 mo (including both 2- and 9-mo assessments; previously reported by Vitiello et al.26) and at 18 mo for the two active intervention arms compared to EOC. Although insomnia treatment effects were attenuated at 18 mo compared to the data over 9 mo, they were larger for the CBT-PI versus EOC comparison than for CBT-P versus EOC, and greatest for the subset of participants with both high levels of baseline insomnia and pain severity. Pain severity also showed moderate effect size benefits for pain outcomes over 9 mo and at 18 mo for CBT-PI versus EOC in the high insomnia and pain severity subgroup. The CBT-P versus EOC group comparisons showed little signs of improvement for either time or subgroup analysis, and in fact indicated a worsening in pain severity scores over time in the high insomnia and pain participants.
Figure 2.
Comparison of unadjusted effect sizes over 9 mo (including both 2- and 9-mo assessments)26 and at 18 mo for primary outcome measures in the total sample (n = 367), for participants with high levels of baseline pain severity (Graded Chronic Pain Score 5+; n = 136), and participants with high levels of baseline pain and insomnia severity (Insomnia Severity Index 15+; n = 98). Decreasing severity scores = improvement. CBT, cognitive behavioral therapy; EOC, education-only control; P, pain alone; PI, insomnia and pain.
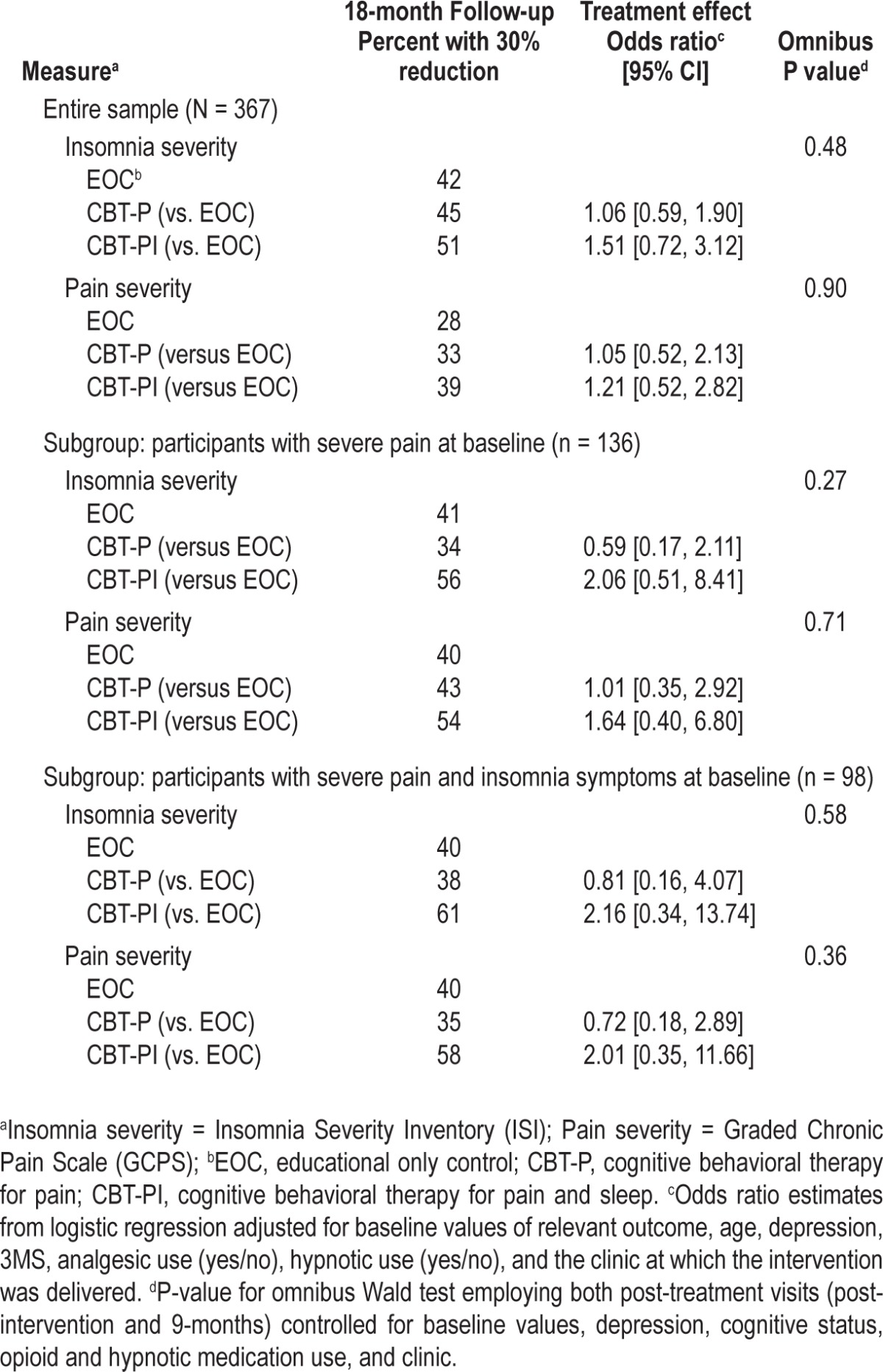
Table 4 summarizes the analysis results of clinically significant (30%) change from baseline for the primary outcomes, insomnia severity, and pain severity.32 In the overall sample 42%, 45%, and 51% of participants, for EOC, CBT-P, and CBT-PI, respectively, had a clinically significant reduction in insomnia severity symptoms from baseline to 18 mo, whereas 28%, 33%, and 39%, respectively, had a clinically significant reduction in pain severity ratings. Odds ratio and Wald test results were consistent with the primary analysis of the continuous outcomes; i.e., there were no statistically significant differences across the three treatment arms in the number of individuals who experienced a clinically significant reduction in insomnia severity or pain severity.
Table 4.
Modified intent-to-treat analysis for clinical significance of primary outcomes for the entire sample, the planned subgroup analysis of participants with severe pain at baseline, and the ad hoc subgroup analysis of participants with severe pain and insomnia symptoms at baseline.
DISCUSSION
The Lifestyles trial was conducted to evaluate the hypothesis that an integrated cognitive behavioral intervention combining treatment for both insomnia and pain would enhance treatment effects for both insomnia and pain outcomes relative to CBT for pain alone or an EOC. Study results indicate that in this large population-based sample of older adults with insomnia symptoms and chronic OA pain, significant improvements in insomnia severity and sleep efficiency observed over the 9 mo following treatment with a combination CBT intervention for pain and sleep were not sustained at 18 mo, nor were there any significant differences between treatment groups for measures of pain severity or arthritis symptoms at either time period. Examination of unadjusted effect size data, however, showed that although insomnia treatment effects were attenuated over time, they were greater for both sleep and pain outcomes in persons receiving the combination CBT-PI intervention. In the subgroup of participants who had a combination of elevated pain plus elevated insomnia symptoms at baseline, unadjusted effect sizes were larger on both outcomes than in the overall sample.
An unexpected finding of the Lifestyles trial was that CBT for pain alone did not show any treatment effects on pain severity compared to EOC. The hypothesized active ingredients included in the CBT-P intervention (e.g., relaxation training and practice, guided imagery, activity pacing)53 were carefully excluded from the EOC protocol, and ongoing treatment fidelity monitoring of all groups ensured that there was no spillover contaminating the EOC condition. It may be that the CBT-P intervention was briefer than other CBT treatments that have shown positive effects on pain.54,55 However, in primary care settings under the current healthcare funding system, it is unlikely that interventions lasting longer than 6 weeks could have widespread adoption. It may also be that CBT-I offers a stronger test against a credible education-based attention control because it contains treatment components (e.g., sleep restriction or stimulus control instructions)56 that are based on known physiological mechanisms underlying sleep, whereas such mechanisms are less well understood for chronic pain. Because in this study we were unable to separately test the treatment effects of CBT-I alone, future studies are needed to determine the interaction between CBT for pain and CBT for insomnia delivered separately and in combination. The lack of compelling CBT-P treatment effects, however, suggests that an argument could be made for future research focused solely on the effect on pain of insomnia-focused treatment alone, without the additional time, expense, and patient burden of combining CBT-I with behavioral strategies previously developed for treating chronic pain.
It is well established that CBT interventions for sleep are effective for the treatment of insomnia and their widespread use has been recommended in the general population where insomnia symptoms are prevalent.7 The current study suggests that the need for widespread treatment may be overstated. Lifestyles was designed as a population-based trial with broad entry criteria to treat OA-related insomnia and pain complaints in a primary care population and was designed to examine both efficacy and effectiveness, rather than recruiting only the more severe insomnia cases typically treated in smaller efficacy trials. Our data suggest that for a significant number of these individuals, their sleep and pain complaints may well be transient and consequently, response to CBT interventions might be expected to be modest. We observed meaningful regression to the mean between screening and baseline (median = 65.8 days) for both pain and insomnia severity scores.28 These pretreatment decreases led many participants who had been identified by population-based screening to enter treatment with subclinical levels of insomnia and pain, reducing potential for detecting improvement in these outcomes. Prevalence estimates based on screening at a single point in time may thus overestimate the need for population-based treatment because a large segment of prevalent cases may improve to subclinical levels of insomnia and pain severity without any intervention.
The presence of moderate to large effect sizes in both insomnia and pain at 18 mo among that subset of individuals with elevated baseline pain and insomnia symptoms further suggests that baseline severity may need to be above some minimal threshold for reciprocal and durable effects of treating sleep and pain to be observed. These observations have potential implications for targeting interventions in primary care settings. Specialty studies have shown analgesic effects from CBT interventions for pain, and it has been suggested that insomnia treatment may enhance these treatment effects. The current study illustrates that it is less clear how such interventions should be translated into clinical practice and what associated long-term benefits may be expected from such treatment for conditions comorbid with insomnia such as chronic pain. Although further research is needed, the fluctuating nature of both OA pain and insomnia symptoms may explain why benefits for pain have been reported in previous smaller clinical trials that enrolled more severely impaired patients based on physician referrals or from specialty sleep clinics, but not in a general population-based trial such as ours with broad eligibility criteria.
Previous studies have emphasized the durability of treatment effects from CBT-I, whereas we observed some decline in insomnia treatment effect at long-term follow-up. This may be in part because our follow-up period was longer than has been previously reported, with the exception of a single trial with individuals with clinically diagnosed insomnia.11 Generalizability of results from that trial to a broader range of persons with comorbid OA pain and insomnia may be limited. It is also conceivable that dividing focus between insomnia treatment and pain treatment in the CBT-PI intervention may have diluted the insomnia treatment efficacy of that integrated intervention arm. However, decline in sleep outcomes over time may not be surprising given that OA is a degenerative condition, and a behavioral sleep protocol places many lifestyle demands (e.g., sleep restriction, consistent sleep scheduling) on people that may be difficult to sustain for a long period of time in the face of advancing age. Perhaps more surprising is the fact that treatment effect sizes for the CBT-PI intervention compared to education only and CBT for pain alone remained as large as they did over time in this aging population of individuals with progressive joint disease.
It is important to note some study limitations. Although subjects were initially identified for recruitment using an ICD-9 OA code in the Group Health electronic records, we could not separate individuals who had received that code during a clinic visit as a rule-out diagnosis from those with definitive clinical symptoms of OA. For this reason, inclusion in the trial required that participating individuals know that they had OA and were reporting a certain severity level of pain, which may reduce generalizability of the sample. Exclusionary screening was done primarily through patient records and not by clinical interview, so it is also possible that additional comorbidities above those noted in Table 1 may have been present in the study sample, and composition of the study sample could well have diluted treatment efficacy. A much higher than projected intraclass correlation (correlations between individuals in the same intervention group) created by our cluster randomized design may have reduced our ability to detect significant treatment-related changes in pain and insomnia. Future population-based trials might consider the implications of potentially high intra-class correlations from group interventions on the precision of intervention effect estimates. Long-term data on treatment adherence was not collected, so it is unknown to what extent continued improvement (or nonimprovement) in sleep and pain was associated with CBT treatment recommendations. This information is not widely available in the insomnia treatment literature, and its addition to future studies would enhance interpretation of study outcomes. Although we controlled for baseline use of analgesics and hypnotics in analyses of long-term outcomes, changes in participants' medication scheduling or dosing over time were not analyzed, so it is unknown whether or how such changes in medications may have affected longitudinal study results. Finally, because we did not adjust P values for multiple comparisons, the results of secondary outcomes and the subgroup analysis should be interpreted cautiously due to the potential for increased type-1 error.
In conclusion, our results point to the need to target individuals with more severe and persistent insomnia and pain symptoms in future research, and ultimately in clinical practice if insomnia interventions are to be delivered on a large scale and expected to produce enduring effects. This observation may be less important for randomized trials conducted in specialty settings that treat more severe and chronic cases, but is highly relevant to targeting services for sleep disorders in future community-based trials and ultimately to widespread deployment of CBT-I based treatments in healthcare systems.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was supported by NIH grant R01-AG031126. Dr. Von Korff has received funding from research grants awarded to Group Health Research Institute by Johnson and Johnson and by Pfizer. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Martha Cagley, Fredda Jaffe, Shirley Meyer, Amy Cunningham, Katie Saunders, Janyce Vick, Kendra Wight, and Patty Yarbro for their invaluable assistance in conducting this study.
REFERENCES
- 1.Sarzi-Puttini P, Cimmino MA, Scarpa R, et al. Osteoarthritis: An overview of the disease and its treatment strategies. Semin Arthritis Rheum. 2005;35(Suppl 1):1–10. doi: 10.1016/j.semarthrit.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: Results of the 2003 National Sleep Foundation Sleep in America survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Blay SL, Andreoli SB, Gastal FL. Chronic painful physical conditions, disturbed sleep and psychiatric morbidity: Results from an elderly survey. Ann Clin Psychiatry. 2007;19:169–74. doi: 10.1080/10401230701468099. [DOI] [PubMed] [Google Scholar]
- 4.Moffitt PF, Kalucy EC, Kalucy RS, Baum FE, Cooke RD. Sleep difficulties, pain and other correlates. J Intern Med. 1991;230:245–9. doi: 10.1111/j.1365-2796.1991.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 5.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: Evidence from national survey data. Arthritis Rheum. 2009;60:3546–53. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- 6.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30:263–73. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 7.Espie CA. “Stepped care”: A health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep. 2009;32:1549–58. doi: 10.1093/sleep/32.12.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Evidence-based psychological treatments for insomnia in older adults. Psychol Aging. 2007;22:18–27. doi: 10.1037/0882-7974.22.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein K. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998-2004) Sleep. 2006;29:1398–414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 10.Siebern AT, Manber R. New developments in cognitive behavioral therapy as the first-line treatment of insomnia. Psychol Res Behav Manag. 2011;4:21–8. doi: 10.2147/PRBM.S10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 12.Belleville G, Cousineau H, Levrier K, St-Pierre-Delorme M-E. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev. 2011;31:638–52. doi: 10.1016/j.cpr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Edinger JD, Olsen MK, Stechuchak KM, et al. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: A randomized clinical trial. Sleep. 2009;32:499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan K, Gregory P, Tomeny M, David B, Gascoigne C. Self-help treatment for insomnia symptoms associated with chronic conditions in older adults: A randomized controlled trial. J Am Geriatr Soc. 2012;60:1803–10. doi: 10.1111/j.1532-5415.2012.04175.x. [DOI] [PubMed] [Google Scholar]
- 15.Rybarczyk B, Mack L, Harris JH, Stepanski E. Testing two types of self-help CBT-I for insomnia in older adults with arthritis or coronary artery disease. Rehabil Psychol. 2011;56:257–66. doi: 10.1037/a0025577. [DOI] [PubMed] [Google Scholar]
- 16.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–92. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Haack M, Lee E, Cohen DA, Mullington JM. Activation of the prostaglandin system in response to sleep loss in healthy humans: potential mediator of increased spontaneous pain. Pain. 2009;145:136–41. doi: 10.1016/j.pain.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Tiede W, Magerl W, Baumgärtner U, Durrer B, Ehlert U, Treede RD. Sleep restriction attenuates amplitudes and attentional modulation of pain-related evoked potentials, but augments pain ratings in healthy volunteers. Pain. 2010;148:36–42. doi: 10.1016/j.pain.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 20.Tang NK, Goodchild CE, Sanborn AN, Howard J, Salkovskis PM. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: A multilevel daily process study. Sleep. 2012;35:675–87A. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 22.Currie SR, Wilson KG, Pontefract AJ, deLaplante L. Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol. 2000;68:407–16. doi: 10.1037//0022-006x.68.3.407. [DOI] [PubMed] [Google Scholar]
- 23.Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: A randomized clinical trial. Arch Intern Med. 2005;165:2527–35. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 24.Jungquist CR, O'Brien C, Matteson-Rusby S, et al. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010;11:302–9. doi: 10.1016/j.sleep.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]
- 26.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for co-morbid insomnia and osteoarthritis pain in primary care: The Lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61:947–56. doi: 10.1111/jgs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCurry SM, Von Korff M, Vitiello MV, et al. Frequency of comorbid insomnia, pain, and depression in older adults with osteoarthritis: Predictors of enrollment in a randomized treatment trial. J Psychosom Res. 2011;71:296–9. doi: 10.1016/j.jpsychores.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Von Korff M, Vitiello MV, McCurry SM, et al. Group interventions for co-morbid insomnia and osteoarthritis pain in primary care: The Lifestyles cluster randomized trial design. Contemp Clin Trials. 2012;33:759–68. doi: 10.1016/j.cct.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Von Korff M, Ormel J, Keefe F, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50:133–49. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 30.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of Research Diagnostic Criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM. Insomnia: Psychological assessment and management. New York, NY: Guilford Press; 1993. [Google Scholar]
- 32.Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146:238–44. doi: 10.1016/j.pain.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Guillemin F, Coste J, Pouchot J, Ghézail M, Bregeon C, Sany J. The AIMS2-SF: A short form of the Arthritis Impact Measurement Scales 2. French Quality of Life in Rheumatology Group. Arthritis Rheum. 1997;40:1267–74. doi: 10.1002/1529-0131(199707)40:7<1267::AID-ART11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 34.Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 35.Ren XS, Kazis L, Meenan RF. Short-form Arthritis Impact Measurement Scales 2: Tests of reliability and validity among patients with osteoarthritis. Arthritis Care Res. 1999;12:163–71. doi: 10.1002/1529-0131(199906)12:3<163::aid-art3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a Geriatric Depression Screening Scale: A preliminary report. J Psych Res. 1982-83;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 37.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 38.Van Buuren S, Brand JPL, Groothuis-Oudshoorn CGM, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76:1049–64. [Google Scholar]
- 39.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 40.Royston P. Multiple imputation of missing values: Update of ice. Stata J. 2005;5:188–201. [Google Scholar]
- 41.Friedman LM, Furberg CD, Demets DL. Fundamentals of clinical trials. 4th ed. New York: Springer; 2010. [Google Scholar]
- 42.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 44.Li KH, Raghunathan TE, Rubin DB. Large-sample significance levels from multiply imputed data using moment-based statistics and an F reference distribution. J Am Statl Assoc. 1991;84:1065–73. [Google Scholar]
- 45.Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. New York: John Wiley & Sons; 2002. [Google Scholar]
- 46.Rotnitzky A, Jewell NP. Hypothesis testing of regression parameters in semiparametric generalized linear models for cluster correlated data. Biometrika. 1990;77:485–97. [Google Scholar]
- 47.Wald A. Tests of statistical hypotheses concerning several parameters when the number of observations is large. Trans Am Math Soc. 1943;43:426–82. [Google Scholar]
- 48.Mancl LA, DeRouen TA. A covariance estimator for GEE with improved small-sample properties. Biometrics. 2001;57:126–34. doi: 10.1111/j.0006-341x.2001.00126.x. [DOI] [PubMed] [Google Scholar]
- 49.Feng Z, McLerran D, Grizzle J. A comparison of statistical methods for clustered data analysis with Gaussian error. Stat Med. 1996;15:1793–806. doi: 10.1002/(SICI)1097-0258(19960830)15:16<1793::AID-SIM332>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 50.Murray DM, Varnell SP, Blitstein JL. Design and analysis of group-randomized trials: A review of recent methodological developments. Am J Public Health. 2004;94:423–32. doi: 10.2105/ajph.94.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hedges LV. Effect sizes in cluster-randomized designs. J Educ Behav Stat. 2007;32:341–70. [Google Scholar]
- 52.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: An orientation. Am J Epidemiol. 2003;157:364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 53.Keefe FJ, Abernethy AP, Campbell LC. Psychological approaches to understanding and treating disease-related pain. Ann Rev Psychol. 2005;56:601–30. doi: 10.1146/annurev.psych.56.091103.070302. [DOI] [PubMed] [Google Scholar]
- 54.Warsi A, LaValley MP, Wang PS, Avorn J, Solomon DH. Arthritis self-management education programs: A meta-analysis of the effect on pain and disability. Arthritis Rheum. 2003;48:2207–13. doi: 10.1002/art.11210. [DOI] [PubMed] [Google Scholar]
- 55.Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: A systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641–9. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- 56.Schwartz DR, Carney CE. Mediators of cognitive-behavioral therapy for insomnia: A review of randomized controlled trials and secondary analysis studies. Clin Psychol Rev. 2012;32:664–75. doi: 10.1016/j.cpr.2012.06.006. [DOI] [PubMed] [Google Scholar]