Abstract
Hemocyanins, the huge oxygen-transporting glycoproteins of some mollusks, are used as immunomodulatory proteins with proven anti-cancer properties. The biodiversity of hemocyanins has promoted interest in identifying new anti-cancer candidates with improved immunological properties. Hemocyanins promote Th1 responses without known side effects, which make them ideal for long-term sustained treatment of cancer. In this study, we evaluated a novel hemocyanin from the limpet/gastropod Fissurella latimarginata (FLH). This protein has the typical hollow, cylindrical structure of other known hemocyanins, such as the keyhole limpet hemocyanin (KLH) and the Concholepas hemocyanin (CCH). FLH, like the KLH isoforms, is composed of a single type of polypeptide with exposed N- and O-linked oligosaccharides. However, its immunogenicity was significantly greater than that of KLH and CCH, as FLH induced a stronger humoral immune response and had more potent anti-tumor activity, delaying tumor growth and increasing the survival of mice challenged with B16F10 melanoma cells, in prophylactic and therapeutic settings. Additionally, FLH-treated mice demonstrated increased IFN-γ production and higher numbers of tumor-infiltrating CD4+ lymphocytes. Furthermore, in vitro assays demonstrated that FLH, but not CCH or KLH, stimulated the rapid production of pro-inflammatory cytokines (IL-6, IL-12, IL-23 and TNF-α) by dendritic cells, triggering a pro-inflammatory milieu that may explain its enhanced immunological activity. Moreover, this effect was abolished when deglycosylated FLH was used, suggesting that carbohydrates play a crucial role in the innate immune recognition of this protein. Altogether, our data demonstrate that FLH possesses increased anti-tumor activity in part because it activates a more potent innate immune response in comparison to other known hemocyanins. In conclusion, FLH is a potential new marine adjuvant for immunization and possible cancer immunotherapy.
Introduction
Adjuvants are key components of successful cancer vaccines, and biomedical research aimed at developing new adjuvants has directly improved the therapeutic efficacy of cancer vaccines [1], [2]. These substances, apart from enhancing antigen immunogenicity, are key factors in modulating the magnitude, type and profile of the immune response, thereby increasing the protective quality of a vaccine [3]. In particular, there is an increasing demand for safe and non-toxic substances that can boost cellular and humoral immune responses against cancer [4], [5]. Among potent immunostimulants, hemocyanins, the large oxygen-transporting glycoproteins found in mollusks, have been shown to trigger a strong Th1 biased cellular and humoral response against certain cancers with negligible toxic side effects, thus making them ideal for long-term repetitive treatments [6], [7].
Hemocyanins have been used clinically as non-specific immunostimulants, with beneficial outcomes, to prevent the progression of superficial bladder cancer [8], [9]. Moreover, these proteins have been fundamental tools as carriers/adjuvants for producing antibodies against tumor-associated antigens, in therapeutic vaccines against cancer [7]. Other therapeutic strategies using hemocyanins include the generation of ex vivo autologous tumor antigen-loaded dendritic cells (DCs) for inducing polyclonal T cell expansion [10]–[12]. Furthermore, studies have demonstrated that hemocyanins contribute to the reversal of the tolerogenic profile of DCs in cancer patients toward a more immunostimulatory profile [13]. The hemocyanin from the gastropod Megathura crenulata, also known as keyhole limpet hemocyanin (KLH), has mainly been used for these applications [14], [15]. However, additional hemocyanin candidates with greater immunogenicity and invariable composition are desirable. The disadvantage of KLH is related to its composition, as it comprises 2 isoforms which coexist in variable proportions in preparations of the agent [16], [17]. Accordingly, several hemocyanins from other species of gastropods have been studied [18], [19], although only the hemocyanin from Concholepas concholepas (CCH) has been preclinically evaluated in a murine model of superficial bladder cancer [20].
Gastropod hemocyanins are enormous glycoproteins (4 to 8 MDa) formed by a complex arrangement of 10 subunits that are self-assembled into hollow cylinders 35 nm in diameter, which are referred to as decamers [21]. This structure is easily observed by negative staining using transmission electron microscopy (TEM). Each subunit, ranging from 350 to 450 kDa, includes eight globular folded domains known as functional units (FUs), and each of them is capable of reversibly binding one oxygen molecule through a pair of copper atoms. In KLH and CCH, the decamers may associate in pairs to form huge molecules called didecamers [22]. Closer analysis has revealed that KLH and CCH differ significantly in their quaternary structures [23] and conformational stabilities [24]. Despite the structural differences between KLH and CCH, we previously demonstrated that both hemocyanins stimulate a Th1 response, suggesting an evolutionarily shared mechanism [20]. An additional feature of the hemocyanins is its carbohydrate content, which can be as high as 9% (w/w), with mannose as the most abundant oligosaccharide [14]. It has been shown that KLH induces the maturation of human DCs through the mannose receptor [13]. In addition, hemocyanins are processed very slowly by DCs, which facilitates more robust and sustained antigen presentation to CD4+ lymphocytes and results in augmented immunogenicity [25].
In this work, we characterize a hemocyanin isolated from the gastropod Fissurella latimarginata, termed FLH, which has not been previously evaluated as an immunostimulant for cancer therapy. We studied the biochemical properties of FLH, along with the immunotherapeutic value of FLH in the B16F10 mouse melanoma model, and our results demonstrate that FLH is more immunogenic than KLH and CCH and leads to decreased tumor growth and improved survival of mice. Moreover, in vitro stimulation of DCs showed that FLH, but not KLH or CCH, could induce the rapid production of pro-inflammatory cytokines. This effect was abolished when deglycosylated FLH was used, indicating that unique glycosylation of this protein may result in its increased immunological when compared to other hemocyanins.
Materials and Methods
Ethics statement
The study was carried out in strict accordance with the Guidelines for the Care and Use of Laboratory Animals (National Commission for Scientific and Technological Research, CONICYT, of Chile) The CONICYT Committee on Animal Welfare approved all animal protocols used in this study (FONDECYT 1110651). The conditions of the animals were monitored daily. Humane endpoints to reduce pain or distress were used during the survival study, considering the following criteria: physical appearance, behavior, movement, hydration, stool consistency and tumor burden. Animals were euthanized by cervical dislocation. All efforts were made to minimize suffering and the procedure was performed under anesthesia if necessary.
C57BL/6 and BALB/c mice were obtained from the Universidad de Chile (Santiago, Chile) and GrupoBios (Santiago, Chile) respectively. C3H/HeJ mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and were bred at Biosonda Corp. The experimental mice were housed at 22 to 24°C with a light/dark cycle of 12/12 h. Sterile water and food were available ad libitum.
Hemocyanin sources
The hemocyanins from Fissurella latimarginata (FLH), Fissurella cumingi (FCH) and Fissurella maxima (FMH) were purified by Biosonda Corp., (Santiago Chile). Hemocyanins were obtained under sterile and pyrogen-free conditions and purified by precipitation at 50% saturation with ammonium sulfate, using the basic procedure of Herkowitz et al. [26], and were suspended in Tris buffer (50 mM Tris pH 7.4, mM CaCl2, 5 mM MgCl2 and 0.15 mM NaCl). The hemocyanin from Concholepas concholepas suspended in PBS (0.1 M sodium phosphate, 0.15 M NaCl; pH 7.2) was provided by the Biosonda Corp. Lyophilized KLH in PBS was purchased from Thermo Scientific (Waltham, Massachusetts, USA) and Calbiochem (Merck, Darmstadt, Germany). Deglycosylated FLH (Ox-FLH) was produced by sodium periodate treatment using the procedure and controls of Arancibia et al. [25]. The endotoxin contents of KLH, CCH and FLH preparations were determined using a PyroGene Recombinant Factor C Endotoxin Detection Assay kit (Lonza Group, Walkersville, USA). All chemicals were analytical grade reagents, and the solutions were prepared using water for human irrigation (Baxter Healthcare Corp., Charlotte, NC, USA) and were filtered through a 0.2-µm membrane filter (Millipore, Billerica, MA, USA).
Cell line
Mouse B16F10 melanoma cells were grown at 37°C in a humidified atmosphere (10% CO2 in air) in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS; HyClone, St. Louis, MO, USA), 100 U/ml penicillin, 100 µg/ml streptomycin, 2.5 µg/ml Fungizone, 1 mM sodium pyruvate and 0.1 mM non-essential amino acids (Invitrogen), as described previously [25]. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay (Sigma Aldrich, St. Louis, MO) according to manufacturer instructions was used to determine viability of B16F10 cells.
Transmission electron microscopy (TEM)
The hemocyanin samples were negatively stained as described previously [23]. Briefly, aliquots of FLH (100 µg/ml) were applied to parlodion-coated copper grid. The proteins were stained with a 1–2% aqueous uranyl acetate solution and then examined and photographed at 80 kV using a Philips Tecnai-12 electron microscope at the Electron Microscopy Facility of the Pontificia Universidad Católica de Chile.
Oligosaccharide analyses
As a first step the nature of the exposed oligosaccharides present in the hemocyanins was determined using the biotinylated lectins (Vector Labs Inc. California, USA) concanavalin A (ConA) and peanut agglutinin (PNA), as described previously [27]. Briefly, the samples (20 µg) were placed on a nitrocellulose membrane and blocked with 3% BSA in Tris-buffered saline (TBS). The membranes were then incubated with the biotinylated lectins (1 µg/ml) in TBS containing 0.3% BSA and 0.05% Tween 20, washed and incubated using 0.1 units streptavidin-ALP and then washed and developed using NBT-BCIP (Thermo Scientific). As a control, the biotinylated lectin was omitted.
The oligosaccharide profiling by MALDI-TOF-MS analysis of purified FLH and CCH was performed by GlycoSolutions Corp., (Marlborough, MA, USA). Briefly, oligosaccharides were released using non-reductive β-elimination, derivatized with 2- aminobenzoic acid, and then analyzed in negative ion mode MALDI-TOF-MS using 2,5-dihydroxybenzoic acid as the matrix. Data analysis for MALDI-TOF-MS data was performed using GlycoMod (expasy.org). A tolerance of ±3 daltons was used. The derivative mass for the 2-aminobenzoic acid was 137 g/mol.
Electrophoretic methods
The native electrophoresis of proteins was performed using a horizontal gel chamber and 1.5% agarose gels prepared in 70 mM Tris and 261 mM boric acid pH 7.4 [27]. The proteins were visualized with Coomassie blue staining. SDS-PAGE analyses were performed using gradient separating gels (5–15%) according to the standard procedure [28]. Molecular masses were estimated based on band mobility with a calibration curve obtained from CCH and KLH as well as pre-stained markers (Invitrogen). In all of these analyses, FCH and FMH were also incorporated as controls.
HPLC analyses
FLH was subjected to anion exchange chromatography (IEX) on a MonoQ 5/5 column (GE Healthcare, Wisconsin, USA) and eluted with a linear NaCl gradient (0 to 0.7 M). The FLH subunit dissociation procedure was performed according to the method of Swerdlow et al. [16]. Briefly, purified FLH in buffer A (130 mM glycine-NaOH, pH 9.6, 10 mM EDTA) was loaded into a MonoQ HPLC column equilibrated with buffer A and eluted with a linear gradient of 0–70% buffer A containing 1.0 M NaCl. Chromatography was monitored at 280 nm, and elution fractions were collected and analyzed by SDS-PAGE [28].
Humoral immune response determination
Two-month-old female BALB/c, C57BL/6 or C3H/HeJ mice were used. Three mice per group were immunized as follows. On day 1, the animals received 200 µg of FLH or the same amount of CCH or KLH as a control in 100 µl PBS intraperitoneally (ip), and the immunization was repeated on day 15. Ten days after, the mice were bled, and sera were obtained to determine the humoral immune response against each hemocyanin using an ELISA as previously described [29]. Briefly, 96-well polystyrene plates (Thermo Scientific) were incubated with 100 µl/well of a 10 µg/ml solution of each hemocyanin in PBS, and then were blocked with 1% PBS-casein. Serial 2-fold dilutions of the immune sera in blocking buffer were incubated for 1.5 h at 37°C. The plates were washed and 100 µl/well of goat anti-mouse IgG serum conjugated with alkaline phosphatase (ALP) (Thermo Scientific) diluted 1/2,500 in blocking buffer was then added to the wells and incubated at room temperature. The plates were then washed and developed by adding pNPP. The absorbance was measured at 405 nm.
To evaluate the carrier property of FLH, the hemocyanins were coupled to 2,4-dinitrofluorobenzene (DNFB) according to Sanger [30]. Briefly, 5 mg of each protein was dissolved in 1 M sodium bicarbonate. Then, 25 µl DNFB was added and mixed vigorously for 45 min at 37°C. Finally, the samples were dialyzed against PBS. Groups of mice were injected ip with 300 µg of the DNFB-conjugated hemocyanins in the presence of complete Freund's adjuvant (Pierce-Endogen) for the first immunization (day 1) and with incomplete Freund's adjuvant (Pierce-Endogen) for the second (day 18) immunization. Ten sera were obtained. The presence of specific IgG antibodies against DNFB was detected in the sera using an indirect ELISA as described above, although the plates were incubated with DNFB coupled to bovine sera albumin (BSA). Titers were defined as the reciprocal of the serum dilution with half of the maximum absorbance.
Experimental immunotherapy schedule
Two-month-old C57BL/6 female mice were either primed with each hemocyanin or left untreated and then challenged with B16F10 melanoma cells using the method of Moltedo et al. with modifications [20]. In brief, 2 weeks prior to tumor implantation, groups of 7 mice were randomized and primed subcutaneously (sc) with 400 µg/100 µl PBS containing FLH, Ox-FLH, CCH or KLH independently or with PBS as the vehicle control. After day 20, the mice were challenged with an intralesional sc injection of 1.5×105 melanoma cells in 100 µl DMEM into the right flank. Immunotherapy was administered for 6 consecutive days or 3 times every 7 days after challenge with the tumor cells. For the experiments using unprimed mice, a group of 5 mice was randomized and injected in the right flank with 1.5×105 B16F10 cells, and 7, 14 and 21 days later, a subcutaneous injection with 1 mg FLH/100 µl PBS was administered intralesionally. The control mice received 100 µl PBS. Tumor dimensions were measured every 3 days, through day 25 (prior to the exponential growth of the tumor cells), and the tumor volume was calculated using the ellipsoid formula.
Tumor infiltration analysis
Groups of 5 C57BL/6 mice were challenged via a sc injection of 1.5×105 melanoma cells in 100 µl and were treated with FLH or PBS as described above. Fresh tumor-infiltrating lymphocytes, natural killer (NK) cells and macrophages were prepared from the tumors at day 18 of the bioassay, as described by Junankar et al. [31]. The cells were stained for specific cell surface markers using anti-CD4/CD3, anti-CD8/CD3, anti-CD19, anti-NK1.1 and anti-F4/80 antibodies (BD Biosciences Pharmingen, USA). The data were acquired on a FACScan flow cytometer (Becton-Dickinson, USA) and were analyzed using WinMDI and FlowJo software (Los Angeles, CA, USA). The results of the experiments are expressed as the percentages of tumor infiltrate-specific cells among the total immune cell population.
Cytokine determination in mice sera and DC cultures
To measure the level of serum IFN-γ, the experimental and control mice were bled on day 13 of the bioassays. Supernatants for cytokine secretion were obtained from C57Bl/6 mouse bone marrow-derived DC cultures, as described by Lutz et al. [32]. Cells were seeded at 1.5×105 cells/ml in 96-well plates and harvested after 24 h of incubation with each hemocyanin in different concentrations (100, 500 and 1,000 µg/ml) and assessed for TNF-α, IL-6, IL-12p40 and IL-23. The purity of the DCs was determined by flow cytometry and found superior to 80%. LPS (5–10 ng/ml) and PBS were used as positive and negative controls, respectively. These cytokines were measured using commercial ELISA kits according to the manufacturer's instructions (R&D systems, Minneapolis, MN, USA). As a control, a sample of the culture medium alone was included.
Statistical analyses
The results of the experiments are expressed as the mean ± SE. Comparisons between groups were made using 1-way or 2-way ANOVAs and the Bonferroni post-test. The survival rate was estimated using the Kaplan-Meier method and the log-rank test. The analyses were performed using GraphPad Prism software (USA). For cytokine determination experiments, the unpaired Student's t-test was used.
Results
FLH consists of a single polypeptide chain
Analysis of purified negatively stained FLH by TEM revealed a homogeneously sized protein with a quaternary structure that corresponded to the classical hollow, cylindrical didecamers of gastropods hemocyanins [21], [23] with an approximate diameter of 350 Å (Figure 1A). As shown in Figure 1B, the subunit composition of FLH in non-reducing conditions included a single polypeptide chain, similar to the closely related hemocyanins isolated from other Fissurella species, such as FCH and FMH; in contrast, KLH and CCH each possess 2 subunits. The molecular weight of the FLH subunit was approximately 350 kDa under reducing conditions (Figure 1C). Analysis using native agarose gels showed that the electrophoretic mobility of FLH was similar to that of KLH (Figure 1D). Additional IEX HPLC analyses with dissociated FLH demonstrated a single elution peak (Figure 1E), which was composed of a single polypeptide chain as determined by SDS-PAGE analysis. A second lower molecular weight band was observed, due to the dissociating condition, which induces some degradation of the protein (Figure 1F). Similar analyses of dissociated KLH and CCH revealed 2 peaks corresponding to 2 subunits [16], [23].
Figure 1. Biochemical characterization of FLH.
(A). TEM of FLH negatively stained. The low-magnification micrograph of the purified protein shows the top (circles) and lateral (rectangles) views of the molecules. The insert shows a high-magnification view of the FLH molecules. (B). SDS-PAGE analysis under non-reducing conditions on a 3–7% polyacrylamide gradient gel stained with Coomassie blue. FLH shows one polypeptide in contrast to CCH and KLH that possess two (arrows). As controls, FCH and FMH were included. (C). SDS-PAGE analysis under reducing conditions on a 3–12% gradient polyacrylamide gel. The two CCH and KLH subunits are noted in contrast to the one subunit of FLH. (D). Native gel agarose electrophoresis. The image shows a single compacted band for each hemocyanin. (E). Chromatography of dissociated FLH. The previously dissociated protein was subjected to IEX HPLC on a MonoQ 5/5 column and eluted with a linear NaCl gradient (0 to 70%, green line). The chromatography was monitored at 280 nm (blue line). (F). SDS-PAGE analysis under reducing conditions of the elution fractions from the chromatography of E. The presence of a single main polypeptide in FLH was confirmed (G). FLH oligosaccharide pattern. Hemocyanins were blotted onto a nitrocellulose membrane, incubated with biotinylated lectins ConA and PNA and developed with avidin-FAL followed by NBT/BCIP detection.
The knowledge of the carbohydrate moieties present in mollusk hemocyanins has been essential for understanding their organization, antigenicity and biomedical properties [33]–[35]. Some hemocyanins possess sugar moieties, such as mannose-rich oligosaccharides and the Thomsen-Friedenreich antigen disaccharide (T antigen disaccharide Galβ1-3GalNAcα1-Ser/Thr), which may play roles in their immune-related properties [36], [37]. To analyze the general glycosylation pattern of FLH, qualitative dot blots were performed using ConA and PNA. The results indicated that FLH possessed both types of carbohydrate moieties, N-linked and O-linked, similar to KLH but different from CCH (Figure 1G). The mass spectrometric analysis of the FLH and CCH oligosaccharides showed the FLH seems to have more microheterogeneity in the N-linked oligosaccharides compared to CCH; the biggest difference between FLH and CCH is the presence of the xylose in the FLH, a sugar also reported in KLH [36], [37]. FLH also showed evidence of oligosaccharides containing sialic acid (although minor, at m/z 2007.6, 2316.8 and 2458.0) which CCH did not. Both CCH and FLH have high-mannose oligosaccharides, as well as fucosylated oligosaccharides (Figure 2); these sugar moieties also has been identified on KLH [36], [37].
Figure 2. N- and O-linked oligosaccharide profile of FLH and CCH.
The structure assignment was based on general knowledge of possible glycosylation in mollusk. The most likely structure was drawn on the spectra; however, additional monosaccharide matches were found for some masses.
Altogether, the biochemical data indicated that FLH is a unique hemocyanin composed of a single subunit. In addition, FLH has a similar carbohydrate composition to KLH but not to CCH, which may be explained by their phylogenetic relationship. M. crenulata and F. latimarginata, which produce KLH and FLH, respectively, are classified within the Archaeogastropoda: Fissurellidae, whereas CCH belongs to the Neogastropoda: Muricidae, a distant group of gastropod mollusks [38].
Superior immunogenicity of FLH versus CCH and KLH
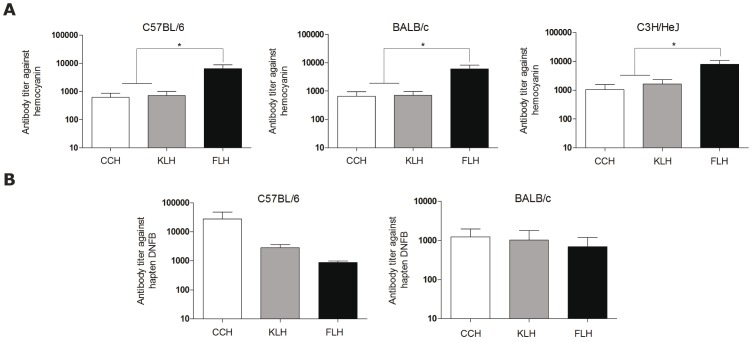
We first evaluated the immunogenicity of FLH and compared it to that of CCH and KLH. For this purpose, mice of different strains (C57BL/6, BALB/c and C3H/HeJ) were injected ip on days 1 and 14 with 200 µg of each hemocyanin without adjuvants. Specific antibody titers were determined by ELISA 10 days after the second immunization. The results showed that FLH produced a significantly higher antibody titer (approximately 1/10,000) than CCH and KLH (approximately 1/1,000) in all of the strains used (Figure 3A). Next, to assess the potential of FLH as a carrier, DNFB was coupled to the hemocyanins in a hapten model. C57BL/6 and BALB/c mice were immunized with 300 µg of each conjugate, with a similar schedule as described above, although CFA and IFA were used in the primary and secondary immunization procedures, respectively. As shown in Figure 3B, the levels of anti-DNFB antibodies were higher in the C57BL/6 mice immunized with CCH-DNFB (approximately 1/10,000) than in the mice immunized with FLH-DNFB and KLH-DNFB (approximately 1/1,000). In contrast, in BALB/c mice, similar titers against DNFB (approximately 1/1,000) were obtained when using all of the hemocyanins as carriers. The titer differences between the mouse strains may be explained by some type of genetic control of the immune response against hemocyanins [39], [40]. In addition, C57BL/6 and BALB/c mice are known to be prone to develop a Th1 and Th2-type activation, respectively. Taken together, these results indicate that FLH generates a more robust specific humoral immune response than CCH and KLH without adjuvant; however, its performance as a carrier for haptens mixed with CFA was similar to that of KLH.
Figure 3. FLH is more immunogenic than CCH and KLH.
(A). Humoral immune response against hemocyanins. Groups of 3 mice were immunized ip with 200 µg of FLH or CCH and KLH in PBS, and the immunization was repeated 15 days later. Ten days later, specific antibody titers were determined by ELISA from serially diluted sera. The mouse IgG antibodies were revealed using goat anti-mouse IgG ALP-conjugated serum; subsequently, pNPP was added. The reaction was read at 405 nm. Data are shown as mean + SEM of n = 3 mice per group and are representative of two independent experiments, *p<0.05 between FLH versus KLH and CCH. (B). Determination of the carrier property of FLH. The hemocyanins were coupled to DNFB; subsequently, groups of 3 C57BL/6 or BALB/c mice were immunized ip with 300 µg of each conjugate with Freund's adjuvants as a repository. Ten days after, the titers against DNFB were determined by ELISA. FLH versus CCH and KLH p not significant.
FLH induces a significant anti-tumor effect in the B16F10 murine melanoma model
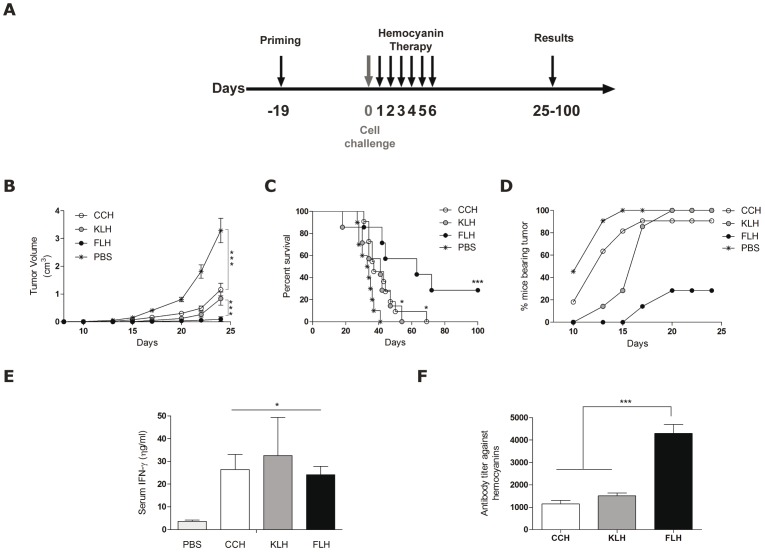
Hemocyanins are well-known immunotherapeutic molecules used in the treatment of superficial bladder cancer; however, these molecules have not been widely investigated for use in other types of cancer. KLH was shown to inhibit the appearance of tumors until 16 days after melanoma HTB68 cell implantation [41]. Furthermore, we showed that both CCH and KLH demonstrated anti-tumor effects in the B16F10 murine melanoma model [25]. Because FLH induced a more robust antibody response when compared to KLH and CCH, it was important to determine whether this property would also translate to improved anti-tumor effects in this melanoma model. Mice previously immunized with FLH, CCH or KLH were challenged with B16F10 melanoma cells and underwent hemocyanin immunotherapy (400 µg/dose) over a period of 6 consecutive days (Figure 4A). Mice treated with FLH demonstrated reduced tumor growth rates, and the percentages of mice bearing tumors decreased significantly in comparison to mice that were treated with CCH and KLH (Figure 4B, 4C and 4D). Additionally, the mouse survival analysis, which was continued until day 100 of the bioassay, indicated that each mouse that received KLH and CCH therapy died by day 70, in contrast to only 70% of the mice subjected to FLH therapy; furthermore, the remaining mice in the FLH group survived more than 100 days after tumor challenge (Figure 4C).
Figure 4. FLH possesses greater anti-tumor activity than CCH and KLH in the B16F10 mouse melanoma model.
(A). The schedule of the bioassay. Groups of 5–7 mice were sc primed with FLH, CCH or KLH (400 µg/100 µl PBS). After 19 days, the mice were challenged sc with 1.5×105 B16F10 melanoma cells, and they then received intralesional injections with 100 µg of each hemocyanin for 6 consecutive days. Data are representative of two independent experiments. (B). Effect of FLH on melanoma growth. The tumor volume was measured every 3 days; ***p<0.001 between CCH, KLH and FLH versus PBS and ***p<0.001 between FLH versus CCH and KLH. (C). Animal survival. Kaplan-Meier survival analysis were compared using the log-rank test; ***p<0.001 FLH versus PBS, *p<0.05 between CCH and KLH versus PBS and *p<0.05 between CCH and KLH versus FLH. (D). Effect of FLH on melanoma growth. Tumor progression was measured for 25 days following the different treatments. (E). Levels of IFN-γ in the sera of mice. The levels of IFN-γ in mouse sera were determined by ELISA on day 13 of the bioassay; *p<0.05 hemocyanins versus the control with PBS. (F). Humoral immune response against hemocyanins. Anti-hemocyanin antibody titers in mouse sera were determined by ELISA on day 13 of the bioassay; ***p<0.001 FLH versus CCH and KLH.
To further investigate the differences observed in the potent effect of FLH and the possible mediators involved, we evaluated the production of IFN-γ and the secretion of anti-hemocyanin antibodies in serum collected on day 13 of the bioassay. No significant differences were observed in the secretion of IFN-γ between hemocyanin treatments, indicating that other differences between the immune response to hemocyanins are involved in the Th1 polarization induced by these proteins (Figure 4E). In addition, the mice that received FLH therapy demonstrated significantly higher antibody titers than those receiving CCH and KLH therapies, which confirmed our previous results and supported the potent immunogenicity of FLH (Figure 4F).
The priming requirements for the anti-tumor effects of KLH and CCH in superficial bladder cancer have been established [20], [42], and we therefore asked whether priming with FLH prior to challenge with melanoma cells would be required for its anti-tumor effects. In therapeutic experiments, mice were challenged with B16F10 cells and after one week the immunotherapy was initiated, with 1 mg/dose of FLH locally at the tumor site on days 7, 14 and 21 post-challenge (Figure 5A). The mice treated with FLH demonstrated a significant delay in tumor growth rate (Figure 5B), fewer of the mice bore tumors (Figure 5D), and 40% of the mice survived up to day 100 of the experiment (Figure 5C). In addition, mice bearing tumors were euthanized on day 18, and the tumor-infiltrating cells were isolated and analyzed by flow cytometry. The data revealed a significant increase in tumor-infiltrating CD4+ lymphocytes, a trend that was similar for NK, CD19 and CD8+ T cells (Figure 5E). As expected, the sera of these mice also contained increased levels of IFN-γ in comparison to the control (Figure 5F). Altogether, these results demonstrated effective anti-tumor activity of FLH in the B16F10 melanoma model.
Figure 5. Immunotherapeutic effect of FLH in the B16F10 mouse melanoma model does not require priming.
(A). Schedule of the bioassay. (B). Effect of FLH on melanoma growth. Tumor growth was followed every 3 days, and each group contained 7 mice; ***p<0.001 between FLH and PBS. (C). Effect of FLH on the survival of mice. The log-rank test was used to compare the curves; *p<0.05 between FLH and PBS. (D). Kinetics of tumor appearance in mice. The percentages of mice bearing tumors were determined visually and by palpation. (E). Tumor-infiltrating immune cells. The quantitative analysis of tumor infiltration by CD8/CD3+, CD11c+, NK1.1+, F4/80+, CD4/CD3+ and CD19+-positive cells in single-cell suspensions derived from B16F10 tumors was performed on day 18 of the bioassay. The results are expressed as the percentages of total viable immune cells. Each group consisted of 3–4 mice; *p<0.05. (F). IFN-γ measurements from mouse sera were obtained on day 18 of the bioassay (same mice and day as the experiment shown in E); *p<0.05 FLH versus PBS.
Deglycosylated FLH demonstrates an attenuated anti-tumor effect
Previous authors have suggested that the anti-tumor effects of hemocyanins may be mediated by their glycosylation [14], [37]. Therefore, a deglycosylated form of FLH (Ox-FLH) was produced to evaluate the role of FLH oligosaccharides in tumor immunotherapy. Two groups of mice were immunized in parallel with FLH and Ox-FLH in the same schedule shown in Figure 4 (Figure 6A). The results demonstrated that Ox-FLH treatment led to a similar delay in tumor growth as native FLH in comparison to the PBS control until day 25 (Figure 6B). However, the survival rates of mice treated with native FLH were significantly improved compared to those of the Ox-FLH and PBS groups until day 100 (Figure 6C). These results suggest that the anti-tumor effect of Ox-FLH is less durable, and that oligosaccharides and/or structural features affected by the chemical treatment of FLH, are fundamental for its anti-tumor effects. However, chemical treatment of FLH did not alter antibody production in challenged mice, and Ox-FLH treatment led to a slightly higher antibody titer compared to FLH (Figure 6D). It is known that the fixation of proteins enhances their resistance to lysosomal proteolysis and therefore increases their immunogenicity [43]. Of note, we did not detect evident allergic reactions or toxic effects in mice treated with FLH or Ox-FLH throughout our studies. In addition, the cytotoxic effect of Ox-FLH on B16F10 melanoma cells cultured in vitro was determined by the MTT assay and we did not found a cytotoxic effect.
Figure 6. Effect of periodate treatment on anti-tumor properties of FLH in the B16F10 mouse melanoma model.
(A). Schedule of the bioassay. Groups of 5–7 mice were sc primed with FLH or Ox-FLH (400 µg/100 µl PBS). After 19 days, the animals were challenged sc with 1.5×105 B16F10 melanoma cells, and they then received intralesional injections of 100 µg of each hemocyanin for 6 consecutive days. (B). Effect of Ox-FLH on melanoma growth. Tumor growth was followed every 3 days until day 25, and each group consisted of 5 mice; ***p<0.001 between FLH and Ox-FLH versus PBS. (C). Effect of Ox-FLH on the survival of mice. Mouse survival was recorded until day 100 of the bioassay. The survival curves were compared using the log-rank test; ***p<0.001 FLH versus PBS, *p<0.05 between Ox-FLH versus PBS. (D). Ox-FLH was more immunogenic than its native counterpart; *p<0.05 between FLH and Ox-FLH.
Deglycosylation of FLH abolishes robust DC maturation
Because DCs play a pivotal role in the regulation of immune responses and tumor immunosurveillance [44], [45], we next investigated the effects of FLH on murine DCs cultured in vitro. In contrast to KLH and CCH, we observed that FLH induced the dose-dependent secretion of several pro-inflammatory cytokines, including IL-6, TNF-α, IL-12p40 and IL-23 (Figure 7A). In addition, FLH induced low upregulation of co-stimulatory molecules (CD80, CD86, CD40) and MHC-II in comparison to LPS, which was used as a positive control (Figure 7B).
Figure 7. FLH induces pro-inflammatory cytokines more rapidly than CCH and KLH in DCs, and Ox-FLH abolishes this secretion.
(A). FLH rapidly induced the dose-dependent secretion of pro-inflammatory cytokines. DCs from C57BL/6 mouse strain were cultured with FLH, CCH or KLH (100, 500 or 1,000 µg/ml) for 24 h, and the supernatants were collected to determine the secretion levels of IL-6, TNF-α, IL-12p40 and IL-23 using commercial ELISA kits. PBS was used as the negative control. Data are shown as mean + SEM of three independent experiments. (B). The FLH-induced, reduced up-regulation of DC maturation markers. DCs were cultured in similar conditions as in (A) and were analyzed for maturation markers (CD80, CD86, CD40 and MHC-II) by flow cytometry. (C). Effect of trypsin-digested Ox-FLH and FLH on the secretion levels of pro-inflammatory cytokines. The DCs were cultured for 24 h with Ox-FLH, native FLH or trypsin-treated FLH. The supernatants were obtained and analyzed for IL-6 and IL-12p40 using commercial ELISA kits. Data are shown as mean + SEM of two independent experiments, *p<0.p<0.05 = *, p<0.01 = ** and p<0.001 = ***, unpaired Student's t-tests.
Because chemical deglycosylation of FLH impaired its anti-tumor effect, we evaluated Ox-FLH for its capacity to elicit DC activation. DCs were incubated for 24 h with Ox-FLH or native FLH, and the secretion of IL-6 and IL-12p40 was analyzed. The results showed that the secreted levels of both cytokines were practically abolished when DCs were treated with Ox-FLH (Figure 7C). Furthermore, supporting the fact that glycosylations are crucial for FLH-induced DC maturation, we proceeded to partially digest the protein with trypsin, which generates smaller fragments and disrupts the cylindrical quaternary structure of the protein while leaving glycosylations intact. As shown in Figure 7C, small fragments of FLH maintained their non-specific immunostimulatory effects, and DC maturation was observed in a dose-dependent manner.
Discussion
This study introduced FLH as a novel immunomodulatory hemocyanin from Fissurella latimarginata. FLH demonstrated greater immunostimulatory properties than traditional KLH and CCH, inducing effective anti-tumor immune responses in a melanoma model. We selected the B16F10 melanoma model because the effectiveness of immune therapies in this model may be considered a reasonable predictor of the effectiveness of immune therapies against human tumors [46]. In addition, KLH is currently used to increase the immunogenicity of some specific glycolipid and mucin-like tumor associated antigens to eliminate the recurrence of such tumors [7].
The data shown here indicate that the mechanism underlying the anti-tumor effect of FLH is, at least in part, mediated by glycosylation. Indeed, it has been shown that KLH contains a variety of unique N-glycans with Gal(β1-6)Man- structural determinants [37]. As mentioned above, the presence of cross-reacting epitopes, such as the T-antigen determinant in the bladder and other epithelial cancer cells, could improve the efficacy of KLH as an immunotherapeutic agent for the treatment of superficial bladder cancer [36]. The results showed that while CCH, KLH and FLH exposed mannose-rich oligosaccharides, only KLH and FLH exposed the T-antigen determinant. However, our results showed that KLH and FLH had different anti-tumor behaviors, indicating that the expression of the T-antigen alone does not explain the effects observed with FLH. It is possible that the arrangement of the GalNAc residues between FLH and KLH may be different and more exposed in FLH, allowing their interaction with some APC receptors to stimulate the innate immune response. Using surface plasmon resonance, it has also been shown that differences in the arrangements, but not the quantities, of GalNAc residues on mucin repeats affect their association rate constants with lectin [47].
Studies have shown that the anti-tumor effect of hemocyanins requires previous priming [20], [42], [48]. However, we observed that FLH, unlike CCH and KLH, had potent anti-tumor activity on murine melanoma without previous priming. To explain this effect, we hypothesized that FLH may induce a more potent inflammatory milieu than CCH and KLH. To test this hypothesis, we studied the effects of these hemocyanins on the expression of cytokines and maturation markers by DCs. In contrast to KLH and CCH, FLH activated the rapid production of pro-inflammatory cytokines, such as IL-6, TNF-α and especially high levels of IL-12p40, which play pivotal roles in coordinating innate and adaptive immunity toward a Th1 response [49]. In humans, direct maturation of DC activity has been demonstrated in response to the mannosylated carbohydrates present in KLH [13]. In this respect, we observed that secretion of IL-12p40 and IL-6 by DCs was dependent on the integrity of the glycosylation of FLH, suggesting that these carbohydrates play a crucial role in innate immune recognition of this protein. The mechanisms associated with these effects are still poorly understood; however, it is likely that, besides the mannose receptor, another C-type lectin receptor expressed by DCs may be involved [50]. In support of this idea, some C-type lectin receptors on the surfaces of antigen presenting cells have been associated with potent anti-tumor responses characterized by the secretion of Th1 cytokines and the generation of cytotoxic T-lymphocytes [51]. Interestingly, FLH induced only low up-regulation of DC maturation markers, in contrast to LPS. In this context, we have previously shown that CCH is slowly processed by murine DCs cultured in vitro and neither the up-regulation of MHC molecules nor the up-regulation of costimulatory molecules were observed [25].
We established that FLH is an invariable protein because it is formed by a unique subunit, unlike KLH. This feature of FLH may be advantageous for the development of new experimental vaccines that use hemocyanins as carriers of tumor associated antigens. The main disadvantages of KLH as a carrier for these antigens in human trials relate to its heterogeneity [52], the variability in its proportion of subunits [16] and the low induction of long-lasting titers of IgM- and IgG-specific antibodies [53], [54]. In this respect, the evaluation of FLH as a carrier for the DNFB has demonstrated similar results to those observed with KLH, and FLH was not superior to CCH. This result may be due to the biochemical differences in the residues exposed by different hemocyanins when chemically coupled to DNFB. In support of this idea we previously reported spectral differences between CCH and KLH [55].
Altogether, these results led to speculation as to the intrinsic structural features responsible for the immunogenicity and anti-tumor effects of FLH. We hypothesized that one peculiar characteristic of FLH may be related to its oligosaccharide components, which may act as natural immunostimulants via their interaction with a specific or putative receptor located on the surface of DCs. FLH binding to this receptor may induce IL-12 secretion and the subsequent activation of other innate immune cells, such as NK cells, which then secrete IFN-γ. Subsequently, FLH localized to phagosomes is slowly processed into peptides by DCs, and these xenogenic peptide sequences from FLH may stimulate CD4+ lymphocytes more strongly than the peptide sequences from other hemocyanins, resulting in increased levels of inflammatory cytokines in the regional lymphoid ganglions. Furthermore, this environment could lead to the proliferation of anti-tumor-associated CD8+ lymphocytes through the indirect stimulation of latent specific responses (i.e., the bystander effect), which could break tolerance towards the tumor. This hypothesis is supported by several lines of evidence. First, DCs express a wide repertoire of saccharide-recognizing surface receptors [56]; of these, the mannose receptor is likely partially involved in this process [13], [25]. Second, hemocyanins are slowly processed [25], and third, hemocyanins stimulate T-helper cell responses, supporting the secretion of cytokines such as IFN-γ and IL-2 by tumor reactive T cells localized in the regional lymph nodes [11]. This inflammatory environment enhances NK cell activity in patients with superficial bladder cancer [57] and in mouse models of this cancer [20], and it is known that NK cells are strongly stimulated by IL-2 to differentiate into lymphokine-activated killer cells [58]. Finally, NK cells can delay tumor growth by means of antibody-dependent cell-mediated cytotoxicity of effector cells [59]. In this respect, the anti-tumor activity of KLH has been associated with antibody cross-reactivity between cell-surface tumor antigens and KLH oligosaccharides, which may promote this type of cytotoxicity [60].
In conclusion, the presented results lead us to hypothesize that hemocyanins from the gastropod Fissurella latimarginata, designed as FLH, can induce an inflammatory milieu more rapidly than CCH and KLH, resulting in a potent innate immune response and an enhanced anti-tumor adaptive immune response against melanoma cells. However, in order to generalize the findings, additional tumor models will be necessary. Consequently, FLH is a potential new marine adjuvant for immunization and possible cancer immunotherapy. Finally, a detailed molecular understanding of the receptors and signaling pathways involved in hemocyanin-induced immunostimulatory processes will be required to elucidate the basis for the qualitative and quantitative differences between these interesting and useful proteins.
Acknowledgments
The authors acknowledge the valuable contributions and involvement of Professors María Antonieta Velanzuela and Marcelo Campos at the University of Chile. The authors are grateful to Andres Mori for his help with DC generation, Alejandro Munizaga of Unidad de Microscopía Electrónica, Pontificia Universidad Católica de Chile, for his technical assistance and Gabriel De Ioannes for assisting with the manuscript figures. The authors would also like to gratefully acknowledge the advice and support provided by Elizabeth Higgins and Elisabeth Kast of GlycoSolutions.
Funding Statement
This work was partially supported by research grants from Fundación COPEC-Universidad Católica No. CQ057 and FONDECYT 1110651 to María Inés Becker. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gupta RK, Siber GR (1995) Adjuvants for human vaccines—current status, problems and future prospects. Vaccine 13: 1263–1276. [DOI] [PubMed] [Google Scholar]
- 2. Petrovsky N, Aguilar JC (2004) Vaccine adjuvants: Current state and future trends. Immunol Cell Biol 82: 488–496. [DOI] [PubMed] [Google Scholar]
- 3. Guy B (2007) The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol 5: 505–517. [DOI] [PubMed] [Google Scholar]
- 4. Ragupathi G, Yeung KS, Leung PC, Lee M, Lau CB, et al. (2008) Evaluation of widely consumed botanicals as immunological adjuvants. Vaccine 26: 4860–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pashine A, Valiante NM, Ulmer JB (2005) Targeting the innate immune response with improved vaccine adjuvants. Nat Med 11: S63–68. [DOI] [PubMed] [Google Scholar]
- 6. Slovin SF, Keding SJ, Ragupathi G (2005) Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol 83: 418–428. [DOI] [PubMed] [Google Scholar]
- 7. Del Campo M, Arancibia S, Nova E, Salazar F, Gonzalez A, et al. (2011) Hemocyanins as immunostimulants. Rev Med Chil 139: 236–246. [PubMed] [Google Scholar]
- 8.Arancibia S, Salazar F, Becker M (2012) Hemocyanins in the Immunotherapy of Superficial Bladder Cancer. Janeza, Croatia: INTECH.
- 9. Lammers RJ, Witjes WP, Janzing-Pastors MH, Caris CT, Witjes JA (2012) Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: Results from a prospective randomized phase III trial. J Clin Oncol 30: 2273–2279. [DOI] [PubMed] [Google Scholar]
- 10. Zhang S, Graeber LA, Helling F, Ragupathi G, Adluri S, et al. (1996) Augmenting the immunogenicity of synthetic MUC1 peptide vaccines in mice. Cancer Res 56: 3315–3319. [PubMed] [Google Scholar]
- 11. Shimizu K, Thomas EK, Giedlin M, Mule JJ (2001) Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res 61: 2618–2624. [PubMed] [Google Scholar]
- 12. Lo-Man R, Vichier-Guerre S, Perraut R, Deriaud E, Huteau V, et al. (2004) A fully synthetic therapeutic vaccine candidate targeting carcinoma-associated Tn carbohydrate antigen induces tumor-specific antibodies in nonhuman primates. Cancer Res 64: 4987–4994. [DOI] [PubMed] [Google Scholar]
- 13. Presicce P, Taddeo A, Conti A, Villa ML, Della Bella S (2008) Keyhole limpet hemocyanin induces the activation and maturation of human dendritic cells through the involvement of mannose receptor. Mol Immunol 45: 1136–1145. [DOI] [PubMed] [Google Scholar]
- 14. Harris JR (1999) Negative staining of thinly spread biological particulates. Methods Mol Biol 117: 13–30. [DOI] [PubMed] [Google Scholar]
- 15. Musselli C, Livingston PO, Ragupathi G (2001) Keyhole limpet hemocyanin conjugate vaccines against cancer: The Memorial Sloan Kettering experience. J Cancer Res Clin Oncol 127: R20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swerdlow RD, Ebert RF, Lee P, Bonaventura C, Miller KI (1996) Keyhole limpet hemocyanin: Structural and functional characterization of two different subunits and multimers. Comp Biochem Physiol B Biochem Mol Biol 113: 537–548. [DOI] [PubMed] [Google Scholar]
- 17. Gebauer W, Stoeva S, Voelter W, Dainese E, Salvato B, et al. (1999) Hemocyanin subunit organization of the gastropod Rapana thomasiana . Arch Biochem Biophys 372: 128–134. [DOI] [PubMed] [Google Scholar]
- 18. Tchorbanov A, Idakieva K, Mihaylova N, Doumanova L (2008) Modulation of the immune response using Rapana thomasiana hemocyanin. Int Immunopharmacol 8: 1033–1038. [DOI] [PubMed] [Google Scholar]
- 19. Dolashka-Angelova P, Stefanova T, Livaniou E, Velkova L, Klimentzou P, et al. (2008) Immunological potential of Helix vulgaris and Rapana venosa hemocyanins. Immunol Invest 37: 822–840. [DOI] [PubMed] [Google Scholar]
- 20. Moltedo B, Faunes F, Haussmann D, De Ioannes P, De Ioannes AE, et al. (2006) Immunotherapeutic effect of Concholepas hemocyanin in the murine bladder cancer model: Evidence for conserved antitumor properties among hemocyanins. J Urol 176: 2690–2695. [DOI] [PubMed] [Google Scholar]
- 21. van Holde KE, Miller KI (1995) Hemocyanins. Adv Protein Chem 47: 1–81. [DOI] [PubMed] [Google Scholar]
- 22. Decker H, Hellmann N, Jaenicke E, Lieb B, Meissner U, et al. (2007) Minireview: Recent progress in hemocyanin research. Integr Comp Biol 47: 631–644. [DOI] [PubMed] [Google Scholar]
- 23. De Ioannes P, Moltedo B, Oliva H, Pacheco R, Faunes F, et al. (2004) Hemocyanin of the molluscan Concholepas concholepas exhibits an unusual heterodecameric array of subunits. J Biol Chem 279: 26134–26142. [DOI] [PubMed] [Google Scholar]
- 24. Idakieva K, Nikolov P, Chakarska I, Genov N, Shnyrov VL (2008) Spectroscopic properties and conformational stability of Concholepas concholepas hemocyanin. J Fluoresc 18: 715–725. [DOI] [PubMed] [Google Scholar]
- 25. Arancibia S, Del Campo M, Nova E, Salazar F, Becker MI (2012) Enhanced structural stability of Concholepas hemocyanin increases its immunogenicity and maintains its non-specific immunostimulatory effects. Eur J Immunol 42: 688–699. [DOI] [PubMed] [Google Scholar]
- 26. Herskowitz H, Harold W, Stavitsky A (1972) Immunochemical and immunogenic properties of a purified keyhole limpet haemocyanin. Immunology 22: 51–61. [PMC free article] [PubMed] [Google Scholar]
- 27. Becker MI, Fuentes A, Del Campo M, Manubens A, Nova E, et al. (2009) Immunodominant role of CCHA subunit of Concholepas hemocyanin is associated with unique biochemical properties. Int Immunopharmacol 9: 330–339. [DOI] [PubMed] [Google Scholar]
- 28. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 29. Oliva H, Moltedo B, De Ioannes P, Faunes F, De Ioannes AE, et al. (2002) Monoclonal antibodies to molluskan hemocyanin from Concholepas concholepas demonstrate common and specific epitopes among subunits. Hybrid Hybridomics 21: 365–374. [DOI] [PubMed] [Google Scholar]
- 30. Sanger F (1945) The free amino groups of insulin. Biochem J 39: 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Junankar SR, Eichten A, Kramer A, de Visser KE, Coussens LM (2006) Analysis of immune cell infiltrates during squamous carcinoma development. J Investig Dermatol Symp Proc 11: 36–43. [DOI] [PubMed] [Google Scholar]
- 32. Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, et al. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223: 77–92. [DOI] [PubMed] [Google Scholar]
- 33. Paccagnella M, Bologna L, Beccaro M, Micetic I, Di Muro P, et al. (2004) Structural subunit organization of molluscan hemocyanins. Micron 35: 21–22. [DOI] [PubMed] [Google Scholar]
- 34. Siddiqui NI, Idakieva K, Demarsin B, Doumanova L, Compernolle F, et al. (2007) Involvement of glycan chains in the antigenicity of Rapana thomasiana hemocyanin. Biochem Biophys Res Commun 361: 705–711. [DOI] [PubMed] [Google Scholar]
- 35. Gatsogiannis C, Markl J (2009) Keyhole limpet hemocyanin: 9-A CryoEM structure and molecular model of the KLH1 didecamer reveal the interfaces and intricate topology of the 160 functional units. J Mol Biol 385: 963–983. [DOI] [PubMed] [Google Scholar]
- 36. Wirguin I, Suturkova-Milosevic L, Briani C, Latov N (1995) Keyhole limpet hemocyanin contains Gal(beta 1-3)-GalNAc determinants that are cross-reactive with the T antigen. Cancer Immunol Immunother 40: 307–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kurokawa T, Wuhrer M, Lochnit G, Geyer H, Markl J, et al. (2002) Hemocyanin from the keyhole limpet Megathura crenulata (KLH) carries a novel type of N-glycans with Gal(beta1-6)Man-motifs. Eur J Biochem 269: 5459–5473. [DOI] [PubMed] [Google Scholar]
- 38.Ponder WF, Lindberg DR, editors (2008) Phylogeny and Evolution of the Mollusca. Berkeley: University of California Press.
- 39. Cerottini JC, Unanue ER (1971) Genetic control of the immune response of mice to hemocyanin. I. The role of macrophages. J Immunol 106: 732–739. [PubMed] [Google Scholar]
- 40. Wells CA, Ravasi T, Faulkner GJ, Carninci P, Okazaki Y, et al. (2003) Genetic control of the innate immune response. BMC Immunol 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rizvi I, Riggs DR, Jackson BJ, McFadden DW (2007) Keyhole limpet hemocyanin: An effective adjunct against melanoma in vivo. Am J Surg 194: 628–632. [DOI] [PubMed] [Google Scholar]
- 42. Lamm DL (2003) Intravesical therapy for superficial bladder cancer: Slow but steady progress. J Clin Oncol 21: 4259–4260. [DOI] [PubMed] [Google Scholar]
- 43. Delamarre L, Couture R, Mellman I, Trombetta ES (2006) Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med 203: 2049–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Banchereau J, Steinman R (1998) Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- 45. Strioga M, Schijns V, Powell DJ Jr, Pasukoniene V, Dobrovolskiene N, et al. (2013) Dendritic cells and their role in tumor immunosurveillance. Innate Immun 19: 98–111. [DOI] [PubMed] [Google Scholar]
- 46. Kochenderfer JN, Gress RE (2007) A comparison and critical analysis of preclinical anticancer vaccination strategies. Exp Biol Med (Maywood) 232: 1130–1141. [DOI] [PubMed] [Google Scholar]
- 47. Kato K, Takeuchi H, Ohki T, Waki M, Usami K, et al. (1972) A lectin recognizes differential arrangements of O-glycans on mucin repeats. Biochem Biophys Res Commun 371: 698–701. [DOI] [PubMed] [Google Scholar]
- 48. Lamm DL, Dehaven JI, Riggs DR (2000) Keyhole limpet hemocyanin immunotherapy of bladder cancer: Laboratory and clinical studies. Eur Urol 37: 41–44. [DOI] [PubMed] [Google Scholar]
- 49. Watford WT, Moriguchi M, Morinobu A, O'Shea JJ (2003) The biology of IL-12: Coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev 14: 361–368. [DOI] [PubMed] [Google Scholar]
- 50. van Vliet SJ, Paessens LC, Broks-van den Berg VC, Geijtenbeek TB, van Kooyk Y (2008) The C-type lectin macrophage galactose-type lectin impedes migration of immature APCs. J Immunol 181: 3148–3155. [DOI] [PubMed] [Google Scholar]
- 51. Qi C, Cai Y, Gunn L, Ding C, Li B, et al. (2011) Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived beta-glucans. Blood 117: 6825–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gathuru JK, Koide F, Ragupathi G, Adams JL, Kerns RT, et al. (2005) Identification of DHBcAg as a potent carrier protein comparable to KLH for augmenting MUC1 antigenicity. Vaccine 23: 4727–4733. [DOI] [PubMed] [Google Scholar]
- 53. Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO (2000) Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine 19: 530–537. [DOI] [PubMed] [Google Scholar]
- 54. Ragupathi G, Cappello S, Yi SS, Canter D, Spassova M, et al. (2002) Comparison of antibody titers after immunization with monovalent or tetravalent KLH conjugate vaccines. Vaccine 20: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 55. Leyton P, Lizama-Vergara P, Campos-Vallette M, Becker M, Clavijo E, et al. (2005) Surface enhanced Raman spectrum of nanometric molecular systems. J Chil Chem Soc 50: 725–730. [Google Scholar]
- 56. Trombetta ES, Mellman I (2005) Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23: 975–1028. [DOI] [PubMed] [Google Scholar]
- 57. Molto LM, Carballido J, Jurincic C, Lapena P, Manzano L, et al. (1991) Keyhole limpet hemocyanine can enhance the natural killer activity of patients with transitional cell carcinoma of the bladder. Eur Urol 19: 74–78. [DOI] [PubMed] [Google Scholar]
- 58. Mule JJ, Yang J, Shu S, Rosenberg SA (1986) The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo: Direct correlation between reduction of established metastases and cytolytic activity of lymphokine-activated killer cells. J Immunol 136: 3899–3909. [PubMed] [Google Scholar]
- 59. Levy EM, Roberti MP, Mordoh J (2011) Natural killer cells in human cancer: From biological functions to clinical applications. J Biomed Biotechnol 2011: 676198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Geyer H, Wuhrer M, Resemann A, Geyer R (2005) Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J Biol Chem 280: 40731–40748. [DOI] [PubMed] [Google Scholar]