Abstract
Background:
Substantial discrepancies exist in the type of sleep studies performed to diagnose pediatric obstructive sleep apnea (OSA) in different countries. Respiratory polygraphic (RP) recordings are primarily performed in sleep laboratories in Europe, whereas polysomnography (PSG) constitutes the majority in the US and Australia. Home RP show consistent apnea-hypopnea index (AHI) underscoring, primarily because the total recording time is used as the denominator when calculating the AHI compared to total sleep time (TST). However, laboratory-based RP are less likely affected, since the presence of sleep technicians and video monitoring may enable more accurate TST estimates. We therefore examined differences in AHI in PSG and in-lab RP, and whether RP-based AHI may impact clinical decision making.
Methods:
Of all the children assessed for possible OSA who underwent PSG evaluation, 100 were identified and divided into 4 groups: (A) those with AHI < 1/h TST (n = 20), (B) 1 ≤ AHI < 5/h TST (n = 40), (C) 5 ≤ AHI < 10/h TST (n = 20), and (D) AHI ≥ 10/h TST (n = 20). Electroencephalography, electrooculography, and electromyography channels were deleted from the original unscored recordings to transform them into RP, and then rescored in random sequence. AHI-RP were compared to AHI-PSG, and therapeutic decisions based on AHI-RP and AHI-PSG were formulated and analyzed using clinical details derived from the patient's clinic letter.
Results:
Bland Altman analysis showed that in lab RP underestimated the AHI despite more accurate estimates of TST. This underestimation was due to missed hypopneas causing arousals without desaturation. Basing the therapeutic management decision on RP instead of PSG results changed the clinical management in 23% of all patients. The clinical management for patients in groups A and D was unaffected. However, 27.5% of patients in group B would have been given no treatment, as they would be diagnosed as having no OSA (AHI < 1/h TST) when they should have received a trial of anti-inflammatory therapy or been referred for ear, nose, and throat (ENT) review. Sixty percent of patients in group C would have received either a trial of medical treatment to treat mild OSA or no treatment, instead of referral to ENT services or commencement of continuous positive airway pressure.
Conclusion:
Apnea-hypopnea index (AHI) is underestimated in respiratory polygraphy (RP), and the disparity in AHI-RP and AHI-polysomnography can significantly affect clinical management decisions, particularly in children with mild and moderate obstructive sleep apnea (1 < AHI < 10/h total sleep time).
Citation:
Tan HL; Gozal D; Ramirez HM; Bandla HPR; Kheirandish-Gozal L. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. SLEEP 2014;37(2):255-260.
Keywords: Pediatric obstructive sleep apnea, respiratory polygraphy, polysomnography
INTRODUCTION
There is a significant discrepancy in the type of sleep studies performed in the context of diagnosing pediatric obstructive sleep apnea (OSA) in different countries. In the US and Australia, polysomnography (PSG) is the accepted gold standard, as recommended by the American Academy of Pediatrics.1–3 In Europe, the vast majority of laboratories perform respiratory polygraphy (RP).4–6 A substantial number of physicians believe that RP is sufficient to diagnose OSA, and the Royal College of Paediatrics and Child Health Working party on Sleep Physiology and Respiratory Control Disorders in Childhood has recommended that RP provides a satisfactory approach to diagnose OSA in uncomplicated children over the age of 2 years.7 However, if the 2 types of diagnostic studies, i.e., RP and PSG, yield different results, then centers that currently perform RP may need to change and adopt PSG as the only valid gold-standard approach. Conversely, if the results derived from RP and PSG are comparable, then performing RP would be both simpler and more cost-effective—a serious consideration in these current economically constrained times.
Considering the potentially significant ramifications of such dilemma, there have been surprisingly few pediatric studies directly comparing RP and PSG,8–10 and none that have exam ined the clinical management implications. Studies comparing home RP with in-lab PSGs have shown that home RPs underscore the apnea-hypopnea index (AHI), primarily because the total recording time (TRT) is used as the denominator when calculating the AHI, as compared to the use of the total sleep time (TST) that can be derived from the PSG.11 However, sleep and wakefulness can be quite accurately determined in children using cardiorespiratory and videotape recordings,12 such that laboratory-based RP should be less affected by this problem, since the presence of a sleep technician and concurrent video monitoring should enable more accurate estimates of TST than those obtained during home-based RPs.
We therefore aimed to determine whether there were significant differences in the AHI obtained from inpatient PSG and RP, and more importantly, whether any existing differences may impact on clinical decision making.
MATERIALS AND METHODS
Inclusion criteria for the present study were: uncomplicated children aged between 2-16 years who were assessed for possible OSA and underwent PSG evaluation at the University of Chicago in 2011 and 2012. Exclusion criteria included patients who had genetic or craniofacial syndromes and any chronic diseases such as cardiac disease, diabetes, cerebral palsy, neuromuscular disease, and chronic lung disease of prematurity. One hundred studies were identified and divided into 4 groups: those with AHI < 1/h TST (n = 20), 1 ≤ AHI < 5/h TST (n = 40), 5 ≤ AHI < 10/h TST (n = 20), and AHI ≥ 10/h TST (n = 20).
The PSGs were scored as per the 2007 American Academy of Sleep Medicine (AASM) guidelines for the scoring of sleep and associated events.13 The standard PSG recordings consisted of 8 standard electroencephalography (EEG) channels, bilateral electrooculography (EOG); electromyography (EMG); 2-lead electrocardiography (ECG); oronasal airflow measurement using thermistor, nasal pressure transducer, and end-tidal CO2; chest and abdominal movement by respiratory inductance plethysmography; and pulse oximetry including pulse waveform. The digital polysomnography equipment used was Poly-smith (Nihon Kohden America Inc, CA, USA).
A respiratory event was scored as an obstructive apnea if it was associated with > 90% fall in signal amplitude for > 90% of the entire event compared to the baseline amplitude, the event lasted ≥ 2 breaths, and there was continued or increased respiratory effort throughout the period of the event. A mixed apnea was scored if there was absent inspiratory effort in the initial part of the event, followed by resumption of inspiratory effort before the end of the event. A central apnea was scored if there was absent respiratory effort throughout the duration of the event, the event lasted ≥ 2 missed breaths, and was associated with an arousal/awakening or ≥ 3% desaturation. A hypopnea was scored if the event was associated with ≥ 50% fall in amplitude of the nasal pressure transducer, lasted ≥ 2 breaths, and was associated with an arousal/awakening or ≥ 3% desaturation. The apnea-hypopnea index (AHI) was calculated as the number of apneas and hypopneas per hour of TST.
The original unscored recording was then accessed, and the EEG, EOG, and EMG channels were deleted such as to transform them into RP. Studies were ordered in random sequence and rescored in a completely blinded fashion.
Power calculations were performed to detect an AHI difference of 1/h TST between the 2 tests; this would require a sample size of 12, such that all groups were in excess of the minimal sample size. The decision was made to include a larger number of subjects in the mild group, as we wanted to ensure that any potential differences in clinical decision processes would be detected with confidence. The AHI obtained when RP scoring was performed were compared to those obtained from the PSG. Statistical analysis was performed using Graphpad Prism version 5.0 (Graphpad software Inc, CA, USA). When data were normally distributed, the paired t-test or 1-way ANOVA was used to compare the groups. When data were not normally distributed, the Mann-Whitney test was used. Spearman correlation was used for the calculation of r and P values. Bland Altman analysis was used to compare the results obtained from the 2 modalities. Stata version 12 (Statacorp, Texas, USA) was used to calculate the sensitivity, specificity, positive and negative predictive values, and receiver operating characteristic curves of RP, using PSG as the gold standard. These were calculated for 3 AHI cutoffs: AHI ≥ 1, AHI ≥ 3, and AHI ≥ 5/h TST.
To further evaluate whether the AHI differences in RP and PSG would affect clinical decision-making, therapeutic decisions were made by a single blinded investigator using clinical details derived from the patient's clinic letter, combined with the results from either the full PSG or RP. The scoring modality was randomly assigned, with half the patients initially being assessed using clinical details and PSG results, while half were assessed with clinical details and the RP results. Three months later, the same therapeutic decision making procedure was repeated for the patients using the same clinical details, but this time using the reciprocal diagnostic modality.
There is no international consensus on the management of children with OSA with regards to AHI cutoff values. For the purposes of this study, we based the therapeutic decision making process on our current clinical practice.14 Children with AHI < 1/h TST were diagnosed as primary snorers. Those children with 1 ≤ AHI < 5/h TST were classified as having mild OSA. If they were symptomatic or exhibited any evidence of OSA morbidity—e.g., systemic or pulmonary hypertension, excessive daytime sleepiness, hyperactivity with or without inattention, academic difficulties, inadequate somatic growth, or enuresis—therapy was initiated with a trial of anti-inflammatory medications, including montelukast, intranasal steroids, or both, with children being then followed-up in clinic. If anti-inflammatory therapy was unsuccessful with no improvement in symptoms or PSG findings and if significant adenotonsillar hypertrophy was present after the trial, referral to the ear, nose and throat (ENT) surgeon would follow. Children with 5 ≤ AHI < 10/h TST and AHI ≥ 10/h TST were classified as having moderate and severe OSA respectively, based on their sleep study; adenotonsillectomy would normally constitute first-line treatment. Continuous positive airway pressure was recommended only if adenotonsillectomy was not performed or there was residual OSA post-adenotonsillectomy. Weight loss was recommended for all overweight children who had a body mass index > 95th centile based on Centers for Disease Control and Prevention growth charts.
RESULTS
Table 1 summarizes the clinical characteristics of the patients studied. Based on the a priori strategy for the present study, the selected subjects were distributed into the 4 severity groups as follows: those with (A) no OSA, AHI < 1/h TST (n = 20), (B) mild OSA, 1 ≤ AHI < 5/h TST (n = 40), (C) moderate OSA, 5 ≤ AHI < 10/h TST (n = 20), and (D) severe OSA, AHI ≥ 10/h TST (n = 20).
Table 1.
Clinical characteristics of the patients
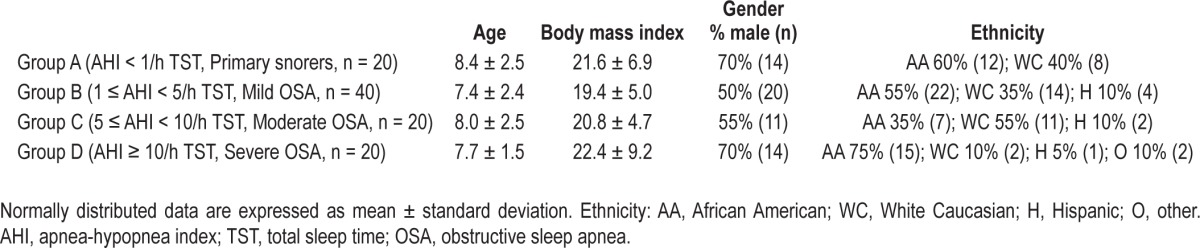
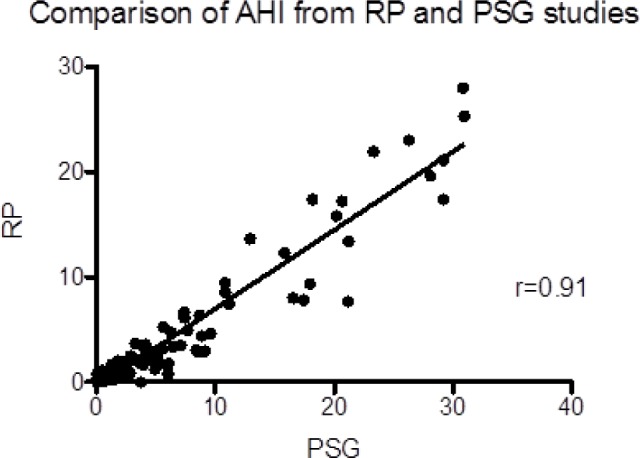
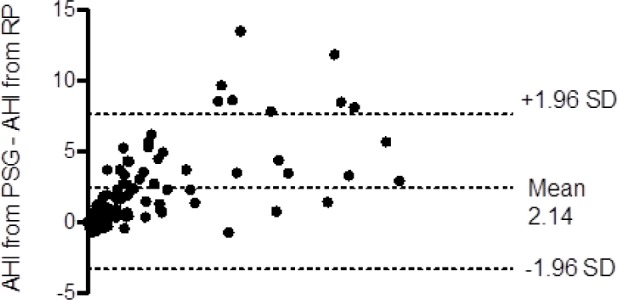
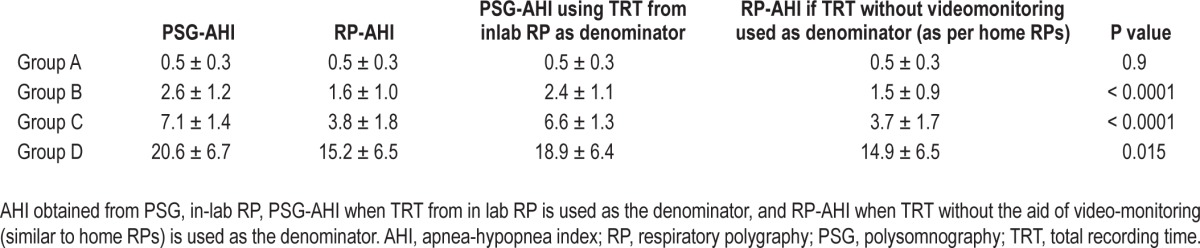
The AHI obtained from RP was strongly correlated to the corresponding PSG AHI (r = 0.91) (Figure 1). However, Bland Altman analysis showed that in-lab RP underestimated the AHI (Figure 2). This underestimation was due to missed hypopneas causing arousals without desaturation (Table 2). There were no significant differences between the PSG and RP obstructive apnea index. The total hypopnea index from RP was significantly lower than the total hypopnea index from PSG in groups B, C, and D. There was no significant difference in the desaturation-related hypopnea index obtained from PSG and RP. To quantify the independent effect of using total recording time on the AHI, Table 3 shows the AHI obtained from PSG, in-lab RP, PSG when the TRT from in-lab RP is used as the denominator, and the RP-AHI when TRT without the aid of video-monitoring (similar to home RPs) is used as the denominator.
Figure 1.
Scatterplot and linear regression on AHI obtained from RPs and corresponding AHI from PSGs. AHI, apnea-hypopnea index; RP, respiratory polygraphy; PSG, polysomnography.
Figure 2.
Bland Altman plot showing that in lab RP underestimates the AHI compared to PSG-derived AHI. AHI, apnea-hypopnea index; RP, respiratory polygraphy; PSG, polysomnography; SD, standard deviation.
Table 2.
Distribution of OAI, THI, DRHI, and THI-RPTRT in PSG and RP
Table 3.
Respiratory disturbance measures (AHI) as calculated form PSG or RP
When a cutoff of PSG-AHI ≥ 1/h TST was used as the cutoff for diagnosing OSA, the sensitivity of RP was 82.5% (95% CI: 72.4-90.1), the specificity was 90% (95% CI: 68.2-98.9), with a positive predictive value of 97.1% (95% CI: 89.8-99.6), negative predictive value of 56.3% (95% CI: 37.7-72.6), and AUC of 0.86 (95% CI: 0.78-0.94). When a cutoff of PSG-AHI ≥ 3/h TST was used, the sensitivity of RP was 70.4% (95% CI: 56.4-82), the specificity was 100% (95% CI: 92.3-100), with a positive predictive value of 100% (95% CI: 90.7-100), negative predictive value of 74.2% (95% CI: 61.5-84.5%), and AUC of 0.85 (95% CI: 0.79-0.91). Finally, when a cutoff of PSG-AHI ≥ 5/h TST was used, the sensitivity of RP was 62.5% (95% CI: 45.8-77.3%), the specificity was 100% (95% CI: 94-100%), with a positive predictive value of 100% (95% CI: 86.3-100%), negative predictive value of 80% (95% CI: 69.2-88.4%), and AUC of 0.81 (95% CI: 0.74-0.89).
The RP-AHI resulted in a change in the classification of severity in 28% of all patients. All 20 patients in group A who were diagnosed as having no OSA by PSG were also correctly classified as having no OSA by RP. Of the 40 patients in group B (mild OSA based on PSG-AHI), 13 (32.5%) were classified as having no OSA by RP, and the rest were classified as having mild OSA (67.5%). Of the 20 patients in group C (moderate OSA based on PSG-AHI), 14 (70%) were classified as mild OSA on RP, 1 (5%) was diagnosed as having no OSA on RP, and the rest classified as moderate OSA (25%). Of the 20 patients in group D (severe OSA based on PSG-AHI), 7 (35%) were classified as moderate OSA on RP and the rest as severe OSA (65%).
When the therapeutic management decision was based on RP instead of the PSG, RP-based results changed the clinical management in 23% of all the patients. There were no differences in clinical management for patients in either group A or group D. However the clinical management of 11 of the 40 patients (27.5%) in group B would have been different, since they would have been given no treatment as they would have been diagnosed as having no OSA (AHI < 1/h TST), instead of receiving a trial of anti-inflammatory therapy (n = 10) or being directly referred for ENT evaluation (n = 1). The clinical management of 12 of the 20 patients in group C (60%) would also have been different, with 2 patients not receiving any treatment as their RP-AHI was < 1, in lieu of being referred to ENT based on the result of their PSG-AHI; furthermore, 9 patients would have received a trial of anti-inflammatory therapy instead of referral to ENT for adenotonsillectomy, and 1 patient would have received a trial of anti-inflammatory therapy instead of being commenced on continuous positive airway pressure (since he had previously had an adenotonsillectomy).
DISCUSSION
The present study shows that RP and PSG are strongly correlated in the AHI values derived from either of these two approaches, and yet greatly differ in their clinical implications. The impetus to perform the present study was our observation on the different practices in the evaluation of snoring children in the US, Australia, and Europe. While in the US and Australia, full PSGs are performed in the diagnosis of pediatric OSA, in Europe, in-lab RPs tend to be the default investigation of choice. If the results from the two approaches are comparable, then RPs would be more cost-effective, less labor-intensive, and less time-consuming to analyze. Furthermore, if the two approaches were not comparable, it would be important to ascertain whether the differences would result in significantly different clinical management outcomes. To the best of our knowledge, this is the first pediatric study to address this latter question.
Our findings show that despite more accurate estimates of TST compared to home-based RPs, in-lab RPs still tend to underestimate the AHI compared to full PSGs. Although this discrepancy does not affect therapeutic decisions at the two ends of the clinical spectrum, i.e., when the child does not have OSA and when the child has severe OSA, the clinical management decision can be markedly affected in children with mild-to-moderate OSA. Indeed, a significant proportion of children with moderate OSA would be classified as having no OSA or mild OSA and would be offered no treatment or a trial of medical therapy, rather than immediate referral to ENT surgeons for adenotonsillectomy. Similarly, a large proportion of children with mild OSA would be diagnosed as primary snorers. Arguably, the impact is more concerning in patients diagnosed as primary snorers, as these children may be discharged from follow-up. Children with moderate OSA classified as mild OSA and offered a trial of medical treatment would normally still be followed up in the sleep clinic, and if the medical treatment was not effective and they remained symptomatic, they would likely still have been referred to the ENT surgeons.
Several limitations of our study should be mentioned and include: (1) The study was retrospective in nature; (2) As there is no internationally agreed consensus on the management of pediatric OSA, the clinical management algorithm and AHI cutoffs used in the study represent the clinical practice of the centers where the authors work. We acknowledge that there is some variation in clinical practice internationally, and not all pediatric sleep centers adopt the same AHI cutoffs. However, even if the percentage of patients affected by RP vs. PSG may vary, the essential conclusions of this study are unlikely to change; (3) The patients studied were children referred to a sleep clinic for investigation of OSA who were otherwise healthy, and therefore results cannot be directly extrapolated to other patient groups.
Alvarez et al. studied 53 children referred for assessment of OSA, who underwent simultaneous RP and PSG.15 In contrast to our findings, Bland Altman analysis of their results showed that RP neither overestimated nor underestimated respiratory events compared to PSG. The best RP-AHI cutoff value based on receiver operating curve calculations emerged as 4.6/h of recording. Possible explanations for this discrepancy with our results include the fact that a thermistor was used instead of a nasal pressure transducer. Thermistors tend to underestimate hypopneas, such that the hypopneas associated with arousals but not with desaturations, which were the primary events that were missed by RPs in our study, may not have been identified in the PSG performed by Alvarez and collaborators. Also of note, the scoring criteria were slightly different and based on the older Rechtschaffen and Kales and American Thoracic Society criteria,16 rather than the AASM 2007 guidelines.13
The majority of published studies on RPs versus PSGs have compared portable home RPs with in-lab PSGs.17–20 Although one can attempt to extrapolate from those findings to draw conclusions regarding in-lab RPs, there are significant differences between portable and in-lab RPs. As mentioned previously, because the TRT rather than the TST is used as the denominator in portable RPs, the AHI is likely to be underestimated. Estimation of TST is likely to be more accurate in in-lab RPs as there is sleep technician and video monitoring. In-lab RPs also tend to use the same equipment and software as PSGs (the difference being that EEG, EMG, and EOG signals are not included in the montage), while portable RPs use equipment and software that is different from PSGs. Portable RPs are usually set up by the parents or caretakers of the child, whereas trained sleep technicians perform the set up in-lab RPs and also monitor the signals throughout the night, making adjustments when necessary. The quality of the signals used for analysis, in particular the thermistor and nasal pressure transducer signals, could therefore differ between the in-lab and in-home studies. Indeed, studies have reported portable RP failure rates ranging from 3% to 18%.21
In adults, the debate over the use of home RP versus in-patient PSG has occupied center stage for almost 20 years.11,22 In 2007, the portable monitoring taskforce of the AASM recommended that portable RPs may be used as an alternative to PSGs for the diagnosis of OSA in patients with a high pre-test probability of moderate to severe OSA, but are not appropriate in patients with significant comorbid medical conditions that may degrade the accuracy of RP and patients suspected of having comorbid sleep disorders. RPs were also deemed as not being appropriate for the screening of asymptomatic populations.21 The recent clinical practice guideline on the diagnosis and management of childhood OSAS by the American Academy of Pediatrics acknowledges there is a “paucity of studies” evaluating ambulatory RPs in children.1 Zucconi and colleagues performed PSGs and portable RPs on 2 consecutive nights in 12 children, and reported that although the sensitivity of RPs was satisfactory, the specificity was poor, leading the authors to conclude that portable RPs could not be advocated for routine use in children in a clinical setting. In contrast, when Rosen et al. studied 55 children in whom PSG and home RP were performed within 3 months, the sensitivity of RPs was 88% and the specificity was 98% with the use of a threshold value for OSA of PSG AHI > 5/h TST.
We are aware of only one study examining the therapeutic decision-making implications when portable RPs are used compared to inpatient PSG.23 This study was conducted in adults, and therapeutic decisions using home RPs had an agreement level of 76% when compared to those made using PSGs. This level of concordance improved to 91% in patients with high AHI, leading the authors to conclude that home RPs were adequate in patients with severe OSA, but deficient in the large population of patients with mild-to-moderate OSA. We should point out, however, that the clinical management of OSA in children is significantly different from adults, with adenotonsillectomy being the recommended first-line treatment in children. Furthermore, a much lower AHI is considered to be normal in pediatrics, reflecting the greater morbidity of children at lower AHI in comparison to adults.24 Therefore, any change in measured AHI is more likely to exert a greater impact on therapeutic decisions in children. Many other factors that can affect clinical management decisions, such as parental wishes, the degree of severity of symptoms, whether there are any other factors such as recurrent tonsillitis, allergies, obesity, and likely compliance of the child with any specific therapy, will also affect outcomes; the present study, being retrospective, could not examine how these factors would play out in the RP vs. PSG comparisons. Variation in clinical practice is also more likely in the mild spectrum of a given clinical condition. A child with an AHI of 1.1 and one with an AHI of 4.9 may both arbitrarily be classified as having mild OSA, but their clinical management can be significantly different from one physician to another.
Clearly, each of the diagnostic modalities under scrutiny has its own advantages and disadvantages. The cost of PSG is higher, requires more resources, takes much longer to score, and requires appropriately trained staff who need to score a certain number of studies annually to maintain their skills. However, PSG may also provide additional valuable information. There is the possibility that children with an undiagnosed seizure disorder could be identified on the EEG leads. Furthermore, the percentage of time spent in the various sleep stages may provide useful additional information to the clinician, and the presence of sleep fragmentation or periodic leg movements is also clinically important. On the other hand, in the current economic climate, even in the US, there is a trend towards exploring the use of home respiratory polygraphy in place of hospital PSG in the diagnosis of OSA.
This study emphasizes the importance of seeking consensus on two important questions: (1) at what severity of OSA does treatment need to be initiated before cardiovascular, metabolic, and neurobehavioral morbidity ensues; and (2) are all hypopnea events similar? Sleep studies undoubtedly provide an objective measure of sleep disturbance, and the currently accepted practice advocating the use of an arbitrary cutoff > 3 SD beyond the mean is not based on end-organ morbidity probabilistic estimates. Since this study has shown that the RP-AHI underestimates the PSG-based AHI, a lower RP-AHI cutoff would have to be ascertained and then could be used if RPs were to be used in clinical practice. However, not every child fulfilling current PSG criteria for OSA demonstrates evidence of end-organ morbidity, raising the question as to whether they should be treated.25,26 Conversely, evidence that some children who snore habitually display neurocognitive consequences or signs of systemic inflammation despite having an PSG-AHI < 1/h TST has also emerged,27,28 raising the possibility of combining both sleep recording measures and clinical and laboratory-based criteria.29 The question on whether a hypopnea which results in an oxygen desaturation ≥ 3% being “equal” to a hypopnea that causes an arousal without a desaturation is also important. The events identified during RPs are those associated with desaturations, whereas events that just result in an arousal may not necessarily be identified. Most of the research on OSA has concentrated on the effects of intermittent hypoxia, and yet there is increasing evidence that sleep fragmentation imposes equally profound effects on outcomes. Tauman et al. proposed the sleep pressure score (SPS) as a measure of disrupted sleep homeostasis.30 Children with high SPS were more likely to have deficits in memory, language, and verbal abilities than children with low SPS, and the association of SPS with deficits in neurobehavioral daytime functions was independent of respiratory disturbance and hypoxemia.31 Thus, RPs may lead to under-appreciating the clinical implications of the disease being evaluated.
In conclusion, the AHI is underestimated in RPs, and this can significantly affect clinical management decisions, particularly in children with mild and moderate OSA (1 < AHI < 10/h TST). Although availability of resources will often dictate clinical practice, this study shows that the type of sleep study performed does impose an effect on the AHI measurements, and that such effect will need to be incorporated in the therapeutic intervention algorithm.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. David Gozal has consulted for Galleon Pharmaceuticals and is supported by National Institutes of Health grants HL-065270. Dr. Leila Kheirandish-Gozal was the recipient of an investigator-initiated grant on the use of montelukast in pediatric OSA. Dr. Tan was supported by the Scadding Morriston Davies Fellowship. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ECG
electrocardiography
- EEG
electroencephalography
- EMG
electromyography
- ENT
ear, nose, and throat
- EOG
electrooculography
- OSA
obstructive sleep apnea
- PSG
polysomnography
- RP
respiratory polygraphy
- SPS
sleep pressure score
- TST
total sleep time
- TRT
total recording time
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.Wise MS, Nichols CD, Grigg-Damberger MM, et al. Executive summary of respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34:389–98AW. doi: 10.1093/sleep/34.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aurora RN, Zak RS, Karippot A, et al. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34:379–88. doi: 10.1093/sleep/34.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurnheer R, Bloch KE, Laube I, Gugger M, Heitz M. Respiratory polygraphy in sleep apnoea diagnosis. Report of the Swiss respiratory polygraphy registry and systematic review of the literature. Swiss Med Wkly. 2007;137:97–102. doi: 10.4414/smw.2007.11654. [DOI] [PubMed] [Google Scholar]
- 5.Alonso-Alvarez ML, Navazo-Eguia AI, Cordero-Guevara JA, et al. Respiratory polygraphy for follow-up of obstructive sleep apnea in children. Sleep Med. 2012;13:611–5. doi: 10.1016/j.sleep.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Lloberes P, Duran-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143–56. doi: 10.1016/j.arbres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Working Party on Sleep Physiology and Respiratory Control Disorders in Childhood. Standards for services for children with disorders of sleep physiology report. Royal College of Paediatrics and Child Health; 2009. [Google Scholar]
- 8.Rosen CL, Larkin EK, Kirchner HL, et al. Prevalence and risk factors for sleep-disordered breathing in 8- to 11-year-old children: association with race and prematurity. J Pediatr. 2003;142:383–9. doi: 10.1067/mpd.2003.28. [DOI] [PubMed] [Google Scholar]
- 9.Zucconi M, Calori G, Castronovo V, Ferini-Strambi L. Respiratory monitoring by means of an unattended device in children with suspected uncomplicated obstructive sleep apnea: a validation study. Chest. 2003;124:602–7. doi: 10.1378/chest.124.2.602. [DOI] [PubMed] [Google Scholar]
- 10.Alonso Alvarez Mde L, Teran Santos J, Cordero Guevara J, et al. [Reliability of home respiratory polygraphy for the diagnosis of sleep apnea-hypopnea syndrome: analysis of costs] Arch Bronconeumol. 2008;44:22–8. [PubMed] [Google Scholar]
- 11.Kirsch DB. PRO: Sliding into home: portable sleep testing is effective for diagnosis of obstructive sleep apnea. J Clin Sleep Med. 2013;9:5–7. doi: 10.5664/jcsm.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morielli A, Ladan S, Ducharme FM, Brouillette RT. Can sleep and wakefulness be distinguished in children by cardiorespiratory and videotape recordings? Chest. 1996;109:680–7. doi: 10.1378/chest.109.3.680. [DOI] [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Kaditis A, Kheirandish-Gozal L, Gozal D. Algorithm for the diagnosis and treatment of pediatric OSA: a proposal of two pediatric sleep centers. Sleep Med. 2012;13:217–27. doi: 10.1016/j.sleep.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Alonso Alvarez ML, Teran Santos J, Cordero Guevara JA, et al. [Reliability of respiratory polygraphy for the diagnosis of sleep apneahypopnea syndrome in children] Arch Bronconeumol. 2008;44:318–23. [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A, editors. A manual of standardized terminology and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 17.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183:1238–44. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 18.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:757–67. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berry RB, Hill G, Thompson L, McLaurin V. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 20.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124:1543–79. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 21.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 22.Parthasarathy S. CON: Thoughtful steps informed by more comparative effectiveness research is needed in home testing. J Clin Sleep Med. 2013;9:9–12. doi: 10.5664/jcsm.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masa JF, Corral J, Pereira R, et al. Therapeutic decision-making for sleep apnea and hypopnea syndrome using home respiratory polygraphy: a large multicentric study. Am J Respir Crit Care Med. 2011;184:964–71. doi: 10.1164/rccm.201103-0428OC. [DOI] [PubMed] [Google Scholar]
- 24.Guilleminault C, Lee JH, Chan A. Pediatric obstructive sleep apnea syndrome. Arch Pediatr Adolesc Med. 2005;159:775–85. doi: 10.1001/archpedi.159.8.775. [DOI] [PubMed] [Google Scholar]
- 25.Gozal D, Kheirandish L. Oxidant stress and inflammation in the snoring child: confluent pathways to upper airway pathogenesis and end-organ morbidity. Sleep Med Rev. 2006;10:83–96. doi: 10.1016/j.smrv.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Kheirandish-Gozal L, Gozal D. The multiple challenges of obstructive sleep apnea in children: diagnosis. Curr Opin Pediatr. 2008;20:650–3. doi: 10.1097/MOP.0b013e328316bdb2. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114:44–9. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- 28.Khalyfa A, Gharib SA, Kim J, et al. Peripheral blood leukocyte gene expression patterns and metabolic parameters in habitually snoring and non-snoring children with normal polysomnographic findings. Sleep. 2011;34:153–60. doi: 10.1093/sleep/34.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozal D, Kheirandish-Gozal L. New approaches to the diagnosis of sleep-disordered breathing in children. Sleep Med. 2010;11:708–13. doi: 10.1016/j.sleep.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Tauman R, O'Brien LM, Holbrook CR, Gozal D. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep. 2004;27:274–8. doi: 10.1093/sleep/27.2.274. [DOI] [PubMed] [Google Scholar]
- 31.O'Brien LM, Tauman R, Gozal D. Sleep pressure correlates of cognitive and behavioral morbidity in snoring children. Sleep. 2004;27:279–82. doi: 10.1093/sleep/27.2.279. [DOI] [PubMed] [Google Scholar]


