Abstract
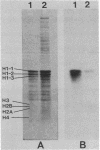
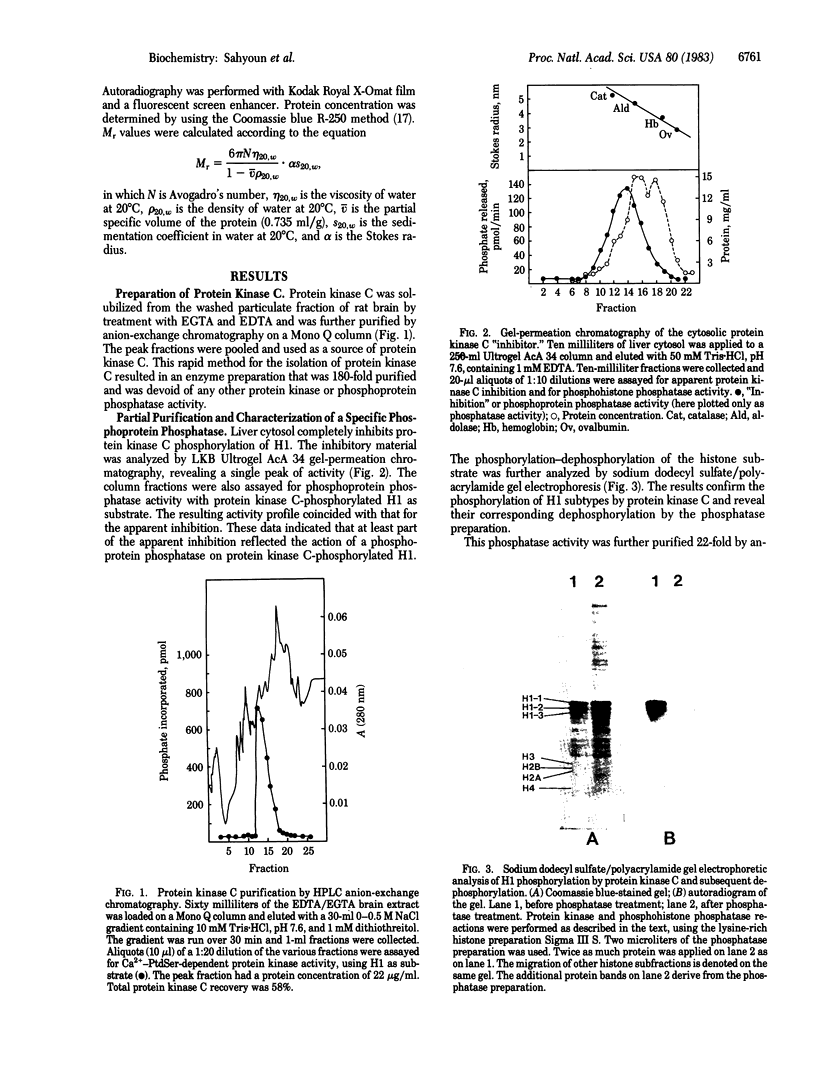
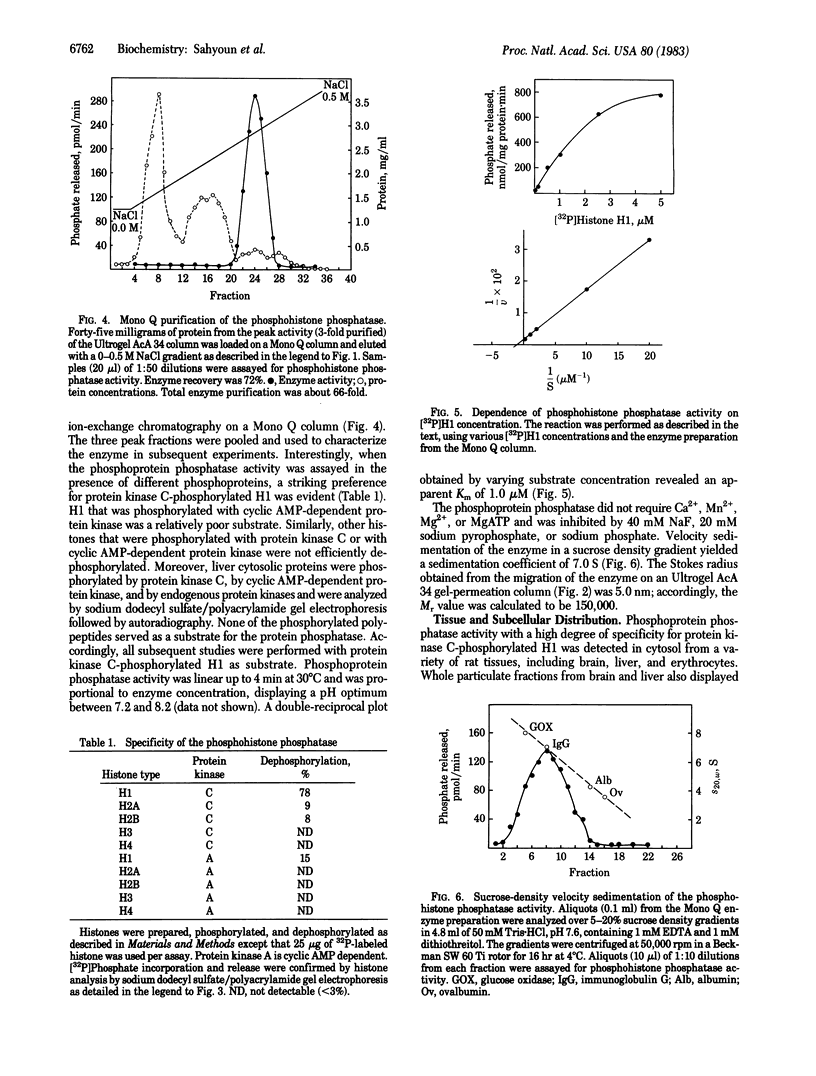
A phosphohistone phosphatase from rat liver cytosol acts specifically on histone H1 that is phosphorylated with the Ca2+-phospholipid-dependent protein kinase (protein kinase C). The apparent Km for 32P-labeled H1 is 1 microM; other histones or cytosolic proteins phosphorylated with protein kinase C or with cyclic AMP-dependent protein kinase are poor substrates for the phosphatase. The enzyme has been partially purified by gel-permeation chromatography and by utilizing a high-performance liquid chromatography ion-exchange column. The physical properties of this enzyme include a Stokes radius of 5.0 nm, a sedimentation coefficient (s20,w) of 7.0 S, and a Mr of 150,000. The detection of protein kinase as well as the specific phosphohistone phosphatase in purified rat liver nuclei suggests a physiologic role for a histone H1 phosphorylation-dephosphorylation cycle mediated by protein kinase C and the corresponding phosphohistone phosphatase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson N. N., Jr, Touster O. Isolation of rat liver plasma membrane fragments in isotonic sucrose. Methods Enzymol. 1974;31:90–102. doi: 10.1016/0076-6879(74)31009-9. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Pollard H. B. The presence of F3-F2a1 dimers and F1 oligomers in chromatin. Biochem Biophys Res Commun. 1975 May 5;64(1):282–288. doi: 10.1016/0006-291x(75)90250-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Dolby T. W., Belmont A., Borun T. W., Nicolini C. DNA replication, chromatin structure, and histone phosphorylation altered by theophylline in synchronized HeLa S3 cells. J Cell Biol. 1981 Apr;89(1):78–85. doi: 10.1083/jcb.89.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst V., Levin D. H., Foulkes J. G., London I. M. Effects of skeletal muscle protein phosphatase inhibitor-2 on protein synthesis and protein phosphorylation in rabbit reticulocyte lysates. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7092–7096. doi: 10.1073/pnas.79.23.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes J. G., Ernst V., Levin D. H. Separation and identification of type 1 and type 2 protein phosphatases from rabbit reticulocyte lysates. J Biol Chem. 1983 Feb 10;258(3):1439–1443. [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Harrison J. J., Schwoch G., Schweppe J. S., Jungmann R. A. Phosphorylative modification of histone H1 subspecies following isoproterenol and N6,O2'-dibutyryl cyclic AMP stimulation of rat C6 glioma cells. J Biol Chem. 1982 Nov 25;257(22):13602–13609. [PubMed] [Google Scholar]
- Harrison J. J., Suter P., Suter S., Jungmann R. A. Isoproterenol-induced selective phosphorylative modification in vivo of rat C6 glioma cell histones. Biochem Biophys Res Commun. 1980 Oct 16;96(3):1253–1260. doi: 10.1016/0006-291x(80)90086-8. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Resink T. J., Cohen P. Reconstitution of a Mg-ATP-dependent protein phosphatase and its activation through a phosphorylation mechanism. FEBS Lett. 1982 Dec 27;150(2):319–324. doi: 10.1016/0014-5793(82)80760-6. [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Takai Y., Kikkawa U., Nishizuka Y. Phosphorylation of calf thymus H1 histone by calcium-activated, phospholipid-dependent protein kinase. Biochem Biophys Res Commun. 1980 Sep 16;96(1):180–187. doi: 10.1016/0006-291x(80)91198-5. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Laks M. S., Harrison J. J., Schwoch G., Jungmann R. A. Modulation of nuclear protein kinase activity and phosphorylation of histone H1 subspecies during the prereplicative phase of rat liver regeneration. J Biol Chem. 1981 Aug 25;256(16):8775–8785. [PubMed] [Google Scholar]
- Langan T. A. Characterization of highly phosphorylated subcomponents of rat thymus H1 histone. J Biol Chem. 1982 Dec 25;257(24):14835–14846. [PubMed] [Google Scholar]
- Link R., Marks F. Histone phosphorylation in phorbol ester stimulated and beta-adrenergically stimulated mouse epidermis in vivo and characterization of an epidermal protein phosphorylation system. Biochim Biophys Acta. 1981 Jul;675(2):265–275. doi: 10.1016/0304-4165(81)90236-1. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D., Sommer K. R., Panyim S., Spiker S., Chalkley R. A modified procedure for fractionating histones. Biochem J. 1972 Sep;129(2):349–353. doi: 10.1042/bj1290349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Ruch P. A., Morris H. P., Jacob S. T. RNA polymerases from a rat hepatoma. Partial purification and comparison of properties with corresponding liver enzymes. Biochim Biophys Acta. 1976 Apr 15;432(1):60–72. doi: 10.1016/0005-2787(76)90041-1. [DOI] [PubMed] [Google Scholar]
- Sperling R., Wachtel E. J. The histones. Adv Protein Chem. 1981;34:1–60. doi: 10.1016/s0065-3233(08)60517-3. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Iwasa Y., Kawahara Y., Mori T., Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979 May 25;254(10):3692–3695. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kawahara Y., Minakuchi R., Sano K., Kikkawa U., Mori T., Yu B., Kaibuchi K., Nishizuka Y. Calcium and phosphatidylinositol turnover as signalling for transmembrane control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1981;14:301–313. [PubMed] [Google Scholar]
- Takai Y., Kishimoto A., Kikkawa U., Mori T., Nishizuka Y. Unsaturated diacylglycerol as a possible messenger for the activation of calcium-activated, phospholipid-dependent protein kinase system. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1218–1224. doi: 10.1016/0006-291x(79)91197-5. [DOI] [PubMed] [Google Scholar]
- Tamura S., Kikuchi H., Kikuchi K., Hiraga A., Tsuiki S. Purification and subunit structure of a high-molecular-weight phosphoprotein phosphatase (phosphatase II) from rat liver. Eur J Biochem. 1980 Mar;104(2):347–355. doi: 10.1111/j.1432-1033.1980.tb04435.x. [DOI] [PubMed] [Google Scholar]
- Tamura S., Kikuchi K., Hiraga A., Kikuchi H., Hosokawa M., Tsuiki S. Characterization of multiple forms of histone phosphatase in rat liver. Biochim Biophys Acta. 1978 Jun 9;524(2):349–356. doi: 10.1016/0005-2744(78)90171-7. [DOI] [PubMed] [Google Scholar]
- Tamura S., Tsuiki S. Purification and subunit structure of rat-liver phosphoprotein phosphatase, whose molecular weight is 260000 by gel filtration (phosphatase IB). Eur J Biochem. 1980 Oct;111(1):217–224. doi: 10.1111/j.1432-1033.1980.tb06096.x. [DOI] [PubMed] [Google Scholar]
- Wattiaux R., Wattiaux-De Coninck S., Ronveaux-dupal M. F., Dubois F. Isolation of rat liver lysosomes by isopycnic centrifugation in a metrizamide gradient. J Cell Biol. 1978 Aug;78(2):349–368. doi: 10.1083/jcb.78.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise B. C., Glass D. B., Chou C. H., Raynor R. L., Katoh N., Schatzman R. C., Turner R. S., Kibler R. F., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. II. Substrate specificity and inhibition by various agents. J Biol Chem. 1982 Jul 25;257(14):8489–8495. [PubMed] [Google Scholar]
- Wise B. C., Raynor R. L., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase from heart. I. Purification and general properties. J Biol Chem. 1982 Jul 25;257(14):8481–8488. [PubMed] [Google Scholar]