Abstract
Wiskott-Aldrich Syndrome protein (WASP) is a key regulator of the actin cytoskeleton in hematopoietic cells. Defective expression of WASP leads to multiple abnormalities in different hematopoietic cells. Despite severe impairment of T cell function, WAS patients exhibit a high prevalence of autoimmune disorders. We attempted to induce EAE, an animal model of organ-specific autoimmunity affecting the CNS that mimics human MS, in Was−/− mice. We describe here that Was−/− mice are markedly resistant against EAE, showing lower incidence and milder score, reduced CNS inflammation and demyelination as compared to WT mice. Microglia was only poorly activated in Was−/− mice. Antigen-induced T-cell proliferation, Th-1 and -17 cytokine production and integrin-dependent adhesion were increased in Was−/− mice. However, adoptive transfer of MOG-activated T cells from Was−/− mice in WT mice failed to induce EAE. Was−/− mice were resistant against EAE also when induced by adoptive transfer of MOG-activated T cells from WT mice. Was+/− heterozygous mice developed an intermediate clinical phenotype between WT and Was−/− mice, and they displayed a mixed population of WASP-positive and -negative T cells in the periphery but not in their CNS parenchyma, where the large majority of inflammatory cells expressed WASP. In conclusion, in absence of WASP, T-cell responses against a CNS autoantigen are increased, but the ability of autoreactive T cells to induce CNS autoimmunity is impaired, most probably because of an inefficient T-cell transmigration into the CNS and defective CNS resident microglial function.
Introduction
Wiskott-Aldrich syndrome (WAS) is a severe X-linked disorder characterized by microthrombocytopenia, eczema, immunodeficiency and increased risk of developing autoimmunity and lymphomas [1]. The protein encoded by the WAS gene, WASP, is a hematopoietic specific regulator of actin nucleation in response to signals arising at the cell membrane [2], [3]. In the last years several WASP mutations have been identified, resulting in a variety of clinical manifestations ranging from the relatively mild X-linked thrombocytopenia (XLT) to the classic full-blown WAS phenotype [4]–[6]. The hallmarks of classical WAS cases are represented by compromised humoral and adaptive immune responses. T-cell defects hamper both effector and helper functions because of the key role of WASP in T-cell activation and actin cytoskeleton remodeling upon TCR engagement [7]. As a consequence of impaired signaling through the TCR and co-stimulatory molecules, WASP-deficient T cells show defective proliferation and decreased secretion of IL-2 and Th-1 cytokines [7], [8]. These findings might help explaining the unbalanced Th1/Th2 cytokine production observed in patients and in the murine counterpart [7]. However, the precise relationship between T-cell abnormalities and autoimmunity in WAS patients remains to be fully elucidated. In fact in these patients, as in several other primary immunodeficient (PID) patients, immunodeficiency is often accompanied by the development of autoimmune disorders [9], reported to affect 25% to 72% of patients [10]–[12], irrespectively of WASP expression levels and disease severity [12].
We thus set out to analyze the role of WASP in a prototypical animal model of organ-specific autoimmunity, experimental autoimmune encephalomyelitis (EAE), an inflammatory demyelinating disease of the CNS mimicking multiple sclerosis (MS) [13]. Much evidence demonstrates that Th-1 and -17 cells activated against the myelin of the CNS drive the pathogenesis of both EAE and MS [14]. Furthermore, migration of autoreactive T cells through the blood-brain-barrier (BBB) into the CNS parenchyma and recruitment of inflammatory cells are crucial steps for the development and perpetuation of these diseases [15]. Because of its complexity, this model allowed us to explore the role of WASP both in the initiation phase of peripheral T-cell activation against self-antigens and in the consequent effector phase, in which autoreactive T cells migrate into the target organ. In the present paper we show that WASP-deficiency impairs the development of EAE by affecting both migration of autoreactive T cells in the CNS and activation of CNS resident cells, such as microglia.
Materials and Methods
Mice, peptides and EAE induction
WAS knock-out mice were generated as previously described [16]. In our study, we used 8–12 week old female BL6-wasnull (Was−/−) mice. Age/gender-matched C57BL/6N WT control mice were purchased from Charles River Laboratories Inc (Calco, Italy). Was+/− heterozygous female mice were generated by crossing Was− male mice and WT female mice and presented both WT and transgenic WAS alleles. EAE was induced as described [17] by subcutaneous immunization with MOG35–55 (100 µg/mouse) in Complete Freund's Adjuvant (CFA, Difco), containing 4 mg/ml of heat-killed Mycobacterium tuberculosis (Difco). All mice were injected i.v. with 200 ng/mouse of Bordetella Pertussis toxin (PTX, List Biological Lab) on day 0 and 2 post-immunization. Passive EAE was induced by i.v. injection of 5×106 activated T-cell blasts, obtained from draining lymph nodes of MOG35–55-immunized mice 7–10 days after challenge and re-stimulated in vitro for three days with MOG35–55 (20 µg/ml) and IL-12 (1 ng/ml), and i.p. injection of 50 ng of PTX [18].
Ethics statement
All mice were housed in specific pathogen free conditions and treated according to protocols approved by the Animal Care and Use Committee of the San Raffaele Scientific Institute (Institutional Animal Care and Use Committee protocols no. 318-406-557).
Pathological studies
Mice were sacrificed 2 and 7 weeks after induction of EAE and brains and spinal cords were removed and fixed in 10% formalin. Four μm paraffin-embedded sections were used for routine Haematoxylin and Eosin (H&E) staining and sections were blindly analyzed (E.F., P.L.P.). Polyclonal/monoclonal specific antibodies for GFAP (Dako, Glostrup, Denmark), MBP (Chemicon, Temecula, CA), CD3 (Thermo-Scientific, Fremont, CA), Iba-1 (Wako, Osaka, Japan) and WASP (503 Ab, a kind gift from Profs H. D. Ochs, Seattle, WA, and L. D. Notarangelo, Boston, MA) have been applied to investigate reactive gliosis, demyelination, T-cell infiltration, presence of macrophages, microglial activation, and WASP expression. Immunostains were revealed by Real EnVision Rabbit HRP Labelled Polymer system (Dako) and Rat on Mouse HRP polymer kit (Biocare medical) using 3,3′-diaminobenzidine tetrahydrocloride (DAB, 0.05%) as a chromogen and Haematoxylin as counterstaining. Double immunostains were performed as previously described [19]. Chromogen reaction was developed by Ferangi Blue Chromogen Kits (Biocare Medical, Concord, CA) and diaminobenzidine. Nuclei were counterstained with methyl green. The degree of inflammation has been expressed as ratio between distribution of the inflammatory cells within CNS parenchyma (measured as total area occupied by inflammatory cells) and the mean area of the spinal cord, obtained analyzing the total area of 8 different spinal cord sections at different levels. Demyelination was evaluated by measuring the percentage of demyelinated areas over the total area of white matter for each single spinal cord section. An average of 12 spinal cord sections for each mouse were studied. Digital images and analysis were obtained by Olympus DP70 camera mounted on an Olympus Bx60 microscope, using CellF Imaging software (Soft Imaging System GmbH).
Antigen-specific T-cell proliferation assay
Draining lymph node cells (LNCs) were isolated from Was−/− and WT mice and cultured in vitro with MOG35–55 (0.1–100 µg/ml), Concanavalin A (ConA; 4 µg/ml) or medium alone. Cells were cultured at a density of 5×105 cells/well in 200 µl RPMI 1640 (EuroClone) supplemented with 10% FBS, L-glutamine (2 mM), sodium pyruvate (1 mM), nonessential amino acids (0.1 mM), penicillin (100 U/ml), streptomycin (100 U/ml), 2-ME (5×10−5 M). After 72 h of incubation at 37°C with 5% CO2, cultures were pulsed for 18 h with 0.5 µCi/well of [3H] thymidine before harvesting.
Cytokine analysis
Splenocytes were harvested from Was−/− and WT mice and cultured in the same conditions as described above at a concentration of 3.5×106 cells/well. Supernatants from in vitro cultured splenocytes were collected after 48 and 96 h and analyzed by multiplex assay (Bioplex, Bio-rad, Hercules, CA) according to the manufacturer's instructions. Serum cytokine concentrations were measured by Bioplex reader (Bio-Rad) on sera diluted 1∶4.
Measurement of serum antibody responses
Blood was collected from the tail vein of Was−/− and WT mice before and 2–7 weeks after the induction of EAE. MOG-specific IgG, IgG1, IgG2a, IgG2b and IgG3 antibodies and total IgE antibodies were measured on serum samples by ELISA assay as described [17]. Briefly, 96-well plates (Immunol, Thermo Labsystems, Franklin, MA) were coated overnight at 4°C with 0.1 ml of MOG35–55 diluted in 0.1 M NaHCO3 buffer pH 9.5 at a concentration of 0.01 mg/ml. The plates were blocked with PBS 10% FBS (blocking buffer) for 2 h. Samples were diluted in blocking buffer at 1∶100 and antibody binding was tested by the addition of peroxidase-conjugated monoclonal goat anti-mouse IgG, IgG1, IgG2a, IgG2b and IgG3 (Southern Biotechnology Associates, Birmingham, AL), each at 1∶5000 dilution in blocking buffer. Enzyme substrate was added and plates were read at 450 nm on a microplate reader.
Flow cytometry analysis
Activated T cells from MOG-immunized WT or Was−/− mice were re-stimulated in vitro for 3 days with MOG35–55 peptide in the presence of antigen presenting cells (irradiated splenocytes). Dead cells were then eliminated by Ficoll-Paque (GE Healthcare) gradient. T-cell blasts so obtained were labeled with monoclonal antibodies for α4-integrin (PS/2 clone, kindly provided by Dr. Eugene Butcher, Stanford University), Lymphocyte Function-associated Antigen (LFA)-1 (anti-αL-chain; clone TIB213 from American Type Culture Collection/ATCC, VA, USA), P-Selectin-Glycoprotein Ligand (PSGL)-1 (clone 4RA10, kindly provided by Dr. Dietmar Vestweber, Max Plank Institute, Germany), L-selectin (Mel-14 clone, ATCC) and CD44 (IM/7 clone, ATCC). Isotype-matched antibodies were used as controls. Antibodies were detected with secondary goat anti-rat PE antibody (Biolegend). At least 10,000 events were collected by flow cytometry (FACScalibur, Becton Dickinson) using the Cell Quest software and analyzed with the FlowJo software (Tree Star Inc.).
Adhesion assay
Recombinant murine Intercellular Cell Adhesion Molecule (ICAM)-1 (1 µg/ml, R&D Systems) was coated over night at 4°C on 18-well glass slides. WT or Was−/− T-cell blasts were collected and re-suspended 4×106/ml in adhesion buffer (PBS + Ca++/Mg++ 1 mM + 10% FBS, pH 7.2). Twenty μl of cell suspension were added to each well and cells were allowed to spontaneously adhere on ICAM-1 for 10 min at 37°C. In some wells, CXCL12 chemokine (R&D Systems) was added at the final concentration of 0.5 µM, and rapidly-induced adhesion was measured after 5 min [20]. Slides were then immediately washed and fixed in ice-cold PBS 1.5% glutaraldehyde and computer-assisted enumeration of bound cells in 0.2 mm2 was performed.
ImageStream data acquisition and analysis
WT or Was−/− T-cell blasts were stimulated with CXCL12 chemokine or control buffer for 5 min at 37°C. Two and a half million cells were then incubated with 10 µg/ml anti-LFA-1 antibody for 30 min on ice. After washing, cells were stained with PE-conjugated secondary antibody. Stained cells were re-suspended in PBS for the ImageStream analysis. Images were acquired on the ImageStream imaging cytometer System 100 (Amnis Corporation, Seattle, WA, USA). Images of fixed cells were collected and analyzed using ImageStream data exploration and analysis software, and LFA-1 clustering was evaluated as previously described [21]. Briefly, cells were divided in uniform (uniform distribution of fluorescence), clustered (small spots of fluorescence), and caps (big clusters of fluorescence) cells in accordance with LFA-1 distribution pattern.
Intravital microscopy in brain pial vessels
Intravital microscopy experiments were performed as described [22], [23]. Briefly, WT mice were injected intraperitoneally with 12 µg lipopolysaccharide (LPS) 5–6 h before starting the intravital experiment. Animals were then anesthetized and a heparinized PE-10 catheter was inserted into the right common carotid artery toward the brain. In order to exclude non-cerebral vessels from the analysis, the right external carotid artery and pterygopalatine artery were ligated. The scalp was reflected and a 24 mm×24 mm coverslip was applied and fixed with silicon grease. A round camera with 11 mm internal diameter was attached to the coverslip and filled with water as previously described [22]. The preparation was placed on an Olympus BX50WI microscope and a water immersion objective with long focal distance (focal distance 3.3 mm, NA 0.5∞) was used. Blood vessels were visualized through the bone by using fluorescent dextran. Two-three million WT or Was−/− T-cell blasts were labelled for 2 min at 37°C with red (5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine (CMTMR), re-suspended in PBS and slowly injected into the carotid artery by a digital pump. Images were visualized and recorded as for mesenteric vessels. Vessel diameter (D), haemodynamic parameters and velocities of rolling were determined by using a PC-based system as previously described [22], [23]. Lymphocytes that remained stationary on venular wall for ≥30 s were considered adherent. At least 100 consecutive cells/venule were examined. Rolling and firm arrest fractions were determined as the percentage of cells that rolled or firmly arrested within a given venule on the total number of cells entering the venule.
Transwell migration assay
To assess LN cell migration in response to CXCL12 (Peprotech, Rocky Hill, NJ), total LN cells were isolated from 15 WT and 15 Was−/− mice 10 days after MOG35–55-immunization and seeded in the upper well of a 5 µm transwell (Corning Costar, Corning, NY) at the concentration of 5×106/ml in 100 µl of medium. In the bottom well, we placed 600 µl of complete medium supplemented with 250 ng/ml of CXCL12. Cells were incubated for 3 h at 37°C, and cells migrated to the lower well were counted. Migration activity was calculated as percentage of migrated cells over the input cell number.
Statistical analysis
Unless otherwise stated, Student's t-test, two tailed, was used to compare the results between two groups. In all tests, P<0.05 was considered statistically significant.
Results
Was−/− mice are resistant against EAE
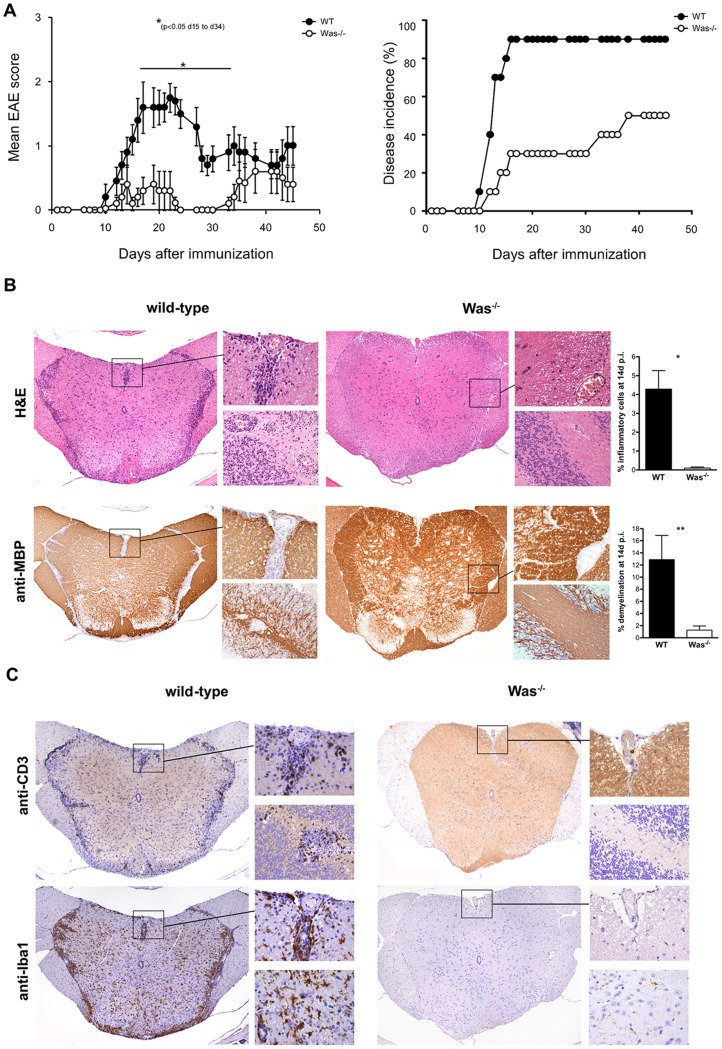
To study the development of autoimmune CNS demyelination in WASP deficiency, we induced chronic EAE with MOG35–55 peptide in Was −/− and WT control mice. In two consecutive experiments, we observed significant resistance against EAE in Was −/− mice (Figure 1A and Table 1). The disease incidence was reduced, the onset of EAE was delayed and the clinical severity was significantly reduced in Was −/− mice as compared to WT mice. In line with these clinical findings, neuropathological analysis of brain and spinal cord obtained from mice during the acute phase of EAE (14 days p.i.) showed a marked reduction of both CNS inflammation and demyelination in Was −/− as compared to WT mice (Figure 1B). In particular, the percentage of inflamed tissue within the CNS parenchyma and the percentage of demyelinated white matter area in the spinal cord of Was −/− mice were significantly reduced (P<0.05 and P<0.01, respectively) compared to those of WT mice (Figure 1C). Of note, immunostains for CD3 and Iba-1 revealed a dramatic decrease of T cells infiltrating the CNS parenchyma (Figure 1C, upper panels) in Was −/− mice as compared to WT mice, and microglial cells, which in WT mice with EAE were intensely activated and expressed WASP (Figure S1), were only weakly or not activated in the CNS of Was −/− mice (Figure 1C, lower panels). The differences in the extent of CNS inflammation and demyelination between Was −/− and WT mice observed 14 days after EAE induction were no longer significant at a later time point of EAE (48 days p.i.) (data not shown), when WT mice were in recovery and the differences in EAE severity between Was −/− and WT became less evident (Figure 1A and Table 1).
Figure 1. Was − /− mice are resistant against EAE.
A, EAE was induced in Was −/− (white circles) and WT mice (black circles) with MOG35–55 peptide. Data represent mean clinical scores of 10 mice/group ± SEM. The experiment is representative of two independent experiments. Statistical analysis was performed with Sum Rank Test. * P<0.05. B, Histopathological analyses were performed on spinal cords and brains obtained from Was −/− and WT mice two weeks after the induction of EAE. Representative 5–8 mice per group were analyzed. H&E staining (upper panels) and anti-MBP immunostain (lower panels) highlight inflammatory changes and myelin damage, respectively. Insets report in detail inflammatory foci and demyelinated areas within spinal cords (upper insets) and brain parenchyma (lower insets), respectively. Graphs show percentage of inflammatory cell infiltration and demyelination in spinal cords of WT and Was −/− mice. * P<0.05; ** P<0.01. C, CD3 (upper panels) and Iba-1 (lower panels) immunostains highlight T-cell infiltration and both macrophages and microglial activation, respectively. Details of the staining as for the previous panels are reported in the insets. In all panels serial sections of representative CNS sample are reported. Panels are 4× and insets 20× original magnification.
Table 1. Clinical traits of EAE in Was −/− and wild type mice.
| Mice | Incidence (%) | Onset (day) | Disease score at peaka) | Maximum disease severity | Cumulative disease score |
| Exp 1 | |||||
| WT | 10/10 (100) | 13±0.6 | 1.7±0.2 | 2.3±0.3 | 28.4±4.7 |
| Was − /− | 5/10 (50) | 22.6±3.9 | 0.3±0.3*** | 1.2±0.4* | 7±2.8*** |
| Exp 2 | |||||
| WT | 10/10 (100) | 9.8±0.6 | 3.4±0.3 | 3.5±0.3 | 47±6.3 |
| Was − /− | 6/10 (60) | 18.5±4.6* | 0.9±0.5*** | 1.7±0.6** | 18.4±8.3* |
Data are shown as mean ± SEM. a)Mice reached a peak of disease severity at day 22 after immunization in Experiment 1 and at day 14 in Experiment 2. * P<0.05, ** P<0.01, *** P<0.005 vs Was −/− group.
Was −/− mice develop a normal peripheral response against myelin antigen
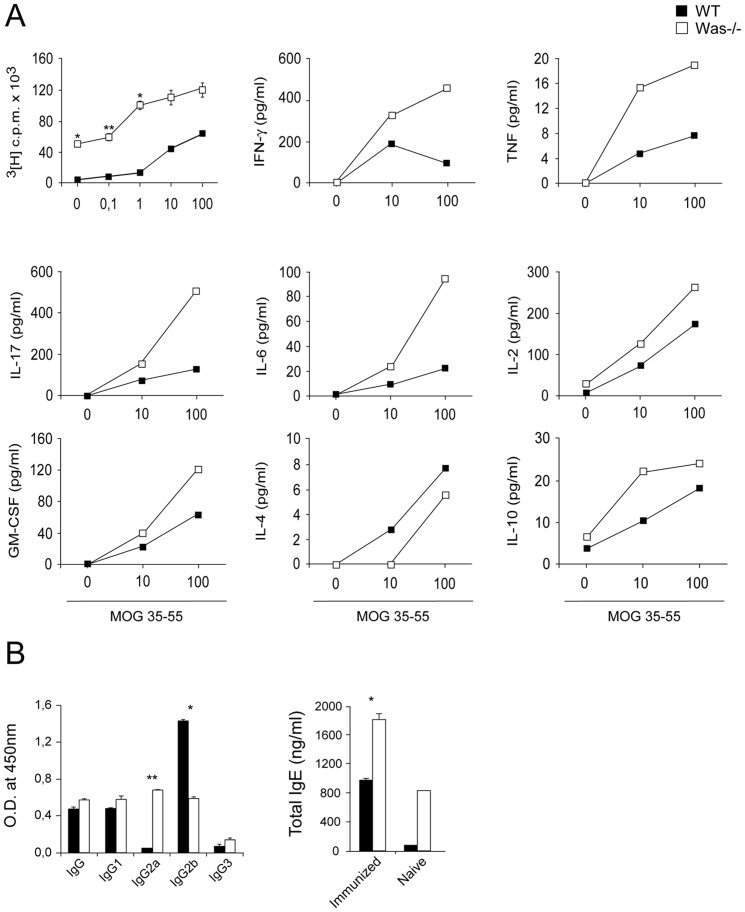
Given the marked resistance against EAE displayed by Was −/− mice, we analyzed the peripheral immune response against myelin antigen in WASP deficiency. We first examined the ability of T cells from Was −/− mice to respond against MOG35–55. Antigen-induced T-cell proliferation in LNCs from mice immunized for EAE was higher in Was −/− mice than in WT mice (Figure 2A). Furthermore, antigen-stimulated spleen cells from Was −/− mice produced more Th-1 and Th-17 cytokines, such as IFN-γ, IL-17, TNF-α and IL-6, which are known to play a key role in the pathogenesis of EAE [24] (Figure 2A). GM-CSF, recently shown to play an essential function in the initiation of autoimmune neuroinflammation in EAE [25], and IL-2 were also increased in splenocytes of Was −/− mice upon antigen stimulation as compared to control mice. Of note, IL-10, known to play suppressive functions, was also found increased in mutant mice.
Figure 2. Peripheral T-cell and antibody response against myelin antigen in Was − /− mice.
A, LNCs and splenocytes were harvested from Was −/− and WT mice (5 mice/group) two weeks after EAE induction and pooled cells were cultured in vitro with increasing doses of MOG35–55 peptide or medium alone. [3H] thymidine was added to LNCs cultures 72 h after stimulation (upper left corner). Cytokine levels were measured on supernatants from splenocytes 48 h after stimulation. B, Antigen-specific IgG antibodies (left panel) and total IgE antibodies (right panel) were measured in duplicate in sera obtained from mice (10 mice/group) 6 weeks after EAE induction. Data are representative of one of the two independent experiments that gave similar results. * P<0.05; ** P<0.01.
The analysis of the IgG antibody response against MOG35–55 revealed that there were not significant differences in serum antigen-specific IgG antibody titers among Was−/− and WT mice. However, the analysis of IgG antibody isotypes showed a decrease of antigen-specific IgG2b and an increase of IgG2a antibody titers in the serum of Was−/− mice as compared to those of WT mice (Figure 2B). The increased antibody switch towards the IgG2a isotype and the decrease in IgG2b subclass observed in Was−/− mice could be related to the higher levels of IFN-γ produced by activated splenocytes of Was−/− mice, as this cytokine is known to promote antibody class switch towards IgG2a while inhibiting IgG2b production [26]. Additionally, total IgE antibody concentrations were increased in the serum of both immunized and non-immunized Was−/− mice as compared to WT mice.
Defects in both autoreactive T cells and host compartments contribute to the resistance against EAE of Was−/− mice
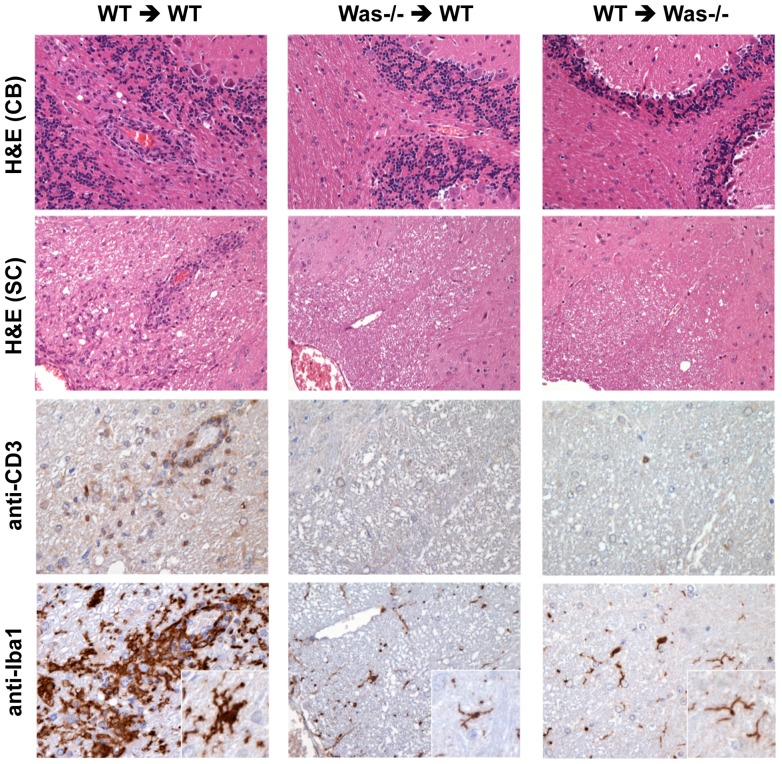
As we did not observe defects in priming of autoreactive T cells in Was−/− mice, we wanted to ascertain the ability of these cells in inducing disease when transferred in non-immunized recipient mice. Adoptive transfer of MOG35–55-activated T cells from WT mice induced EAE in the majority of WT recipient mice (3/5, 60%), and neuropathological analysis of spinal cord and brains obtained 3 weeks after adoptive transfer showed infiltrating T cells and macrophages in the CNS parenchyma, along with microglia activation (Table 2 and Figure 3, left panels). Conversely, T cells from Was−/− mice failed to induce EAE in WT recipient mice (0/4, Table 2). Neuropathological analysis of spinal cords and brains of these treated mice confirmed the clinical results, as we could observe complete absence of infiltrating T cells and macrophages, along with absence of microglia activation (Figure 3, middle panels). These results demonstrate that, despite a normal and even increased generation of Th-1 and Th-17 response against MOG35–55 in Was−/− mice, autoreactive T cells from these mice were not able to induce EAE even when adoptively transferred in WT recipient mice. Surprisingly, adoptive transfer of myelin autoreactive T cells from WT mice failed to induce disease in Was−/− mice (0/5, Table 2 and Figure 3, right panels), and, accordingly, infiltrating T cells were absent in the CNS of these treated mice and microglia was scarcely/not activated.
Table 2. Passive transfer of EAE.
| Donor | Recipient | Number of animals | Incidence (%) | Day of onset | Maximum disease severity |
| WT | WT | 5 | 3/5 (60) | 14±0a) | 1.3±0.3a) |
| WT | Was−/− | 5 | 0/5 (0) | - | 0 |
| Was−/− | WT | 4 | 0/4 (0) | - | 0 |
EAE was induced with injection of 5×106 T-cell blasts per mouse derived from WT or Was−/− mice actively immunized with MOG35–55 peptide. a)Data represent mean ± SEM for those mice that developed the disease.
Figure 3. Histological features of brain and spinal cord sections from passively induced EAE (WT in WT, WT in Was−/− and Was−/− in WT).
Samples were obtained 3 weeks after transfer and stained with H&E (upper panels; CB, cerebellum and SC, spinal cords), anti-CD3 and anti-Iba1 (lower panels). Insets show in detail representative microglial cells within spinal cords. Upper panels are from 10×, lower panels from 20× and insets from 40× original magnification.
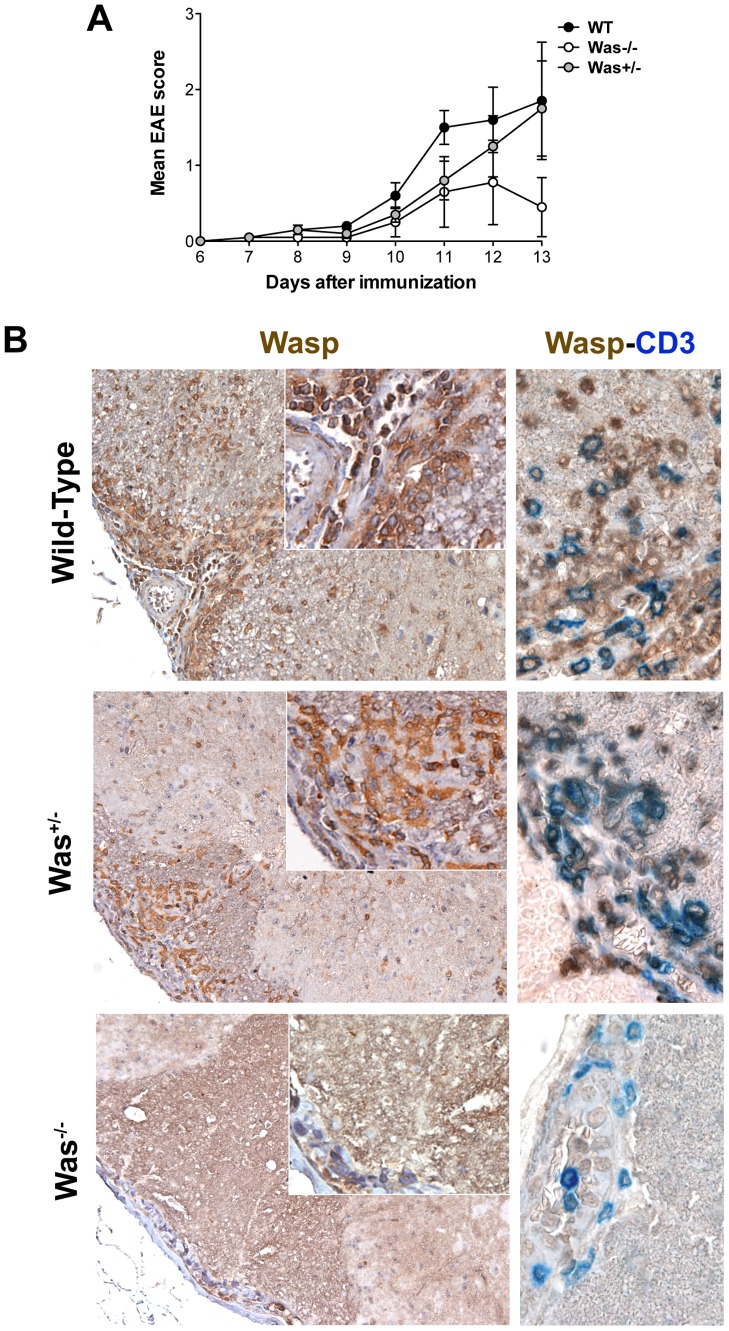
On the other hand, absence of disease transfer in WT recipient mice injected with Was−/− T cells suggests a defective migration of Was−/− T cells in the CNS of recipient mice. To verify this hypothesis, we induced EAE in Was+/− heterozygous mice, in which WASP-positive and -negative cells coexist, and evaluated their clinical scores and WASP expression in T cells from peripheral lymphoid organs and CNS (Figure 4). In parallel, WT and Was−/− mice were immunized as controls. As we reported in our previous experiments, Was−/− mice were resistant to EAE and showed only mild or no clinical score, while WT animals started showing signs of the disease at day 10 post-immunization and were all affected by day 13 (Figure 4A). Conversely, Was+/− heterozygous mice showed a clinical phenotype intermediate between WT and Was−/− mice (Figure 4A). In these mice a mixed population of WASP-positive and -negative T cells persisted in the periphery before and after MOG-immunization (Figure S1). Neuropathological analysis of spinal cords from Was+/− heterozygous mice conducted 14 days after EAE induction revealed that inflammatory infiltrates expressed WASP. Conversely, in Was−/− mice immunized for EAE, in which we did not detect inflammatory infiltrates within the CNS parenchyma, as expected there was no expression of WASP, and the few CD3-positive cells found in their spinal cords were localized in the vessels or in the leptomeningeal space (Figure 4B, bottom row). These results strongly suggest that WASP is required for the proper migration of immune cells from peripheral lymphoid tissues into the CNS (Figure 4B, middle row).
Figure 4. Was+/− heterozygous mice are not resistant to EAE induction.
A, EAE was induced in Was−/− (white circles), Was+/− (grey circles) and WT mice (black circles) with MOG35–55 peptide. Data represent mean clinical scores of 5 mice/group ± SEM. B, Histopathological analyses were performed on spinal cords obtained from Was−/−, Was+/− and WT mice 14 days after the induction of EAE. WASP (left panels) and WASP-CD3 (right panels) immunostains highlight WASP expression in total CNS infiltrates and in T-cell infiltrates, respectively. Details of the staining are reported in the insets. In all panels serial sections of representative CNS samples are reported. Panels for WASP single stainings are 20×, insets 40× and WASP-CD3 double stainings 60× original magnification.
Taken together, these results suggest that deficiency of WASP affecting both the migration of autoreactive T cells into the CNS parenchyma and the activation of host compartment cells, such as microglia, could be responsible for the resistance against EAE observed in Was−/− mice.
Was−/− T-cell blasts had no reduction of integrin-dependent adhesion in vitro and in vivo
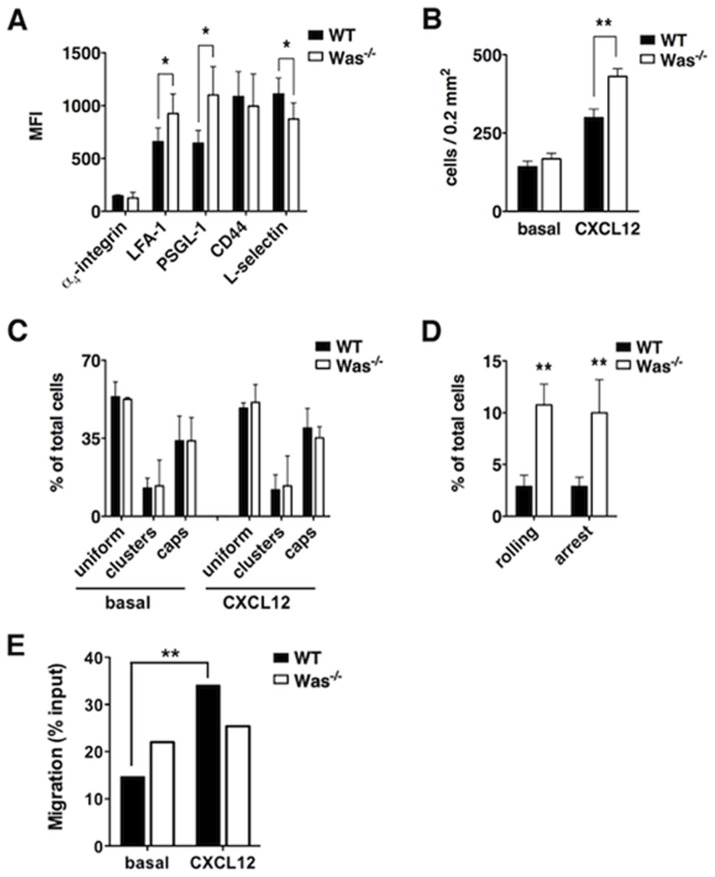
Because adoptive transfer of autoreactive T cells from Was−/− mice in WT recipient mice failed to induce EAE (Table 2 and Figure 3), we sought investigating whether the adhesive capacities of Was−/− T cells were compromised. We first performed flow cytometric analysis and found that T cells from Was−/− mice express significantly higher levels of integrin LFA-1 and mucin PSGL-1, but had lower L-selectin expression (Figure 5A). These results suggested that Was−/− T-cell blasts have a more activated phenotype and presumably higher adhesion capacity in inflamed vessels when compared to WT cells. No differences in the expression of α4-integrin and CD44 were found between the two populations (Figure 5A). Because LFA-1 integrin has been previously shown to mediate T-cell adhesion in inflamed brain vessels [22], we next analyzed the ability of T-cell blasts from WT and Was−/− mice to adhere in vitro on purified ICAM-1 after CXCL12-mediated triggering of adhesion. Our results showed that Was−/− T cells display a significantly higher adhesion on purified ICAM-1 when compared to WT cells (Figure 5B), suggesting that LFA-1 integrin is already activated in Was−/− T cells. Interestingly, the increased adhesiveness was not associated to an increase in LFA-1 clustering on Was−/− T-cell surface, as evaluated with ImageStream analysis (Figure 5C), suggesting that activated Was−/− T cells may have an increase in LFA-1 affinity compared to WT cells.
Figure 5. Adhesive properties of MOG35–55-primed WT and Was−/− T-cell blasts.
A, The expression of LFA-1, α4-integrin, PSGL-1, CD44 and L-selectin was analyzed by flow cytometry on MOG35–55-primed T-cell blasts obtained form WT or Was−/− immunized mice. The mean fluorescence intensity (MFI) of staining is shown. B, WT or Was−/− MOG35–55-primed T-cell blasts were left to adhere spontaneously on slides coated with purified ICAM-1, with or without CXCL12. C, WT or Was−/− MOG35–55-primed T-cell blasts were stimulated with CXCL12 chemokine or control buffer and then labeled with anti-LFA-1 antibody and LFA-1 distribution on cell surface was analyzed with the ImageStream system. D, WT or Was−/− MOG35–55-primed T-cell blasts were injected in the right carotid of LPS-treated mice to analyze their interaction with brain pial vessels expressing ICAM-1, VCAM-1 and endothelial selectins [22]. Rolling interactions and stable adhesions were evaluated by analyzing at least 100 cells/venule. In all panels, data are mean ± SD of three independent experiments. E, WT or Was−/− LN cells isolated from MOG35–55-immunized mice were seeded in transwells and induced to migrate in the presence or absence of CXCL12 for 3 h. Frequency of cells migrated to the bottom wells of transwell plates are indicated in the graph. Each bar is representative of a pool of 15 mice. Statistical analysis was performed with Fisher's Exact Test. ** P<0.01.
We next studied the adhesion capacity of Was−/− T cells in vivo by performing intravital microscopy in inflamed pial venules in an experimental model mimicking vascular inflammation occurring during early phases of EAE [22]. In this model, pial vessels express adhesion molecules such as ICAM-1, vascular cell adhesion molecule (VCAM)-1, E-selectin and P-selectin, as previously described [22], [27]. In line with the data obtained in in vitro adhesion assays as well as in flow cytometry experiments, we found that Was−/− T cells had an increased ability to roll and adhere in inflamed pial vessels when compared to WT cells (Figure 5D). In order to test whether increased adhesion capacity of Was−/− T cells corresponds to an augmented migratory ability of these cells, we performed a transwell migration assay in response to CXCL12 in WT and Was−/− LN cells isolated from MOG35–55-immunized mice (Figure 5E). Our results show that even if spontaneous migration was increased in Was−/− LN cells, the specific migration to CXCL12 was dramatically reduced in Was−/− LN cells, as compared to WT cells. Taken together, these results show that Was−/− T cells have increased adhesion capacity compared with WT T cells but reduced ability to transmigrate in response to chemokine signals.
Discussion
The data reported in this study provide evidence that WASP is required for the development of EAE. In the absence of WASP, mice were markedly resistant against EAE. Resistance against EAE was present despite the increased autoreactive Th-1 and Th-17 cell responses against the self myelin antigen MOG35–55 developed by Was−/− mice.
WASP-deficient mice develop overt signs of autoimmunity only later in life in some specific background [28], even though most of these mice exhibit elevated titers of circulating autoantibodies in their sera [19], [28]–[30]. In our study, we used an induced model of organ-specific T cell-mediated autoimmunity to analyze the role of WASP in the development of autoimmunity. Several groups reported that WASP-deficient T cells fail to undergo a normal polarization, and that cytokine secretion from these cells is impaired [31]–[33]. This could either result from an indirect effect of WASP on gene transcription factors associated with cytokine production, or indicate an important post-transcriptional role for WASP in T-cell effector functions [8]. Moreover, in vivo evidence revealed that immune cells of WASP-deficient mice have motility defects, as DC homing from the periphery is kinetically and quantitatively impaired [34], [35]. Was−/− mice also display impaired DC priming of T cells due to defects in cell-to-cell interactions [34], [36]. In our model, LNCs from Was−/− mice proliferated more than those from wild type mice when stimulated with the self myelin antigen, and splenocytes produced more IFN-γ, IL-17, IL-6 and GM-CSF, cytokines that are all known to have a crucial role in the development of EAE. Of note IL-10, which is known to play a suppressive function in several autoimmune disorders including EAE, was also increased in Was−/− mice. The mechanisms underlying the increased immune responses against self MOG still need to be elucidated. However, it is possible that the reduced function and homeostasis of naturally occurring regulatory T cells (Tregs), which has been previously reported in Was−/− mice and WAS patients [29], [37]–[39], could be associated with these findings. However, even in the presence of an increased self antigen response in Was−/− mice, they were still resistant to develop EAE, indicating that impaired effector functions of Was−/− T cells are likely responsible of the absence of disease in the CNS of Was−/− mice.
In fact, although myelin-autoreactive Was−/− T cells exhibited an increased activation, the absence of WASP hampered the ability of these cells to induce CNS disease, also after passive transfer into recipient WT mice. Both the ability of T cells to traffic through the inflamed BBB into the CNS parenchyma and to migrate to and from secondary lymphoid organs, such as lymph nodes, might be impaired in WASP deficient mice, as proven by the fact that Was+/− heterozygous mice are not resistant to EAE induction and present only WASP-positive infiltrates in the CNS parenchyma. These defects appear not be associated with a decreased expression of adhesion molecules in Was−/− T cells. Indeed, we did not observe in Was−/− T cells defective expression of molecules such as LFA-1, integrin α4β7 and CD44, in accordance with data already published [40]. Nevertheless, we observed a dramatic decrease of infiltrating T cells and macrophages in the CNS of Was−/− mice, both in active induced EAE and adoptive transfer EAE. These data point to an inefficient recruitment of cells and macrophages in the CNS of Was−/− mice. However, WASP-deficient T cells display an increased chemokine-induced adhesion on ICAM-1 and a more efficient sticking on inflamed endothelium in brain microcirculation when compared to WT blasts. These data are in agreement with recent results showing that WASP-deficient T cells display an increased adhesion on ICAM-1 in vitro [41]. The increased adhesion of Was−/− T cells is in agreement with our recent data showing that CDC42, which is an upstream regulator of WASP, is involved in the negative control of integrin activation and T-cell adhesion in vitro and in vivo [20]. Thus, the lack of WASP inhibits CDC42 function leading to an increase of integrin activation and T-cell adhesion. Despite their increased capacity to adhere in vitro and in vivo, Was−/− T-cell blasts may still be unable to penetrate into the CNS, as shown by their inability to transfer EAE and by their defective capacity to migrate in response to CXCL12. In fact, it has been previously shown that T cells from Was−/− mice or from WAS patients display a reduced chemokine-induced chemotaxis due to defective cytoskeletal remodeling [42], [43]. Moreover, WASP has a well-established role in cytoskeletal organization, suggesting that Was−/− T cells could efficiently adhere in inflamed brain vessels, but fail to spread on the vascular endothelium, a process which is crucial for adhesion strengthening and subsequent lymphocyte transmigration through the endothelial wall [44], [45]. The observation that MOG35–55-primed WT T-cell blasts failed to induce EAE in Was−/− mice could also suggest that WASP is also involved in brain endothelium cytoskeleton remodeling, which is known to be critical for leukocyte transendothelial migration into the tissues [45]. Thus, according with what has been described in other contexts, cytoskeleton modifications required for an efficient entry into the CNS might be altered in WASP-deficiency, and therefore inhibit inflammatory cell transmigration into the CNS parenchyma. Additionally, the inability of MOG35–55-primed WT T-cell blasts in transferring EAE in Was−/− mice could be due to defects in host immune cells, such as CNS resident microglia. In fact, microglial proliferation has been associated with brain lesions in multiple sclerosis [46], [47] and a role of WASP in microglial activation has been reported [48]. In support of these data we also have observed that activated microglia expresses WASP in the CNS of MOG35–55-immunized WT mice (data not shown). Lack of WASP could compromise the activation ability of microglial cells and thus contribute to EAE resistance in Was−/− mice, both in active and adoptive transfer EAE.
Another hypothesis that could explain the resistance against EAE observed in Was−/− mice is that the absence of WASP may hamper the ability of T and B cells to egress from lymph nodes and reach the target organ, the CNS. Indeed, it has been demonstrated that in Was−/− mice B cells display a defective migratory ability towards sphingosine 1-phosphate (S1P), a crucial chemoattractant for the positioning of B cells in the splenic marginal zone (MZ) [9]. S1P and its receptors play also an important role in the egress of B and T cells from lymph nodes into the lymphatic circulation (reviewed in [49]). This molecule has recently become an important target in the therapy of CNS autoimmunity, and Fingolimod (FTY720), which upon in vivo phosphorylation resembles S1P and impairs lymphocyte trafficking through the receptor subtype S1P1, has shown therapeutic efficacy in EAE [50] and, most importantly, in MS patients [51].
Conclusions
Although WASP deficiency does not impair the development of myelin-specific autoreactive T cells, it does importantly blunt the ability of these cells to induce disease in the target organ, the CNS. Additionally, the absence of WASP in CNS microglial cells could limit their activation and contribute to EAE resistance in WASP-deficient animals. Understanding the role of WASP in the development of CNS autoimmunity might lead to a deeper comprehension of the molecules and pathways involved in the complex pathology of EAE and MS. Modulation of WASP activation could then become a target for the treatment of CNS inflammatory diseases.
Supporting Information
Frequency of WASP-positive T cells in Was+/− heterozygous mice. Frequency of WASP-positive T cells evaluated by flow cytometry in peripheral blood of Was+/− heterozygous mice before EAE challenge and in spleen and lymph nodes of the same Was+/− heterozygous mice 14 days after EAE challenge (panel A) and representative histograms showing WASP expression in Was−/−, Was+/− and WT mice on peripheral blood samples before EAE challenge (panel B).
(TIFF)
Acknowledgments
We thank Renato Longhi for the synthesis of MOG35–55 peptide.
Funding Statement
This work was supported by grants from Italian Telethon Foundation (to AV), AISM-FISM (to RP and GC), Ministero della Salute - Grant Giovani Ricercatori (to RP and MB); European Research Council grant 261079- NEUROTRAFFICKING (GC); National Multiple Sclerosis Society (NMSS), New York, NY, USA (GC); Fondazione Cariverona (GC). SM is supported by a Research Fellowship FISM - Fondazione Italiana Sclerosi Multipla - cod. 2011/B/6. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ochs HD, Thrasher AJ (2006) The Wiskott-Aldrich syndrome. J Allergy Clin Immunol 117: 725–738. [DOI] [PubMed] [Google Scholar]
- 2. Stewart DM, Treiber-Held S, Kurman CC, Facchetti F, Notarangelo LD, et al. (1996) Studies of the expression of the Wiskott-Aldrich syndrome protein. The Journal of clinical investigation 97: 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, et al. (1996) Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell 84: 723–734. [DOI] [PubMed] [Google Scholar]
- 4. Ancliff PJ, Blundell MP, Cory GO, Calle Y, Worth A, et al. (2006) Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood 108: 2182–2189. [DOI] [PubMed] [Google Scholar]
- 5. Notarangelo LD, Mazza C, Giliani S, D'Aria C, Gandellini F, et al. (2002) Missense mutations of the WASP gene cause intermittent X-linked thrombocytopenia. Blood 99: 2268–2269. [DOI] [PubMed] [Google Scholar]
- 6. Villa A, Notarangelo L, Macchi P, Mantuano E, Cavagni G, et al. (1995) X-linked thrombocytopenia and Wiskott-Aldrich syndrome are allelic diseases with mutations in the WASP gene. Nat Genet 9: 414–417. [DOI] [PubMed] [Google Scholar]
- 7. Bosticardo M, Marangoni F, Aiuti A, Villa A, Grazia Roncarolo M (2009) Recent advances in understanding the pathophysiology of Wiskott-Aldrich syndrome. Blood 113: 6288–6295. [DOI] [PubMed] [Google Scholar]
- 8. Thrasher AJ, Burns SO (2010) WASP: a key immunological multitasker. Nat Rev Immunol 10: 182–192. [DOI] [PubMed] [Google Scholar]
- 9. Westerberg LS, de la Fuente MA, Wermeling F, Ochs HD, Karlsson MC, et al. (2008) WASP confers selective advantage for specific hematopoietic cell populations and serves a unique role in marginal zone B-cell homeostasis and function. Blood 112: 4139–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA (1994) A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr 125: 876–885. [DOI] [PubMed] [Google Scholar]
- 11. Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, et al. (2003) Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics 111: e622–627. [DOI] [PubMed] [Google Scholar]
- 12. Imai K, Morio T, Zhu Y, Jin Y, Itoh S, et al. (2004) Clinical course of patients with WASP gene mutations. Blood 103: 456–464. [DOI] [PubMed] [Google Scholar]
- 13. Steinman L (2008) A rush to judgment on Th17. J Exp Med 205: 1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH (2010) T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol 162: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Larochelle C, Alvarez JI, Prat A (2011) How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett 585: 3770–3780. [DOI] [PubMed] [Google Scholar]
- 16. Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, et al. (1999) Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med 190: 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, et al. (2006) A key regulatory role for histamine in experimental autoimmune encephalomyelitis: disease exacerbation in histidine decarboxylase-deficient mice. J Immunol 176: 17–26. [DOI] [PubMed] [Google Scholar]
- 18. Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, et al. (2002) De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J Immunol 168: 4173–4183. [DOI] [PubMed] [Google Scholar]
- 19.Bosticardo M, Draghici E, Schena F, Sauer AV, Fontana E, et al.. (2011) Lentiviral-mediated gene therapy leads to improvement of B-cell functionality in a murine model of Wiskott-Aldrich syndrome. The Journal of allergy and clinical immunology 127: 1376–1384 e1375. [DOI] [PubMed]
- 20. Bolomini-Vittori M, Montresor A, Giagulli C, Staunton D, Rossi B, et al. (2009) Regulation of conformer-specific activation of the integrin LFA-1 by a chemokine-triggered Rho signaling module. Nature immunology 10: 185–194. [DOI] [PubMed] [Google Scholar]
- 21. Lapilla M, Gallo B, Martinello M, Procaccini C, Costanza M, et al. (2011) Histamine regulates autoreactive T cell activation and adhesiveness in inflamed brain microcirculation. Journal of leukocyte biology 89: 259–267. [DOI] [PubMed] [Google Scholar]
- 22. Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, et al. (2002) Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric G(i)-linked receptors. Journal of immunology 168: 1940–1949. [DOI] [PubMed] [Google Scholar]
- 23. Battistini L, Piccio L, Rossi B, Bach S, Galgani S, et al. (2003) CD8+ T cells from patients with acute multiple sclerosis display selective increase of adhesiveness in brain venules: a critical role for P-selectin glycoprotein ligand-1. Blood 101: 4775–4782. [DOI] [PubMed] [Google Scholar]
- 24. Steinman L (2007) A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature medicine 13: 139–145. [DOI] [PubMed] [Google Scholar]
- 25. Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, et al. (2011) RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol 12: 560–567. [DOI] [PubMed] [Google Scholar]
- 26. Snapper CM, Paul WE (1987) Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236: 944–947. [DOI] [PubMed] [Google Scholar]
- 27. Kerfoot SM, Kubes P (2002) Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. Journal of immunology 169: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 28. Nikolov NP, Shimizu M, Cleland S, Bailey D, Aoki J, et al. (2010) Systemic autoimmunity and defective Fas ligand secretion in the absence of the Wiskott-Aldrich syndrome protein. Blood 116: 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, et al. (2007) Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest 117: 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerberg LS, Dahlberg C, Baptista M, Moran CJ, Detre C, et al.. (2012) Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B cell development and function. Blood. [DOI] [PMC free article] [PubMed]
- 31. Morales-Tirado V, Johannson S, Hanson E, Howell A, Zhang J, et al. (2004) Cutting edge: selective requirement for the Wiskott-Aldrich syndrome protein in cytokine, but not chemokine, secretion by CD4+ T cells. J Immunol 173: 726–730. [DOI] [PubMed] [Google Scholar]
- 32. Morales-Tirado V, Sojka DK, Katzman SD, Lazarski CA, Finkelman FD, et al. (2009) Critical requirement for the Wiskott-Aldrich syndrome protein in Th2 effector function. Blood 115: 3498–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trifari S, Sitia G, Aiuti A, Scaramuzza S, Marangoni F, et al. (2006) Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. J Immunol 177: 7451–7461. [DOI] [PubMed] [Google Scholar]
- 34. Bouma G, Burns S, Thrasher AJ (2007) Impaired T-cell priming in vivo resulting from dysfunction of WASp-deficient dendritic cells. Blood 110: 4278–4284. [DOI] [PubMed] [Google Scholar]
- 35. de Noronha S, Hardy S, Sinclair J, Blundell MP, Strid J, et al. (2005) Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood 105: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 36. Pulecio J, Tagliani E, Scholer A, Prete F, Fetler L, et al. (2008) Expression of Wiskott-Aldrich syndrome protein in dendritic cells regulates synapse formation and activation of naive CD8+ T cells. J Immunol 181: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 37. Adriani M, Aoki J, Horai R, Thornton AM, Konno A, et al. (2007) Impaired in vitro regulatory T cell function associated with Wiskott-Aldrich syndrome. Clinical immunology 124: 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marangoni F, Trifari S, Scaramuzza S, Panaroni C, Martino S, et al. (2007) WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. The Journal of experimental medicine 204: 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maillard MH, Cotta-de-Almeida V, Takeshima F, Nguyen DD, Michetti P, et al. (2007) The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. The Journal of experimental medicine 204: 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snapper SB, Meelu P, Nguyen D, Stockton BM, Bozza P, et al. (2005) WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. Journal of leukocyte biology 77: 993–998. [DOI] [PubMed] [Google Scholar]
- 41. Lafouresse F, Cotta-de-Almeida V, Malet-Engra G, Galy A, Valitutti S, et al. (2012) Wiskott-Aldrich syndrome protein controls antigen-presenting cell-driven CD4(+) T-cell motility by regulating adhesion to intercellular adhesion molecule-1. Immunology 137: 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haddad E, Zugaza JL, Louache F, Debili N, Crouin C, et al. (2001) The interaction between Cdc42 and WASP is required for SDF-1-induced T-lymphocyte chemotaxis. Blood 97: 33–38. [DOI] [PubMed] [Google Scholar]
- 43. Gallego MD, de la Fuente MA, Anton IM, Snapper S, Fuhlbrigge R, et al. (2006) WIP and WASP play complementary roles in T cell homing and chemotaxis to SDF-1alpha. International immunology 18: 221–232. [DOI] [PubMed] [Google Scholar]
- 44. Thrasher AJ (2002) WASp in immune-system organization and function. Nature reviews Immunology 2: 635–646. [DOI] [PubMed] [Google Scholar]
- 45. Ley K, Laudanna C, Cybulsky MI, Nourshargh S (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews Immunology 7: 678–689. [DOI] [PubMed] [Google Scholar]
- 46. Selmaj K, Raine CS, Cannella B, Brosnan CF (1991) Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. The Journal of clinical investigation 87: 949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Almolda B, Gonzalez B, Castellano B (2011) Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Frontiers in bioscience : a journal and virtual library 16: 1157–1171. [DOI] [PubMed] [Google Scholar]
- 48. Sato M, Ogihara K, Sawahata R, Sekikawa K, Kitani H (2007) Impaired LPS-induced signaling in microglia overexpressing the Wiskott-Aldrich syndrome protein N-terminal domain. International immunology 19: 901–911. [DOI] [PubMed] [Google Scholar]
- 49. Hla T, Brinkmann V (2012) Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology 76: S3–8. [DOI] [PubMed] [Google Scholar]
- 50. Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, et al. (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 108: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kappos L, Antel J, Comi G, Montalban X, O'Connor P, et al. (2006) Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 355: 1124–1140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Frequency of WASP-positive T cells in Was+/− heterozygous mice. Frequency of WASP-positive T cells evaluated by flow cytometry in peripheral blood of Was+/− heterozygous mice before EAE challenge and in spleen and lymph nodes of the same Was+/− heterozygous mice 14 days after EAE challenge (panel A) and representative histograms showing WASP expression in Was−/−, Was+/− and WT mice on peripheral blood samples before EAE challenge (panel B).
(TIFF)