Abstract
The mechanism of contrast enhancement of tumors using magnetic resonance imaging was investigated in MCF7 human breast cancer implanted in nude mice. Dynamic contrast-enhanced images recorded at high spatial resolution were analyzed by an image analysis method based on a physiological model, which included the blood circulation, the tumor, the remaining tissues, and clearance via the kidneys. This analysis enabled us to map in rapidly enhancing regions within the tumor, the capillary permeability factor (capillary permeability times surface area per voxel volume) and the fraction of leakage space. Correlation of these maps with T2-weighted spin echo images, with histopathology, and with immunohistochemical staining of endothelial cells demonstrated the presence of dense permeable microcapillaries in the tumor periphery and in intratumoral regions that surrounded necrotic loci. The high leakage from the intratumoral permeable capillaries indicated an induction of a specific angiogenic process associated with stress conditions that cause necrosis. This induction was augmented in tumors responding to tamoxifen treatment. Determination of the distribution and extent of this stress-induced angiogenic activity by contrast-enhanced MRI might be of diagnostic and of prognostic value.
Full text
PDF
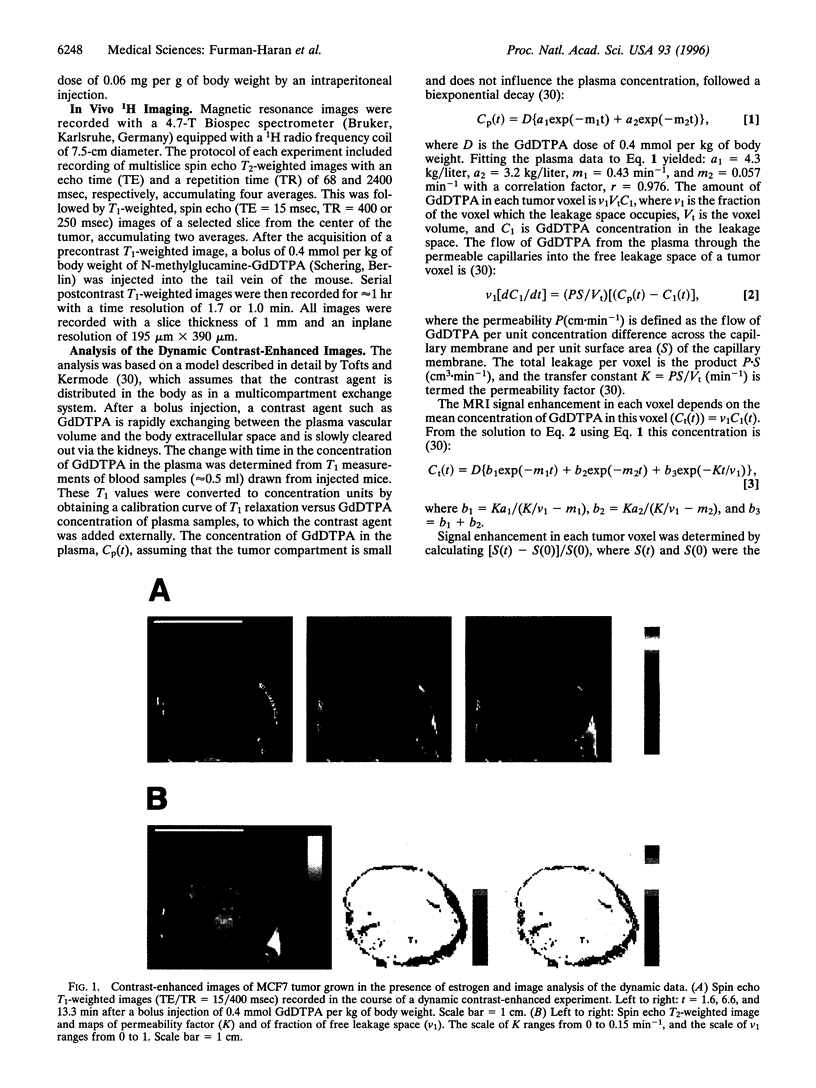
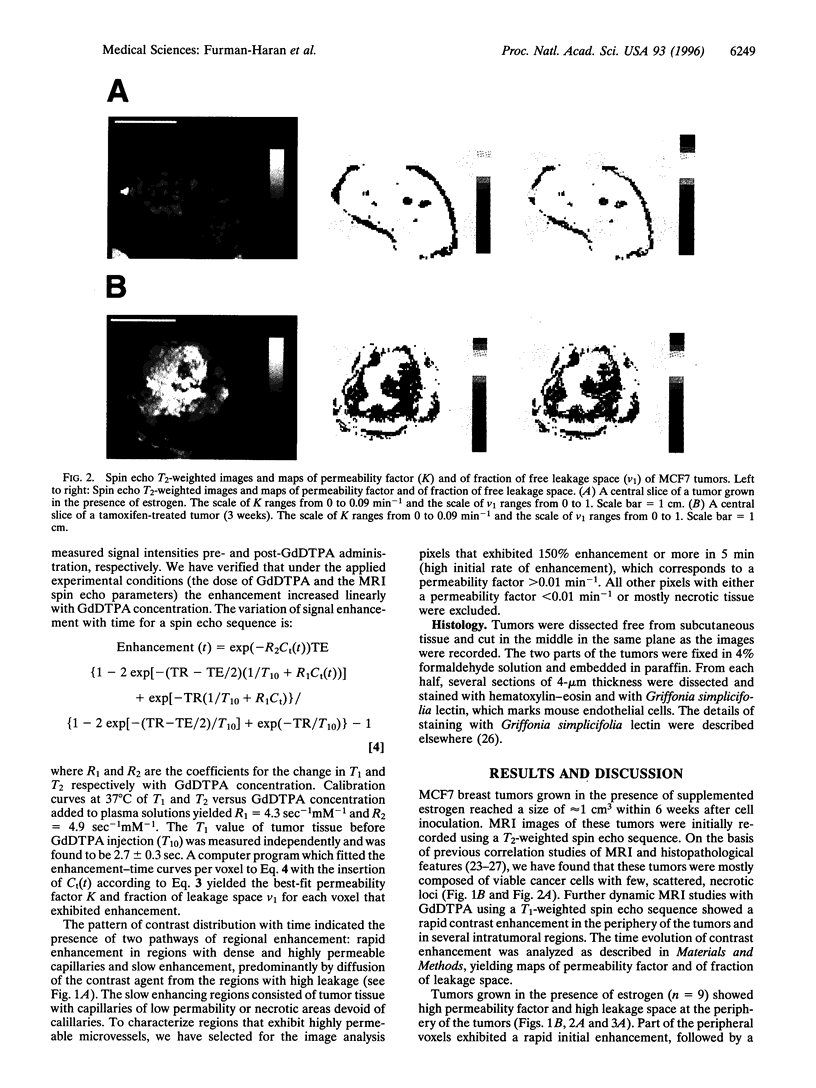
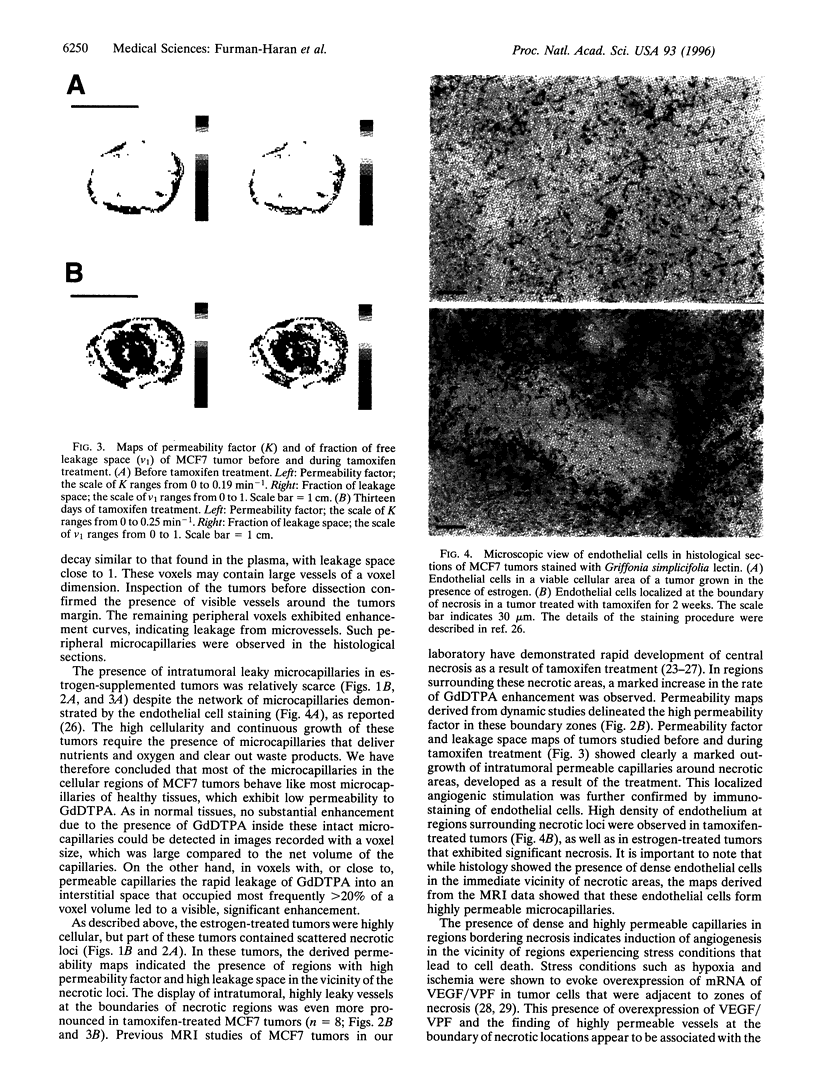

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosari S., Lee A. K., DeLellis R. A., Wiley B. D., Heatley G. J., Silverman M. L. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol. 1992 Jul;23(7):755–761. doi: 10.1016/0046-8177(92)90344-3. [DOI] [PubMed] [Google Scholar]
- Brix G., Semmler W., Port R., Schad L. R., Layer G., Lorenz W. J. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr. 1991 Jul-Aug;15(4):621–628. doi: 10.1097/00004728-199107000-00018. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Senger D. R., Dvorak H. F. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993 Oct 1;53(19):4727–4735. [PubMed] [Google Scholar]
- Degani H., Furman E., Fields S. Magnetic resonance imaging and spectroscopy of MCF7 human breast cancer: pathophysiology and monitoring of treatment. Clin Chim Acta. 1994 Jul;228(1):19–33. doi: 10.1016/0009-8981(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- Furman-Haran E., Margalit R., Maretzek A. F., Degani H. Angiogenic response of MCF7 human breast cancer to hormonal treatment: assessment by dynamic GdDTPA-enhanced MRI at high spatial resolution. J Magn Reson Imaging. 1996 Jan-Feb;6(1):195–202. doi: 10.1002/jmri.1880060135. [DOI] [PubMed] [Google Scholar]
- Furman E., Margalit R., Bendel P., Horowitz A., Degani H. In vivo studies by magnetic resonance imaging and spectroscopy of the response to tamoxifen of MCF7 human breast cancer implanted in nude mice. Cancer Commun. 1991 Sep;3(9):287–297. doi: 10.3727/095535491820873001. [DOI] [PubMed] [Google Scholar]
- Furman E., Rushkin E., Margalit R., Bendel P., Degani H. Tamoxifen induced changes in MCF7 human breast cancer: in vitro and in vivo studies using nuclear magnetic resonance spectroscopy and imaging. J Steroid Biochem Mol Biol. 1992 Sep;43(1-3):189–195. doi: 10.1016/0960-0760(92)90207-y. [DOI] [PubMed] [Google Scholar]
- Gowland P., Mansfield P., Bullock P., Stehling M., Worthington B., Firth J. Dynamic studies of gadolinium uptake in brain tumors using inversion-recovery echo-planar imaging. Magn Reson Med. 1992 Aug;26(2):241–258. doi: 10.1002/mrm.1910260206. [DOI] [PubMed] [Google Scholar]
- Hanna S. L., Reddick W. E., Parham D. M., Gronemeyer S. A., Taylor J. S., Fletcher B. D. Automated pixel-by-pixel mapping of dynamic contrast-enhanced MR images for evaluation of osteosarcoma response to chemotherapy: preliminary results. J Magn Reson Imaging. 1993 Nov-Dec;3(6):849–853. doi: 10.1002/jmri.1880030609. [DOI] [PubMed] [Google Scholar]
- Haran E. F., Maretzek A. F., Goldberg I., Horowitz A., Degani H. Tamoxifen enhances cell death in implanted MCF7 breast cancer by inhibiting endothelium growth. Cancer Res. 1994 Nov 1;54(21):5511–5514. [PubMed] [Google Scholar]
- Heywang-Köbrunner S. H. Contrast-enhanced magnetic resonance imaging of the breast. Invest Radiol. 1994 Jan;29(1):94–104. doi: 10.1097/00004424-199401000-00019. [DOI] [PubMed] [Google Scholar]
- Hoffmann U., Brix G., Knopp M. V., Hess T., Lorenz W. J. Pharmacokinetic mapping of the breast: a new method for dynamic MR mammography. Magn Reson Med. 1995 Apr;33(4):506–514. doi: 10.1002/mrm.1910330408. [DOI] [PubMed] [Google Scholar]
- Holmgren L., O'Reilly M. S., Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995 Feb;1(2):149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- Horak E. R., Leek R., Klenk N., LeJeune S., Smith K., Stuart N., Greenall M., Stepniewska K., Harris A. L. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992 Nov 7;340(8828):1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Kaiser W. A., Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology. 1989 Mar;170(3 Pt 1):681–686. doi: 10.1148/radiology.170.3.2916021. [DOI] [PubMed] [Google Scholar]
- Kennedy S. D., Szczepaniak L. S., Gibson S. L., Hilf R., Foster T. H., Bryant R. G. Quantitative MRI of Gd-DTPA uptake in tumors: response to photodynamic therapy. Magn Reson Med. 1994 Mar;31(3):292–301. doi: 10.1002/mrm.1910310308. [DOI] [PubMed] [Google Scholar]
- Larsson H. B., Stubgaard M., Frederiksen J. L., Jensen M., Henriksen O., Paulson O. B. Quantitation of blood-brain barrier defect by magnetic resonance imaging and gadolinium-DTPA in patients with multiple sclerosis and brain tumors. Magn Reson Med. 1990 Oct;16(1):117–131. doi: 10.1002/mrm.1910160111. [DOI] [PubMed] [Google Scholar]
- Macchiarini P., Fontanini G., Hardin M. J., Squartini F., Angeletti C. A. Relation of neovascularisation to metastasis of non-small-cell lung cancer. Lancet. 1992 Jul 18;340(8812):145–146. doi: 10.1016/0140-6736(92)93217-b. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Su M. Y., Jao J. C., Nalcioglu O. Measurement of vascular volume fraction and blood-tissue permeability constants with a pharmacokinetic model: studies in rat muscle tumors with dynamic Gd-DTPA enhanced MRI. Magn Reson Med. 1994 Dec;32(6):714–724. doi: 10.1002/mrm.1910320606. [DOI] [PubMed] [Google Scholar]
- Tofts P. S., Berkowitz B., Schnall M. D. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumors using a permeability model. Magn Reson Med. 1995 Apr;33(4):564–568. doi: 10.1002/mrm.1910330416. [DOI] [PubMed] [Google Scholar]
- Tofts P. S., Kermode A. G. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991 Feb;17(2):357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- Vartanian R. K., Weidner N. Correlation of intratumoral endothelial cell proliferation with microvessel density (tumor angiogenesis) and tumor cell proliferation in breast carcinoma. Am J Pathol. 1994 Jun;144(6):1188–1194. [PMC free article] [PubMed] [Google Scholar]
- Vaupel P., Kallinowski F., Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989 Dec 1;49(23):6449–6465. [PubMed] [Google Scholar]
- Weidner N., Carroll P. R., Flax J., Blumenfeld W., Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993 Aug;143(2):401–409. [PMC free article] [PubMed] [Google Scholar]
- Weidner N., Folkman J., Pozza F., Bevilacqua P., Allred E. N., Moore D. H., Meli S., Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992 Dec 16;84(24):1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- Weidner N., Semple J. P., Welch W. R., Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991 Jan 3;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- Weidner N. Tumor angiogenesis: review of current applications in tumor prognostication. Semin Diagn Pathol. 1993 Nov;10(4):302–313. [PubMed] [Google Scholar]