Abstract
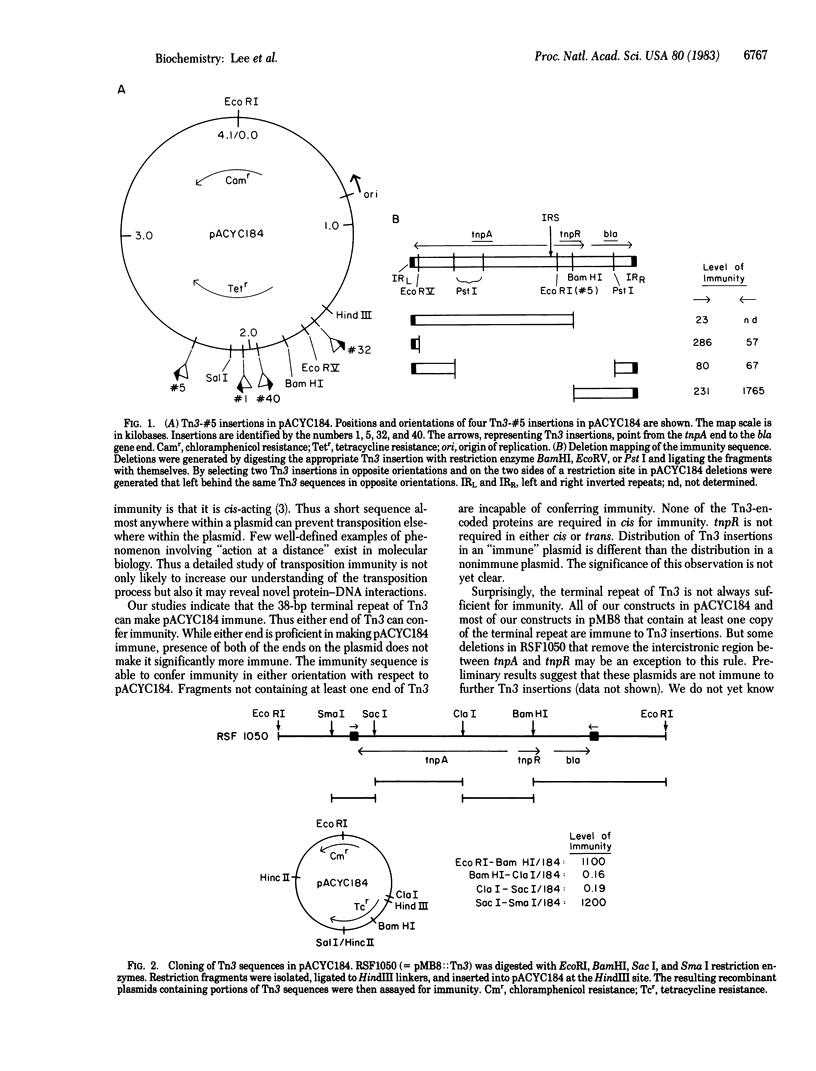
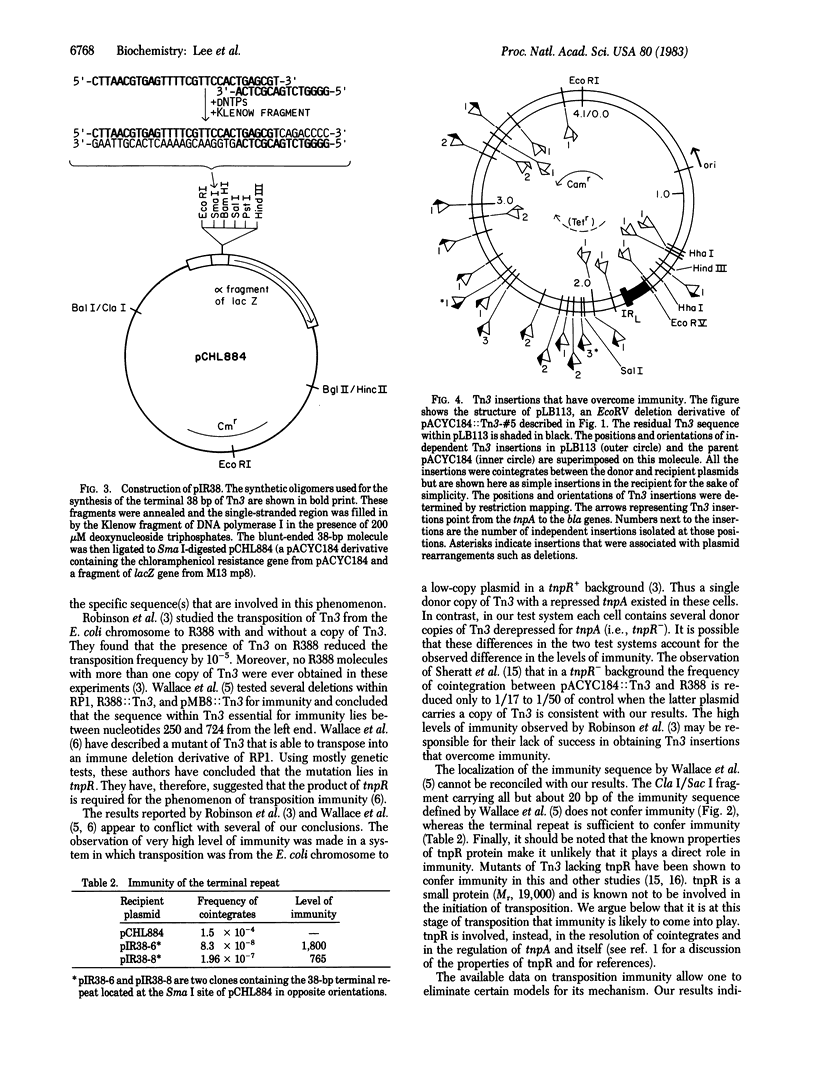
A plasmid containing transposon Tn3 is immune to further insertions of Tn3. This phenomenon works in cis and is referred to as transposition immunity. We have used the ability of Tn3 to form cointegrates between two plasmids to develop a quantitative assay to detect transposition immunity. Presence of Tn3 on both the plasmids reduces the cointegration frequency to less than 1/100 of parental. Using this assay, we have determined that (i) tnpR is not required for immunity, (ii) only the terminal 38 base pairs of Tn3 need be present to confer immunity, and (iii) other parts of Tn3 appear not to confer immunity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Bennett P. M., Higginson S., Richmond M. H. Regional preference of insertion of Tn501 and Tn802 into RP1 and its derivatives. Mol Gen Genet. 1978 Nov 9;166(3):313–320. doi: 10.1007/BF00267624. [DOI] [PubMed] [Google Scholar]
- Grinsted J., de la Cruz F., Altenbuchner J., Schmitt R. Complementation of transposition of tnpA mutants of Tn3, Tn21, Tn501, and Tn1721. Plasmid. 1982 Nov;8(3):276–286. doi: 10.1016/0147-619x(82)90065-8. [DOI] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M., Wishart W., Ohtsubo H., Heffron F., Ohtsubo E. Plasmid cointegrates and their resolution mediated by transposon Tn3 mutants. Gene. 1981 Nov;15(2-3):103–118. doi: 10.1016/0378-1119(81)90120-7. [DOI] [PubMed] [Google Scholar]
- Muster C. J., MacHattie L. A., Shapiro J. A. p lambda CM system: observations on the roles of transposable elements in formation and breakdown of plasmids derived from bacteriophage lambda replicons. J Bacteriol. 1983 Feb;153(2):976–990. doi: 10.1128/jb.153.2.976-990.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo H., Ohmori H., Ohtsubo E. Nucleotide-sequence analysis of Tn3 (ap): implications for insertion and deletion. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1269–1277. doi: 10.1101/sqb.1979.043.01.144. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Grinsted J., Richmond M. H. The stable carriage of two TnA units on a single replicon. Mol Gen Genet. 1978 Apr 17;160(3):339–346. doi: 10.1007/BF00332978. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Richmond M. H. Inhibition of TnA translocation by TnA. J Bacteriol. 1977 Jan;129(1):407–414. doi: 10.1128/jb.129.1.407-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R., Altenbuchner J., Wiebauer K., Arnold W., Pühler A., Schöffl F. Basis of transposition and gene amplification by Tn1721 and related tetracycline-resistance transposons. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):59–65. doi: 10.1101/sqb.1981.045.01.011. [DOI] [PubMed] [Google Scholar]
- Sherratt D., Arthur A., Burke M. Transposon-specified, site-specific recombination systems. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):275–281. doi: 10.1101/sqb.1981.045.01.040. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. Translocation specificity of the Tn3 element: characterization of sites of multiple insertions. Cell. 1980 Jan;19(1):151–160. doi: 10.1016/0092-8674(80)90396-7. [DOI] [PubMed] [Google Scholar]
- Wallace L. J., Ward J. M., Richmond M. H. The location of sequences of TnA required for the establishment of transposition immunity. Mol Gen Genet. 1981;184(1):80–86. doi: 10.1007/BF00271199. [DOI] [PubMed] [Google Scholar]
- Wallace L. J., Ward J. M., Richmond M. H. The tnpR gene product of TnA is required for transposition immunity. Mol Gen Genet. 1981;184(1):87–91. doi: 10.1007/BF00271200. [DOI] [PubMed] [Google Scholar]


