Abstract
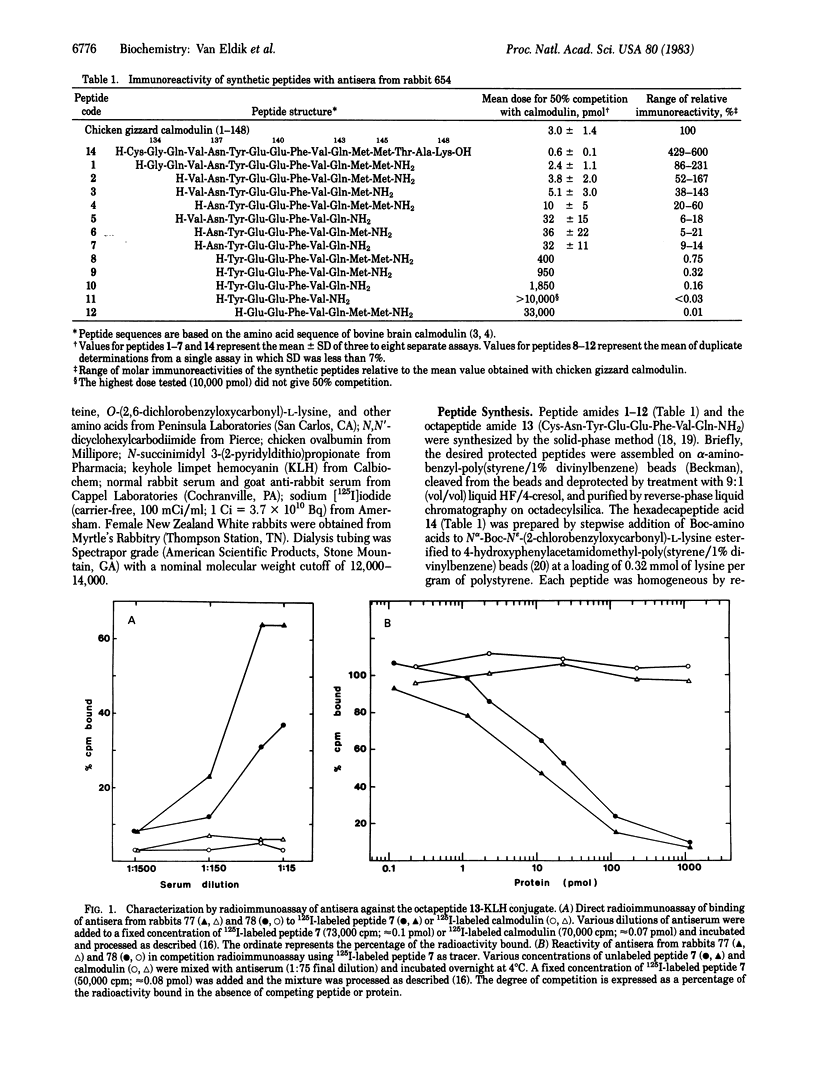
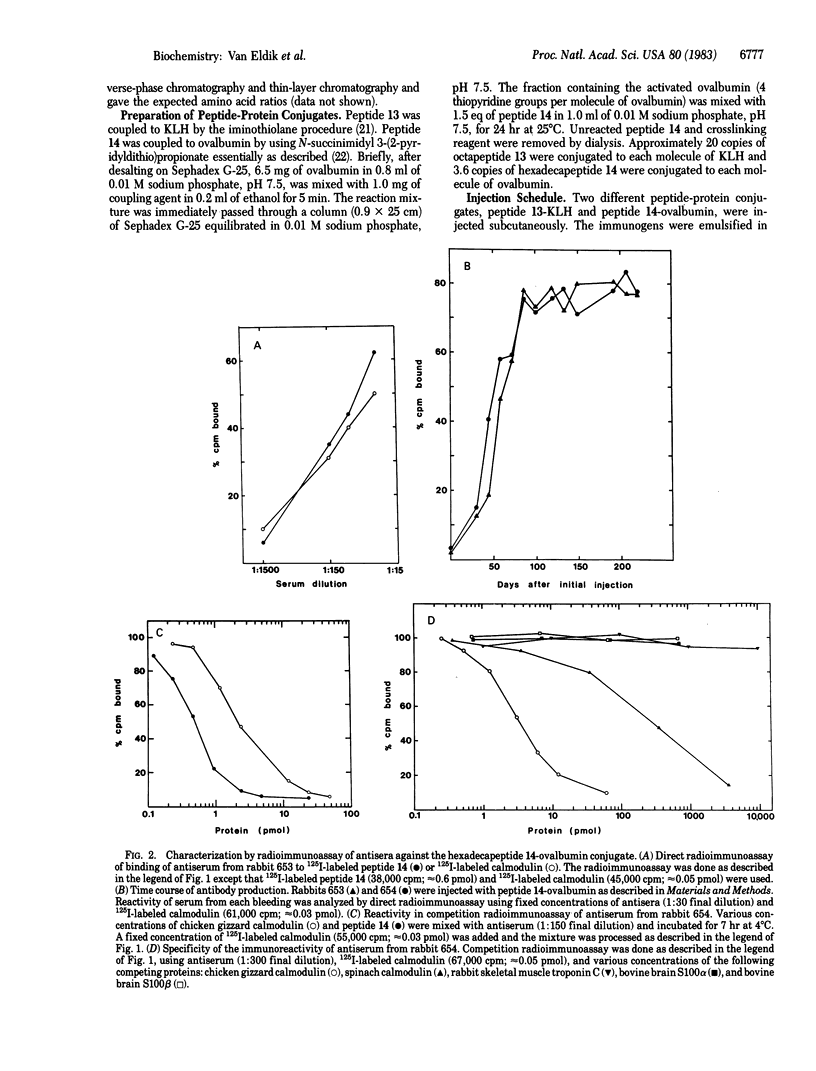
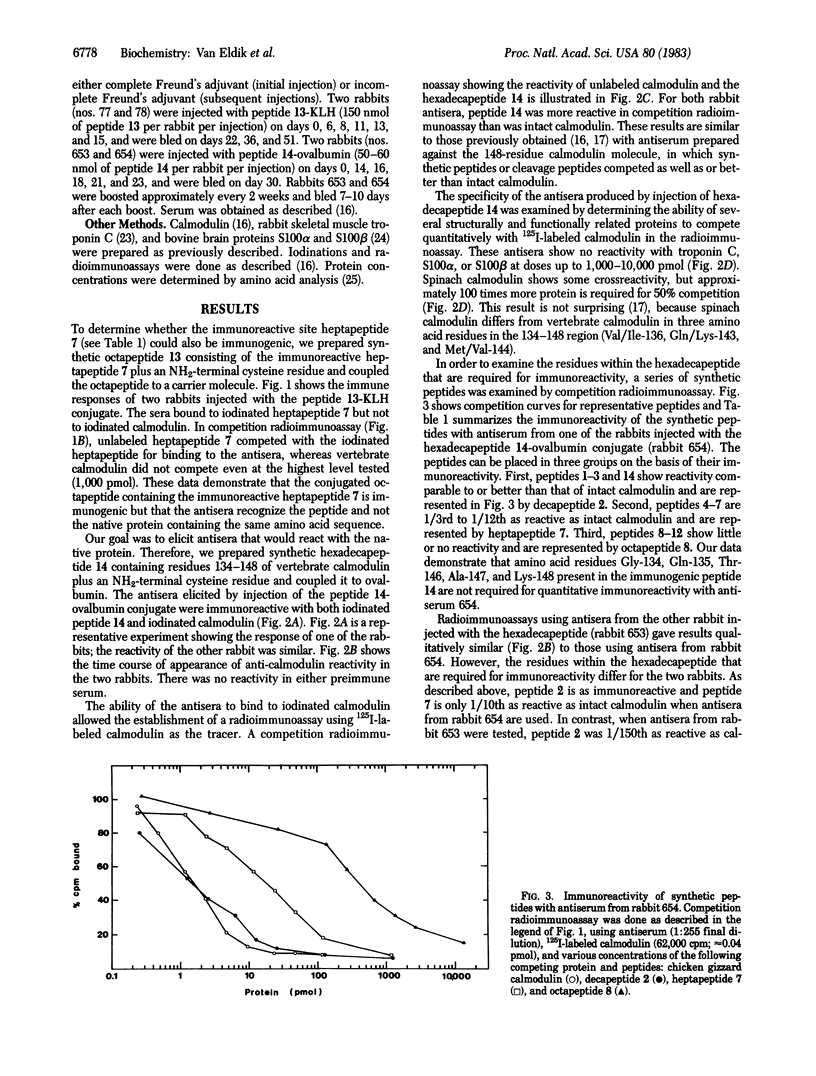
Site-directed antisera against vertebrate calmodulin were elicited in rabbits by injection of a synthetic immunogen containing the pentadecapeptide Gly-Gln-Val-Asn-Tyr-Glu-Glu-Phe-Val-Gln-Met-Met-Thr-Ala-Lys-OH, which corresponds to residues 134-148 of vertebrate calmodulin. A major immunoreactive region (residues 127-144) of calmodulin is found in the COOH-terminal structural domain and an immunoreactive site for one antiserum is contained in the heptapeptide Asn-Tyr-Glu-Glu-Phe-Val-Gln-NH2, which corresponds to residues 137-143 of vertebrate calmodulin. This immunoreactive heptapeptide was conjugated to a carrier protein by adding a cysteine residue to the NH2 terminus of the peptide and coupling the Cys-heptapeptide to the carrier through the thiol group of the cysteine residue. Injection of this Cys-heptapeptide-protein conjugate into rabbits yielded antisera that react with the heptapeptide but not with native calmodulin. Thus, the immunoreactive heptapeptide that is exposed on the surface of calmodulin is immunogenic, but it is not sufficient to elicit antibodies that react with native calmodulin. However, when the Cys-pentadecapeptide corresponding to residues 134-148 and containing the immunoreactive heptapeptide sequence was conjugated to a carrier protein and injected into rabbits, antisera were elicited that react with the intact calmodulin molecule. The affinities and specificities of these antisera for calmodulin are similar to those of antisera elicited by injection of the intact protein and are sufficient for their use in radioimmunoassays. These results indicate that the successful engineering of site-directed antisera against proteins by using synthetic peptide immunogens may require an appropriate intramolecular environment that allows the peptide region to closely approximate the spatial orientation it adopts in the intact protein.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess W. H. Characterization of calmodulin and calmodulin isotypes from sea urchin gametes. J Biol Chem. 1982 Feb 25;257(4):1800–1804. [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedman J. R., Jackson R. L., Schreiber W. E., Means A. R. Sequence homology of the Ca2+-dependent regulator of cyclic nucleotide phosphodiesterase from rat testis with other Ca2+-binding proteins. J Biol Chem. 1978 Jan 25;253(2):343–346. [PubMed] [Google Scholar]
- Grand R. J., Shenolikar S., Cohen P. The amino acid sequence of the delta subunit (calmodulin) of rabbit skeletal muscle phosphorylase kinase. Eur J Biochem. 1981 Jan;113(2):359–367. doi: 10.1111/j.1432-1033.1981.tb05074.x. [DOI] [PubMed] [Google Scholar]
- King T. P., Li Y., Kochoumian L. Preparation of protein conjugates via intermolecular disulfide bond formation. Biochemistry. 1978 Apr 18;17(8):1499–1506. doi: 10.1021/bi00601a022. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Lerner R. A. Tapping the immunological repertoire to produce antibodies of predetermined specificity. Nature. 1982 Oct 14;299(5884):593–596. doi: 10.1038/299592a0. [DOI] [PubMed] [Google Scholar]
- Marshak D. R., Watterson D. M., Van Eldik L. J. Calcium-dependent interaction of S100b, troponin C, and calmodulin with an immobilized phenothiazine. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6793–6797. doi: 10.1073/pnas.78.11.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. R., Erickson B. W., Ryabtsev M. N., Hodges R. S., Merrifield R. B. Tert-butoxycarbonylaminoacyl-4-(oxymethyl)-phenylacetamidomethyl-resin, a more acid-resistant support for solid-phase peptide synthesis. J Am Chem Soc. 1976 Nov 10;98(23):7357–7362. doi: 10.1021/ja00439a041. [DOI] [PubMed] [Google Scholar]
- Ohga K., Incefy G. S., Fok K. F., Erickson B. W., Good R. A. Radioimmunoassays for the thymic hormone serum thymic factor (FTS). J Immunol Methods. 1983 Feb 25;57(1-3):171–184. doi: 10.1016/0022-1759(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa T., Ericsson L. H., Walsh K. A., Schreiber W. E., Fischer E. H., Titani K. Complete amino acid sequence of human brain calmodulin. Biochemistry. 1982 May 11;21(10):2565–2569. doi: 10.1021/bi00539a041. [DOI] [PubMed] [Google Scholar]
- Toda H., Yazawa M., Kondo K., Honma T., Narita K., Yagi K. Amino acid sequence of calmodulin from scallop (Patinopecten) adductor muscle. J Biochem. 1981 Nov;90(5):1493–1505. doi: 10.1093/oxfordjournals.jbchem.a133616. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Characterization of a calcium-modulated protein from transformed chicken fibroblasts. J Biol Chem. 1979 Oct 25;254(20):10250–10255. [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M., Fok K. F., Erickson B. W. Elucidation of a minimal immunoreactive site of vertebrate calmodulin. Arch Biochem Biophys. 1983 Dec;227(2):522–533. doi: 10.1016/0003-9861(83)90481-2. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Reproducible production of antiserum against vertebrate calmodulin and determination of the immunoreactive site. J Biol Chem. 1981 May 10;256(9):4205–4210. [PubMed] [Google Scholar]
- Watterson D. M., Burgess W. H., Lukas T. J., Iverson D., Marshak D. R., Schleicher M., Erickson B. W., Fok K. F., Van Eldik L. J. Towards a molecular and atomic anatomy of calmodulin and calmodulin-binding proteins. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:205–226. [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- Yazawa M., Yagi K. The amino acid sequence of the calmodulin obtained from sea anemone (metridium senile) muscle. Biochem Biophys Res Commun. 1980 Sep 16;96(1):377–381. doi: 10.1016/0006-291x(80)91225-5. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Yagi K., Toda H., Kondo K., Narita K., Yamazaki R., Sobue K., Kakiuchi S., Nagao S., Nozawa Y. The amino acid sequence of the Tetrahymena calmodulin which specifically interacts with guanylate cyclase. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1051–1057. doi: 10.1016/0006-291x(81)90725-7. [DOI] [PubMed] [Google Scholar]


