Abstract
Objective:
We used quantitative genetic models to assess whether sleep duration modifies genetic and environmental influences on depressive symptoms.
Method:
Participants were 1,788 adult twins from 894 same-sex twin pairs (192 male and 412 female monozygotic [MZ] pairs, and 81 male and 209 female dizygotic [DZ] pairs] from the University of Washington Twin Registry. Participants self-reported habitual sleep duration and depressive symptoms. Data were analyzed using quantitative genetic interaction models, which allowed the magnitude of additive genetic, shared environmental, and non-shared environmental influences on depressive symptoms to vary with sleep duration.
Results:
Within MZ twin pairs, the twin who reported longer sleep duration reported fewer depressive symptoms (ec = -0.17, SE = 0.06, P < 0.05). There was a significant gene × sleep duration interaction effect on depressive symptoms (a'c = 0.23, SE = 0.08, P < 0.05), with the interaction occurring on genetic influences that are common to both sleep duration and depressive symptoms. Among individuals with sleep duration within the normal range (7-8.9 h/night), the total heritability (h2) of depressive symptoms was approximately 27%. However, among individuals with sleep duration within the low (< 7 h/night) or high (≥ 9 h/night) range, increased genetic influence on depressive symptoms was observed, particularly at sleep duration extremes (5 h/night: h2 = 53%; 10 h/night: h2 = 49%).
Conclusion:
Genetic contributions to depressive symptoms increase at both short and long sleep durations.
Citation:
Watson NF; Harden KP; Buchwald D; Vitiello MV; Pack AI; Stachan E; Goldberg J. Sleep duration and depressive symptoms: a gene-environment interaction. SLEEP 2014;37(2):351-358.
Keywords: Sleep Duration, Twins, Monozygotic, Dizygotic, Depression, Symptoms
INTRODUCTION
Depression and sleep are tightly intertwined. Insomnia and hypersomnia are disease-defining symptoms for major depressive disorder and sleep-wake disturbance is a risk factor for depression onset, recurrence, and severity.1,2 Objectively measured sleep disturbances predict poor treatment outcomes in patients with depression; in particular, short sleep duration is a risk factor for poor depression treatment outcome.3 Insufficient sleep is associated with suicidality, even after controlling for depression symptomology, sleepiness, and insomnia.4,5 High suicidality is associated with increased discrepancy between weekday and weekend sleep duration, a common metric of chronic sleep debt.5
The optimal amount of sleep needed to maintain physiological homeostasis is individualized and influenced by both genetic and environmental factors. The physiologically normal “sleep fraction” in humans is between 29% and 33% of the sleep-wake cycle, or 7 to 7.9 hours under conditions of environmental and temporal isolation.6,7 The heritability of sleep duration is between 31% and 55%, suggesting a substantial amount of sleep need is genetically determined, but environmental factors also contribute.8–11 Modern society, with its ubiquitous technology and countless competing interests for time, along with the zeitgeist deemphasizing sleep's importance, creates an environment that promotes sleep deprivation.12 Currently, about one-third of the working population sleeps ≤ 6 hours per night. Over the past century, habitual sleep duration has dropped an estimated 1.5 to 2 hours per night.13–15 Meanwhile, human physiological sleep need remains unchanged. This growing disconnect between sleep need and sleep actualization has substantial adverse consequences for cognitive functioning and metabolic, cardiovascular, immunological, and psychological health.3,16–22
This study examines the association between habitual sleep duration and depressive symptoms using a genetically informed twin design. Twins, if reared together, are identical in age and typically well matched for shared family background and numerous childhood and adolescent exposures. As such, twin comparisons can be used to control for third-variable confounders that typically differ among unrelated individuals. This approach is particularly helpful when investigating the relationship between sleep duration and depression because many subjective and objective aspects of these phenotypes are genetically influenced.23,24 Twin studies can also be used to estimate gene × environment interaction (G×E) effects. In the current paper, we examine sleep duration as an “environmental” moderator of the heritability of depressive symptoms, while also modeling genetic influences on habitual sleep duration. Previous studies suggest age, gender, and depression recurrence influence the heritability of major depressive disorder by way of G×E effects.25 Therefore, the goal of the current study was to: (1) determine the magnitude of genetic and environmental influences on sleep duration and depressive symptoms, and (2) determine if sleep duration moderates genetic influences on depressive symptoms.
METHODS
Participants
The University of Washington Twin Registry is a community-based sample of twins constructed using data from the Washington State Department of Licensing. The minimum age for participation is 18 years. As of September 2013, the Registry consisted of over 8,000 pairs. Zygosity is determined using previously validated self-report methods that are correct ≥ 95% of the time.26,27 Every twin enrolled in the Registry completes a recruitment survey. In 2006 and 2008, an additional health survey was mailed to more than 4,000 enrolled twins that included sleep duration and depression symptom questions. Since 2009, the recruitment survey has included the same questions. The data collection procedures were approved by the University of Washington Institutional Review Board. All twins were raised together.
Measures
Sleep Duration
Habitual sleep duration was obtained from responses to the question, “On average, how long do you sleep per night?” reported in hours and minutes. For the purposes of our calculations we categorized sleep duration into 3 groups. Normal sleep duration was considered 7-8.9 h because this range encompasses the physiologically normal sleep fraction in humans6,7 and the sleep duration considered normal in previous studies.28–30 We classified sleep duration of < 7 h per night as short sleep and ≥ 9 h per night as long sleep. One exception to these definitions involves our calculation of heritability. Heritability estimates are generated from model parameters that require a single number. Therefore, we defined normal sleep as 8 h/night, short sleep as 5 h/night, and long sleep as 10 h/night for these specific calculations.
Depressive Symptoms
Depressive symptoms were measured using participants' self-report on the modified 3 question Patient Health Questionnaire-9 (mPHQ-9), which asks “In the past 4 weeks, how often have you been bothered by the following problems”: Little interest or pleasure in doing things; Feeling down, depressed, hopeless; and Feeling tired or having little energy. All items were rated on a 4-point Likert scale ranging from 0 = Not at all to 3 = Nearly every day. Responses were summed to yield depressive symptom scores (range = 0 to 9; mean = 1.71; standard deviation (SD) = 1.82; 25th-75th percentile = 0 to 3). As described below in the section labeled “Sensitivity Analysis,” we also conducted a complimentary analysis that omitted the item referring to “feeling tired or having little energy.”
Sociodemographics
Age, sex, and race were self-reported. Race was dichotomized into Caucasian and non-Caucasian (American Indian, Alaska Native, Native Hawaiian, Pacific Islander, Asian, Black or African American, or other) categories. Education was ascertained by the question, “What is the highest level of school you have completed?” A total of 7 responses were possible, ranging from “eighth grade or less” to “graduate or professional degree.” The midpoint was “some college, but no degree or certificate.”
Statistical Analyses
We began by examining zygosity specific twin pair correlations for sleep duration and depressive symptoms. The within-trait, cross-twin correlations (e.g., the correlation between sleep duration in Twin A and sleep duration in Twin B) can be used to evaluate the magnitude of genetic and environmental influences on a given phenotype, while the cross-trait, cross-twin correlations (e.g., the correlation between sleep duration in Twin A and depressive symptoms in Twin B) can be used to evaluate the extent to which the association between sleep duration and depressive symptoms is accounted for by genetic versus environmental pathways.
Next we evaluated these questions more formally with quantitative genetic models using the software program Mplus (Muthén & Muthén, 1998-2012). This approach allows modeling of genetic overlap and moderation effects between sleep duration and depressive symptoms. First, we fit a bivariate twin model, shown in Figure 1. Total variance in each of the observed phenotypes (the boxes labeled “Sleep Duration” and “Depressive Symptoms”) was decomposed into 3 latent factors: additive genetic influences (A), shared environmental influences (i.e., environmental influences that make siblings similar to one another, or C), and non-shared environmental influences (i.e., environmental influences that are unique to each twin, plus measurement error, or E). The ACE components for each phenotype were standardized (mean = 0, SD = 1) and the paths from the ACE components to the phenotype were estimated. The correlation between additive genetic influences (A) in the first and second member of each twin pair was fixed to 1.0 in monozygotic (MZ) twins and 0.5 in dizygotic (DZ) twins, consistent with genetic theory. The correlation between common environmental (C) factors was fixed to 1.0 in all pair types, whereas the correlation between unique environmental (E) factors was fixed to 0 in all pair types. Finally, depressive symptoms were regressed on the ACE components of sleep duration. These cross paths estimate the extent to which genetic and environmental influences on sleep duration also influence depressive symptoms. Previous authors have described the logic and parameterization of twin models in great detail.31
Figure 1.
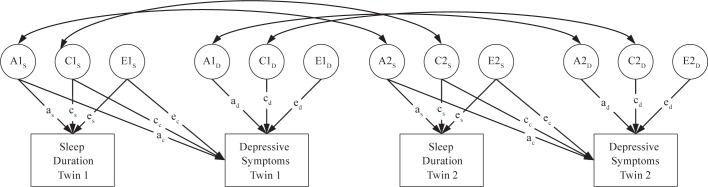
Structural equation model of sleep duration and depressive symptoms in adult twins. A, additive genetic variance; C, shared environmental variance; E, nonshared environmental variance. A, C, and E components standardized (mean = 0, SD = 1). Correlation between A components fixed at 1.0 in monozygotic twins and 0.5 in dizygotic twins. Correlation between C components fixed to 1.0 in all twins. Correlation between E components fixed to 0 in all twins.
We fit an extension of the bivariate twin model that examined the interaction between sleep duration and the genetic influences on depressive symptoms. As illustrated in Figure 2, this interaction model allows the genetic and environmental cross paths between sleep duration and depressive symptoms (pathways labeled: ac + a'c*sleep; cc + c'c*sleep; and ec + e'c*sleep), as well as the residual genetic and environmental variation in depressive symptoms (pathways labeled: ad + a'd*sleep; cd + c'd*sleep; and ed + e'd*sleep), to vary as a function of habitual sleep duration. Note that the cross paths between the ACE components of sleep duration and depressive symptoms represent genetic and environmental influences on depressive symptoms that are shared with (common to) sleep duration, whereas the ACE components of depressive symptoms represent genetic and environmental influences unique to depressive symptoms. Further explanation about using variance components for testing gene-environment interactions are explained in detail elsewhere.32
Figure 2.
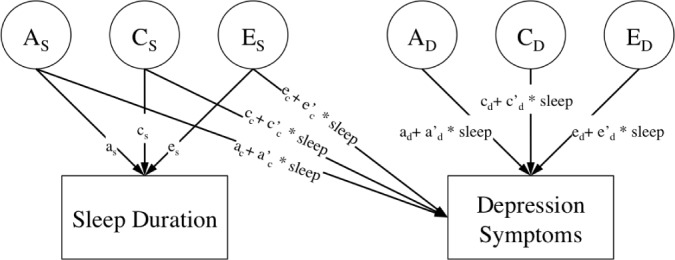
Interaction model of sleep duration and depressive symptoms in adult twins. Only one twin per pair is shown. A, additive genetic variance; C, shared environmental variance; E, nonshared environmental variance. A, C, and E components standardized (mean = 0, SD = 1). Correlation between A components fixed at 1.0 in monozygotic twins and 0.5 in dizygotic twins. Correlation between C components fixed to 1.0 in all twins. Correlation between E components fixed to 0 in all twins.
RESULTS
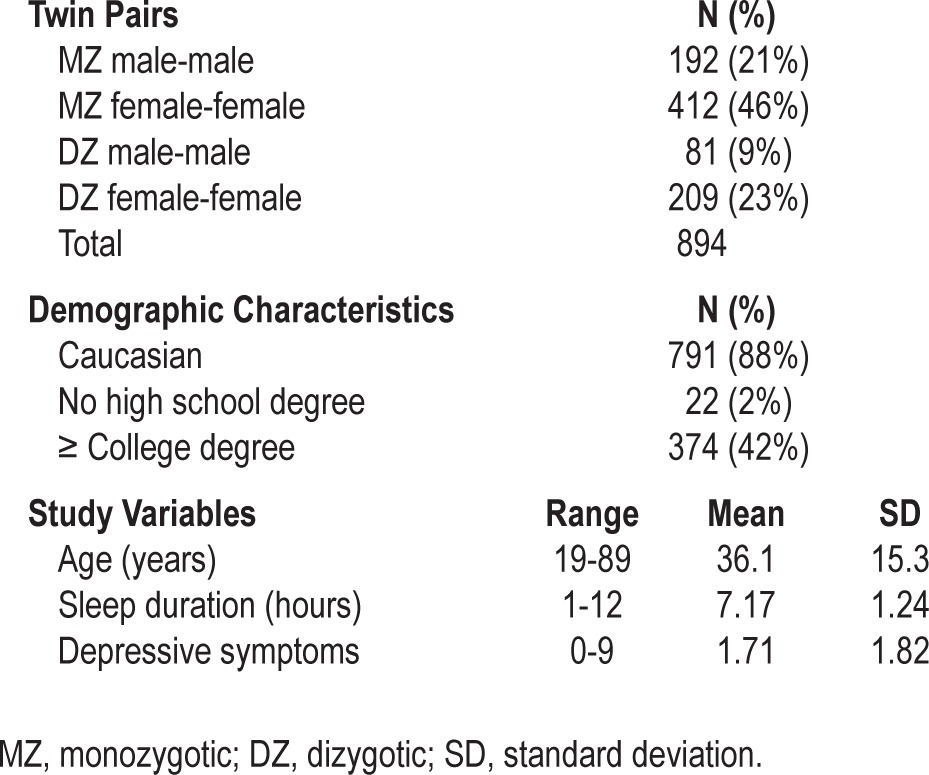
The study sample includes 1,788 individuals from 894 same-sex twin pairs (604 monozygotic [MZ], 290 dizygotic [DZ]). Sample characteristics are summarized in Table 1. Overall, the sample was composed of younger adults (mean = 36.1 years), who were well-educated (42% with a college degree or higher), predominantly Caucasian (88%), and female (69%). The most common twin relationship was female-female MZ pairs (46%). The mean sleep duration in the sample was in the normal range (mean = 7.17 h). Most participants (n = 1,186, 66%) reported “normal” sleep duration (7-8.9 h), while 24% (n = 434) reported “short” sleep (< 7 h) and 9% (n = 166) reported “long” sleep (≥ 9 h).
Table 1.
Sample characteristics
Sleep duration was negatively correlated with depressive symptoms (r = -0.16, P < 0.001). As shown in Figure 3, individuals with normal sleep duration reported significantly fewer depressive symptoms, on average, than individuals with short (b = 0.36, SE = 0.18, P = 0.05) or long (b = 0.86, SE = 0.13, P < 0.0001) habitual sleep duration.
Figure 3.

Mean depressive symptoms by sleep duration classification. Possible range of depressive symptoms = 0 to 9. Bars represent ± 1 SE. Data based on one randomly selected twin per pair. Differences between the groups statistically significant at P ≤ 0.05 (see text).
What are the Magnitudes of Genetic and Environmental Influences on Sleep Duration and Depressive Symptoms?
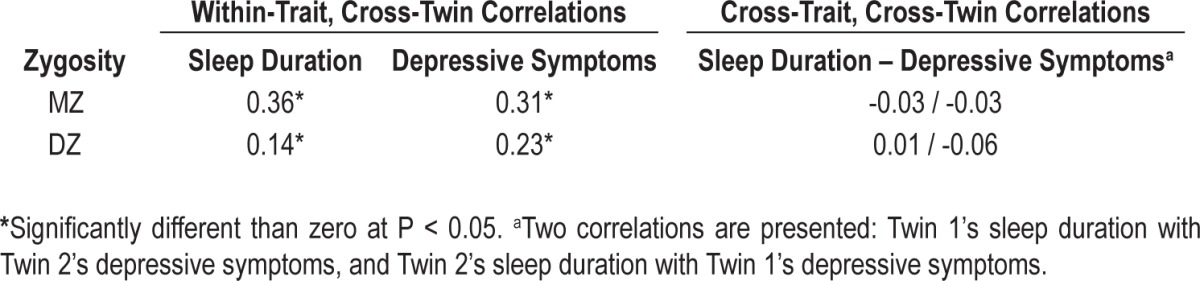
The within-trait and cross-trait twin correlations for sleep duration and depressive symptoms are summarized in Table 2. The MZ correlation for sleep duration (0.36, 95% CI 0.29-0.43) substantially exceeded the DZ correlation (0.14, 95% CI 0.02-0.25), suggesting that familial resemblance in sleep duration was primarily due to genetic influences, with minimal contribution of the shared environment. As expected, the MZ correlation for depressive symptoms (0.31, 95% CI 0.23-0.38) exceeded the DZ correlation (0.23, 95% CI 0.11-0.33), consistent with a genetic contribution to variance in depression. (The significance of the MZ-DZ differences was formally tested with the quantitative genetic models, described below.) Finally, the cross-trait, cross-twin correlations were negligible (and not significantly different than zero) in either MZ or DZ twins. That is, twin A's sleep duration was unrelated to twin B's depressive symptoms (for comparison, the phenotypic correlation between sleep duration and depressive symptoms was -0.11, P < 0.01). These results suggest genetic influences on sleep duration do not account for the association between sleep duration and depressive symptoms.
Table 2.
Within-trait and cross-trait twin correlations for sleep duration and depressive symptoms
These initial results were investigated more formally with quantitative genetic models. The bivariate model (Model 1, Table 3, illustrated in Figure 1) fit the data well (χ2 = 43.87, df = 35, P = 0.15, RMSEA = 0.024, CFI = 0.956, see footnote A).33 In addition to the full bivariate model, we also fit a reduced bivariate model (Model 2, Table 3), in which the shared environmental variance (cs pathway) in sleep duration and the shared environmental pathway between sleep duration and depressive symptoms (cc pathway) were fixed to zero. This reduced model also fit the data well (χ2 = 44.68, df = 37, P = 0.18, CFI = 0.962, RMSEA = 0.022), and the change in model fit was not significant (Δχ2 = 0.81, Δdf = 2, P = 0.67). Consequently, these parameters were fixed to zero in all subsequent models.
Table 3.
Unstandardized parameter estimates from behavioral genetic models of sleep duration and depressive symptoms
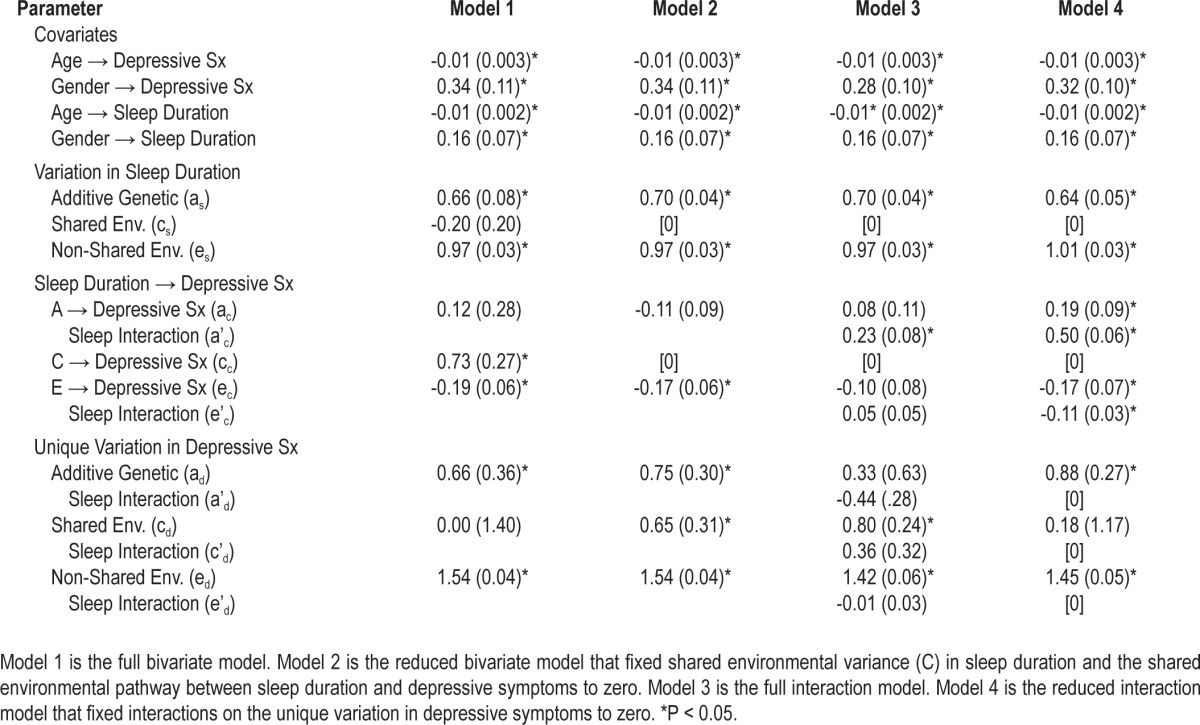
Unstandardized parameter estimates from Model 2 are summarized in Table 3. There were significant effects of age and gender on both sleep duration and depressive symptoms: older adults and males, on average, reported shorter sleep durations and fewer depressive symptoms, consistent with previous studies.34,35 Controlling for age and gender, there were significant additive genetic (as) and non-shared environmental (es) influences on sleep duration: 34% of the variance in sleep duration was due to additive genetic differences [0.702 / (0.702 + 0.972) = 0.34], and the remaining 66% was due to non-shared environmental factors. There was also a significant non-shared environmental pathway (ec) between sleep duration and depressive symptoms: within MZ twin pairs, the twin who reported longer sleep duration reported fewer depressive symptoms (ec = -0.17, SE = 0.06, P < 0.05). Finally, of the residual (unique) variation in depressive symptoms, 17% was due to additive genetic factors [0.752 / (0.752 + 0.652 + 1.542) = 0.17], 13% to shared environmental factors, and the rest (70%) to non-shared environmental factors.
Does Sleep Duration Moderate Genetic Influence on Depressive Symptoms?
Unstandardized parameter estimates from Model 3 are summarized in Table 3. We found evidence for a significant interaction between sleep duration and the genetic cross-path from sleep duration to depressive symptoms (a'c = 0.23, SE = 0.08, P < 0.05). There were no significant interactions on the unique variance components for depressive symptoms (parameters a'd, c'd, and e'd). That is, the genes that were not shared with sleep duration were also not moderated by sleep duration; it was the shared genetic mechanism (genes that overlap between sleep duration and depressive symptoms) that became more influential as individuals diverged from normal sleep. Consequently, we fit a reduced model (Model 4) that fixed the interaction terms on the unique variance components to zero. Results from Model 4 are illustrated in Figure 4. Genetic influences on depressive symptoms were greater among individuals with either high or low sleep duration. That is, as sleep duration diverges from normal (7-8.9 h/night), genetic vulnerabilities common to both sleep duration and depressive symptoms become more influential.
Figure 4.
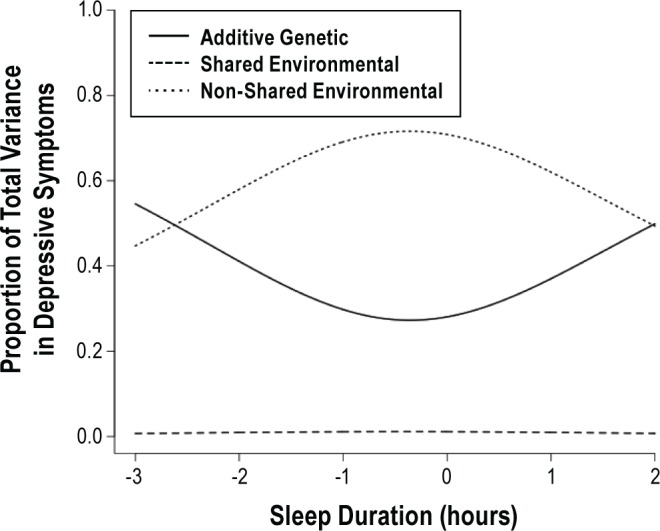
Proportion of total variance in depressive symptoms due to genetic, shared environmental, and non-shared environmental influences, by sleep duration. Implied by parameters from bivariate interaction model (Model 4, Table 3).
To further illustrate these results, we calculated the model-implied heritability of depressive symptoms for individuals with habitual sleep durations of 8 h/night (within our defined range of normal sleep), 5 h/night (within our defined range of short sleep), and 10 h/night (within our defined range of long sleep). Among individuals sleeping 8 h/night, the total heritability of depressive symptoms was approximately 27% (95% CI 0% to 58%). However, among individuals with short or long sleep duration we observed increased genetic influence on depressive symptoms, particularly at sleep duration extremes (5 h/night: h2 = 53% [95% CI 31% to 75%]; 10 h/night: h2 = 49% [95% CI 26% to 72%]). Therefore sleep durations outside the normal range increased the genetic risk for depressive symptoms.
Sensitivity Analysis
Because our depressive symptoms measure, the mPHQ-9, included an item that referred to “feeling tired or having little energy,” we conducted a sensitivity analysis that omitted this item from the mPHQ-9 sum score. Notably, the pattern of results was unchanged: there was a significant interaction between the genetic cross-path and sleep duration (a'c = 0.13, SE = 0.5, P = 0.01), resulting in U-shaped curve, with stronger genetic effects at both short and long sleep durations. Moreover, the interaction between sleep duration and genetic influences unique to depression remained nonsignificant (a'd = 0.01, SD = 0.18, P = 0.96).
DISCUSSION
We found that genetic influences on depressive symptoms were moderated by habitual sleep duration. Both short and long sleep extremes were associated with the highest heritability of depressive symptoms. As sleep duration moved away from the extremes and toward the “normal” range, the effect of the non-shared environment was more strongly associated with depressive symptoms, while genetic factors became less important. These findings show a gene-environment interaction between sleep duration and depressive symptoms.
Although our study does not specify the shared genetic factors that drive this interaction, recent findings suggest candidate genes and pathways. The CLOCK gene encodes a transcription factor that influences both the persistence and period of circadian rhythms and variants in the human CLOCK gene are associated with sleep duration.36–38 A point mutation in DEC2, a gene that regulates both CLOCK and another circadian gene ARNTL, is associated with short habitual sleep duration.39 ARNTL, in turn, encodes a protein that heterodimerizes with CLOCK creating a complex that activates circadian rhythm associated genes including PER1 and is associated with sleep and wake onset times.37 ARNTL, PER2, and NPAS2 form a functional unit in the circadian system and polymorphisms in these genes are associated with seasonal affective disorder.40 The gene ABCC9 encodes an ATP-sensitive potassium channel and a recent GWAS study identified a polymorphism in this gene that explained 5% of the variance in usual sleep duration.41 Variants in ABCC9 have also been found to be associated with depressive symptoms.42 Polymorphisms in 5-hydroxytryptamine transporter linked polymorphic region (5-HTTLPR) are associated with clinical response to sleep deprivation in bipolar depression43 and short sleep with higher depressed mood in young adults.44 Taken together these studies suggest that genes related to circadian rhythms, coupling of cell metabolism to electrical activity, and serotonergic neurotransmission may be central to the gene/environment interaction we report in this study.
Sleep deprivation, either total or partial, has long been considered a treatment option for depression.45–49 Approximately 46% to 70% of depressed patients respond to sleep deprivation, with improvement observed for all signs and symptoms of major depressive disorder.45 However, the therapeutic response is usually temporary, lasting no more than several days, with the vast majority eventually relapsing and some experiencing worsening of their depression.50–52 Further, sleep reduction can provoke manic or hypomanic episodes in predisposed patients.53 At present, there is no convincing mechanism explaining the therapeutic benefits of sleep deprivation, although a number of hypotheses have been proposed including effects on: homeostatic sleep drive,54 cerebral adenosine concentrations,55 and synaptic monoamines—particularly serotonin and dopamine.43,44,56–58 In contrast, the long term effects of habitual short sleep are well-established and include adverse endocrine,59 immune,19,60,61 metabolic,62,63 and functional impairment,64,65 resulting in diabetes,16,66,67 cardiovascular disease,17,21,68–71 obesity,11,72,73 transportation accidents,74–76 and reduced longevity.77 These factors, along with our current findings, raise questions about the value of sleep curtailment as a depression treatment. Alternatively, our study suggests that normalization of sleep duration may reduce genetic risk for depressive symptoms allowing greater influence of environmental factors, such as psychotherapy, on mood. The therapeutic implications of this finding deserve further research.
The “normal” amount of sleep for any individual is age dependent and determined by the amount required to maintain physiological homeostasis and daytime alertness. Studies of humans in environmental isolation suggest 7 to 9 hours per night encompasses normal sleep for the majority of individuals.6,7 This range also includes the amount of sleep considered normal in numerous epidemiological studies assessing the untoward effect of short sleep.10,17,21,77 Because about a third of sleep need is heritable,11 with substantial variability from person to person, the precise amount of sleep needed for optimal health and functioning is best determined by the individual. This would be ascertained by the amount of sleep calculated from bedtime to natural wake time following a period of sleep saturation.
Both insomnia and insufficient sleep represent short sleep, but are physiologically distinct entities. Those with insomnia “can't sleep,” while those with insufficient sleep “won't sleep.” When compared to healthy controls, insomnia patients have higher scores on the multiple sleep latency test,78 an objective measure of sleep-ability where subjects are given four to five opportunities to take a 20-minute nap during the day generating a mean sleep latency score in minutes. This, along with the fact that insomnia patients may misperceive sleep for wakefulness makes insomnia a state of hyperarousal with decreased sleep drive.79,80 In contrast, both acute and chronic sleep deprivation decreases scores on the multiple sleep latency test.81 Therefore, the short sleep of insomnia, a common symptom of depression, is physiologically distinct from the short sleep of sleep deprivation. We did not assess insomnia as a covariate in our analysis, but these distinctions suggest that future studies should assess if insomnia modifies the gene by environment interaction between short sleep and depressive symptoms.
Our study has several limitations. Our twins were predominantly younger adult Caucasian women, and therefore our results should be applied to the general population with caution. In particular, future research should examine the relation between sleep and depressive symptoms in racial/ethnic minorities. It should be noted that our sample was derived from the community and not from a clinical population seeking health-care. Self-reported sleep duration and depressive symptoms are commonly used in observational studies but can be problematic. However, the PHQ-9, from which the mPHQ-9 was derived for this study, is accurate as a screening instrument for depression,82,83 and self-reported sleep duration approximates objective measures of sleep length,84,85 although recent studies suggest it may be biased by overestimation.86 Future studies assessing gene-environment interactions between sleep duration and depression would benefit from direct objective measures or related endophenotype quantification.
In conclusion, this is the first study to demonstrate a gene by environment interaction between habitual sleep duration and depressive symptoms. Both short (< 7 h/night) and long (≥ 9 h/ night) sleep increased the heritability of depressive symptoms, suggesting genetic risk for depressive symptoms increases as twins move away from normal sleep duration (7-8.9 h/night). This works suggests that environmentally mediated treatments for depression may have the greatest opportunity for success when administered in a patient sleeping normal amounts of time. Future research should consider the effects of habitual sleep duration on treatment success.
FOOTNOTE
A. The χ2 statistic tests the discrepancy between the observed data values and the model-expected values, with values not significantly different from zero (P > 0.05) indicating that the model fits the data well. RMSEA (root mean square error of approximation) and CFI (comparative fit index) are alternate indices of model fit, with RMSEA values < 0.06 and CFI values > 0.95, indicating good model fit.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by NIH grants K23HL083350, P30NR011400, and a University of Washington General Clinical Research Center Pilot Grant. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Naismith SL, Rogers NL, Lewis SJ, et al. Sleep disturbance relates to neuropsychological functioning in late-life depression. J Affect Disord. 2011;132:139–45. doi: 10.1016/j.jad.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Troxel WM, Kupfer DJ, Reynolds CF, 3rd, Frank E, Thase ME, Miewald JM, Buysse DJ. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J Clin Psychiatry. 2012;73:478–85. doi: 10.4088/JCP.11m07184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco-Fontecilla H, Alegria AA, Lopez-Castroman J, et al. Short self-reported sleep duration and suicidal behavior: A cross-sectional study. J Affect Disord. 2011;133:239–46. doi: 10.1016/j.jad.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Cho SJ, Cho IH, Kim SJ. Insufficient sleep and suicidality in adolescents. Sleep. 2012;35:455–60. doi: 10.5665/sleep.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitzman ED, Czeisler CA, Zimmerman JC, Ronda JM. Timing of REM and stages 3 + 4 sleep during temporal isolation in man. Sleep. 1980;2:391–407. [PubMed] [Google Scholar]
- 7.Dijk DJ, Duffy JF, Czeisler CA. Age-related increase in awakenings: Impaired consolidation of NREM sleep at all circadian phases. Sleep. 2001;24:565–77. doi: 10.1093/sleep/24.5.565. [DOI] [PubMed] [Google Scholar]
- 8.Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983;6:179–85. doi: 10.1093/sleep/6.3.179. [DOI] [PubMed] [Google Scholar]
- 9.de Castro JM. The influence of heredity on self-reported sleep patterns in free-living humans. Physiol Behav. 2002;76:479–86. doi: 10.1016/s0031-9384(02)00699-6. [DOI] [PubMed] [Google Scholar]
- 10.Watson NF, Buchwald D, Vitiello MV, Noonan C, Goldberg J. A twin study of sleep duration and body mass index. J Clin Sleep Med. 2010;6:11–7. [PMC free article] [PubMed] [Google Scholar]
- 11.Watson NF, Harden KP, Buchwald D, et al. Sleep duration and body mass index in twins: A gene-environment interaction. Sleep. 2012;35:597–603. doi: 10.5665/sleep.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basner M, Fomberstein KM, Razavi FM, et al. American time use survey: Sleep time and its relationship to waking activities. Sleep. 2007;30:1085–95. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webb WB, Agnew HW. Are we chronically sleep deprived? Bull Psychonomic Soc. 1975;6:47–8. [Google Scholar]
- 14.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. Sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Short sleep duration among workers - United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:281–96. [PubMed] [Google Scholar]
- 16.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 17.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 18.Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 19.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 20.De Jonghe S, Van Overmeire I, Poulton S, et al. Structure-activity relationship of short-chain sphingoid bases as inhibitors of sphingosine kinase. Bioorg Med Chem Lett. 1999;9:3175–80. doi: 10.1016/s0960-894x(99)00554-5. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: The sleep heart health study. Sleep. 2006;29:1009–14. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 22.Watson NF. Stroke and sleep specialists: An opportunity to intervene? J Clin Sleep Med. 2010;6:138–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Familial influences on the clinical characteristics of major depression: A twin study. Acta Psychiatr Scand. 1992;86:371–8. doi: 10.1111/j.1600-0447.1992.tb03283.x. [DOI] [PubMed] [Google Scholar]
- 24.Watson NF, Goldberg J, Arguelles L, Buchwald D. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–9. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 25.Sandler M. Multiple facets of the heritability of major depressive disorder. Mind Matters: The Wesleyan Journal of Psychology. 2007;2:57–69. [Google Scholar]
- 26.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28:225–6. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 27.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the vietnam era twin registry: An approach using questionnaires. Clin Genet. 1989;35:423–32. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 28.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: A population-based study. J Clin Sleep Med. 2005;1:357–63. [PubMed] [Google Scholar]
- 30.Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Arch Intern Med. 2006;166:1689–92. doi: 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]
- 31.Neale MC, Maes HHM. Methodology for genetics studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic; 2004. [Google Scholar]
- 32.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–71. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- 33.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 34.Nolen-Hoeksema S. Gender differences in depression. Curr Dir Psychol Sci. 2001;10:173–6. [Google Scholar]
- 35.Yang Y. Is old age depressing? Growth trajectories and cohort variations in late-life depression. J Health Soc Behav. 2007;48:16–32. doi: 10.1177/002214650704800102. [DOI] [PubMed] [Google Scholar]
- 36.Allebrandt KV, Teder-Laving M, Akyol M, et al. Clock gene variants associate with sleep duration in two independent populations. Biol Psychiatry. 2010;67:1040–7. doi: 10.1016/j.biopsych.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 37.Evans DS, Parimi N, Nievergelt CM, et al. Common genetic variants in arntl and npas2 and at chromosome 12p13 are associated with objectively measured sleep traits in the elderly. Sleep. 2013;36:431–46. doi: 10.5665/sleep.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landgraf D, Shostak A, Oster H. Clock genes and sleep. Pflugers Arch. 2012;463:3–14. doi: 10.1007/s00424-011-1003-9. [DOI] [PubMed] [Google Scholar]
- 39.He Y, Jones CR, Fujiki N, et al. The transcriptional repressor dec2 regulates sleep length in mammals. Science. 2009;325:866–70. doi: 10.1126/science.1174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partonen T, Treutlein J, Alpman A, et al. Three circadian clock genes per2, arntl, and npas2 contribute to winter depression. Ann Med. 2007;39:229–38. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- 41.Allebrandt KV, Amin N, Muller-Myhsok B, et al. A k(atp) channel gene effect on sleep duration: From genome-wide association studies to function in drosophila. Mol Psychiatry. 2013;18:122–32. doi: 10.1038/mp.2011.142. [DOI] [PubMed] [Google Scholar]
- 42.Parsons MJ, Lester KJ, Barclay NL, Nolan PM, Eley TC, Gregory AM. Replication of genome-wide association studies (gwas) loci for sleep in the British g1219 cohort. Am J Med Genet B Neuropsychiatr Genet. 2013;162:431–8. doi: 10.1002/ajmg.b.32106. [DOI] [PubMed] [Google Scholar]
- 43.Benedetti F, Serretti A, Colombo C, et al. Influence of a functional polymorphism within the promoter of the serotonin transporter gene on the effects of total sleep deprivation in bipolar depression. Am J Psychiatry. 1999;156:1450–2. doi: 10.1176/ajp.156.9.1450. [DOI] [PubMed] [Google Scholar]
- 44.Carskadon MA, Sharkey KM, Knopik VS, McGeary JE. Short sleep as an environmental exposure: A preliminary study associating 5-httlpr genotype to self-reported sleep duration and depressed mood in first-year university students. Sleep. 2012;35:791–6. doi: 10.5665/sleep.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med Rev. 2002;6:361–77. [PubMed] [Google Scholar]
- 46.Leibenluft E, Wehr TA. Is sleep deprivation useful in the treatment of depression? Am J Psychiatry. 1992;149:159–68. doi: 10.1176/ajp.149.2.159. [DOI] [PubMed] [Google Scholar]
- 47.Papadimitriou GN, Christodoulou GN, Katsouyanni K, Stefanis CN. Therapy and prevention of affective illness by total sleep deprivation. J Affect Disord. 1993;27:107–16. doi: 10.1016/0165-0327(93)90083-v. [DOI] [PubMed] [Google Scholar]
- 48.Pflug B, Tolle R. Therapie endogener depressionen durch schlafentzug. Nervenartz. 1971;42:117–24. [PubMed] [Google Scholar]
- 49.Schulte W. Kombinierte psycho- und pharmakotherapie bei melancholikern. Basel: Karger; 1966. [Google Scholar]
- 50.Fahndrich E. Effects of sleep deprivation on depressed patients of different nosological groups. Psychiatry Res. 1981;5:277–85. doi: 10.1016/0165-1781(81)90074-3. [DOI] [PubMed] [Google Scholar]
- 51.Giedke H, Geilenkirchen R, Hauser M. The timing of partial sleep deprivation in depression. J Affect Disord. 1992;25:117–28. doi: 10.1016/0165-0327(92)90074-g. [DOI] [PubMed] [Google Scholar]
- 52.Gordijn M, Beersma D, Bouhuys N, Korte H, van den Hoofdakker R. A longitudinal study of sleep deprivation responses in depression; the variability is highly related to diurnal mood variability. Acta Neuropsychiatr. 1995;7:58–60. doi: 10.1017/S0924270800037583. [DOI] [PubMed] [Google Scholar]
- 53.Wehr TA. Effects of wakefulness and sleep on depression and mania. Oxford: Oxford University Press; 1990. [Google Scholar]
- 54.Endo T, Schwierin B, Borbely AA, Tobler I. Selective and total sleep deprivation: Effect on the sleep EEG in the rat. Psychiatry Res. 1997;66:97–110. doi: 10.1016/s0165-1781(96)03029-6. [DOI] [PubMed] [Google Scholar]
- 55.Demet EM, Chicz-Demet A, Fallon JH, Sokolski KN. Sleep deprivation therapy in depressive illness and parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:753–84. doi: 10.1016/s0278-5846(99)00039-1. [DOI] [PubMed] [Google Scholar]
- 56.Ebert D, Berger M. Neurobiological similarities in antidepressant sleep deprivation and psychostimulant use: A psychostimulant theory of antidepressant sleep deprivation. Psychopharmacology (Berl) 1998;140:1–10. doi: 10.1007/s002130050732. [DOI] [PubMed] [Google Scholar]
- 57.Neumeister A, Praschak-Rieder N, Hesselmann B, et al. Effects of tryptophan depletion in drug-free depressed patients who responded to total sleep deprivation. Arch Gen Psychiatry. 1998;55:167–72. doi: 10.1001/archpsyc.55.2.167. [DOI] [PubMed] [Google Scholar]
- 58.Smeraldi E, Benedetti F, Barbini B, Campori E, Colombo C. Sustained antidepressant effect of sleep deprivation combined with pindolol in bipolar depression. A placebo-controlled trial. Neuropsychopharmacology. 1999;20:380–5. doi: 10.1016/S0893-133X(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 59.Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 5:31–43. doi: 10.2174/1874306401105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faraut B, Boudjeltia KZ, Vanhamme L, Kerkhofs M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med Rev. 2012;16:137–49. doi: 10.1016/j.smrv.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Miller MA. Association of inflammatory markers with cardiovascular risk and sleepiness. J Clin Sleep Med. 2011;7:S31–3. doi: 10.5664/JCSM.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klingenberg L, Sjodin A, Holmback U, Astrup A, Chaput JP. Short sleep duration and its association with energy metabolism. Obes Rev. 2012;13:565–77. doi: 10.1111/j.1467-789X.2012.00991.x. [DOI] [PubMed] [Google Scholar]
- 63.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sallinen M, Harma M, Akila R, et al. The effects of sleep debt and monotonous work on sleepiness and performance during a 12-h dayshift. J Sleep Res. 2004;13:285–94. doi: 10.1111/j.1365-2869.2004.00425.x. [DOI] [PubMed] [Google Scholar]
- 65.Tomasi D, Wang RL, Telang F, et al. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex. 2009;19:233–40. doi: 10.1093/cercor/bhn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Padilha HG, Crispim CA, Zimberg IZ, et al. A link between sleep loss, glucose metabolism and adipokines. Braz J Med Biol Res. 2011;44:992–9. doi: 10.1590/s0100-879x2011007500113. [DOI] [PubMed] [Google Scholar]
- 67.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–7. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 68.Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among us adults varies by age and sex. Am J Hypertens. 2012;25:335–41. doi: 10.1038/ajh.2011.201. [DOI] [PubMed] [Google Scholar]
- 69.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 70.Eguchi K, Hoshide S, Ishikawa S, Shimada K, Kario K. Short sleep duration is an independent predictor of stroke events in elderly hypertensive patients. J Am Soc Hypertens. 2010;4:255–62. doi: 10.1016/j.jash.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 71.Eguchi K, Pickering TG, Schwartz JE, et al. Short sleep duration as an independent predictor of cardiovascular events in japanese patients with hypertension. Arch Intern Med. 2008;168:2225–31. doi: 10.1001/archinte.168.20.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel SR. Reduced sleep as an obesity risk factor. Obes Rev. 2009;10(Suppl 2):61–8. doi: 10.1111/j.1467-789X.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 73.Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: A randomised controlled trial. Crit Care. 2010;14:R151. doi: 10.1186/cc9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.National Transportation Safety Board. Railroad accident report. Collision of two Canadian National/Illinois Central railway trains near Clarkston, Michigan, November 15, 2001. http://www.ntsb.gov/doclib/reports/2002/rar0204.pdf.
- 75.National Transportation Safety Board. Aircraft accident report: Crash during attempted go-around after landing east coast jets flight 81 Hawker Beechcraft Corporation 125-800A, N818MV Owatonna, Minnesota, July 31, 2008. http://www.ntsb.gov/doclib/reports/2011/aar-11-01.pdf.
- 76.National Transportation Safety Board. Highway accident report: Truck-tractor semitrailer rollover and motorcoach collision with overturned truck, interstate highway 94 near Osseo, Wisconsin, October 16, 2005. http://www.ntsb.gov/doclib/reports/2008/har0802.pdf.
- 77.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 78.Huang L, Zhou J, Li Z, Lei F, Tang X. Sleep perception and the multiple sleep latency test in patients with primary insomnia. J Sleep Res. 2012;21:684–92. doi: 10.1111/j.1365-2869.2012.01028.x. [DOI] [PubMed] [Google Scholar]
- 79.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: Stability and relation to nocturnal sleep. Sleep. 2011;34:1647–52. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Philip P, Sagaspe P, Prague M, et al. Acute versus chronic partial sleep deprivation in middle-aged people: Differential effect on performance and sleepiness. Sleep. 2012;35:997–1002. doi: 10.5665/sleep.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borner I, Braunstein JW, St Victor R, Pollack J. Evaluation of a 2-question screening tool for detecting depression in adolescents in primary care. Clin Pediatr (Phila) 2010;49:947–53. doi: 10.1177/0009922810370203. [DOI] [PubMed] [Google Scholar]
- 83.Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: Validity of a two-item depression screener. Med Care. 2003;41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 84.Hauri PJ, Wisbey J. Wrist actigraphy in insomnia. Sleep. 1992;15:293–301. doi: 10.1093/sleep/15.4.293. [DOI] [PubMed] [Google Scholar]
- 85.Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 86.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: How similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]


