Abstract
Nuclear receptors and their coregulators play a critical role in brain development by regulating the spatiotemporal expression of their target genes. The arginine-glutamic acid dipeptide repeats gene (Rere) encodes a nuclear receptor coregulator previously known as Atrophin 2. In the developing cerebellum, RERE is expressed in the molecular layer, the Purkinje cell layer and the granule cell layer but not in granule cell precursors. To study RERE's role in cerebellar development, we used RERE-deficient embryos bearing a null allele (om) and a hypomorphic allele (eyes3) of Rere (Rere om/eyes3). In contrast to wild-type embryos, formation of the principal fissures in these RERE-deficient embryos was delayed and the proliferative activity of granule cell precursors (GCPs) was reduced at E18.5. This reduction in proliferation was accompanied by a decrease in the expression of sonic hedgehog (SHH), which is secreted from Purkinje cells and is required for normal GCP proliferation. The maturation and migration of Purkinje cells in Rere om/eyes3 embryos was also delayed with decreased numbers of post-migratory Purkinje cells in the cerebellum. During the postnatal period, RERE depletion caused incomplete division of lobules I/II and III due to truncated development of the precentral fissure in the cerebellar vermis, abnormal development of lobule crus I and lobule crus II in the cerebellar hemispheres due to attenuation of the intercrural fissure, and decreased levels of Purkinje cell dendritic branching. We conclude that RERE-deficiency leads to delayed development of the principal fissures and delayed maturation and migration of Purkinje cells during prenatal cerebellar development and abnormal cerebellar foliation and Purkinje cell maturation during postnatal cerebellar development.
Introduction
Cerebellar foliation is a highly conserved feature of the mammalian brain with all mammals having at least ten folia along the anterior-posterior (AP) axis of the cerebellar vermis [1]–[3]. Foliation contributes to expanded surface area, permitting the mammalian cerebellum to accommodate more cells and allowing for increased numbers of sophisticated neural circuits [4]. Some types of neural circuits are located exclusively within specific folia. For example, spinocerebellar mossy fibers project only to the anterior lobules I-V and posterior lobules VIII/IX in the cerebellar vermis of mice [5]–[7]. Since the cerebellum provides a platform for innervations of neural circuits carrying sensory-motor information, it is not surprising that processes that disrupt foliation are frequently associated with abnormal motor behaviors including ataxia and impaired motor coordination [1], [5], [6], [8], [9].
In mice, the cerebellar primordium is formed between embryonic days 9 (E9) and 12 (E12) and is transformed into a cylindrical structure by E15.5 [10]. After birth, the cerebellum grows rapidly with robust expansion of granule cells and begins to form three landmark regions—the central vermis, the lateral hemispheres, and the lateral most flocculi and paraflocculi. A specific set of fissures and folia is found in the vermis and the hemispheres at adulthood. Mice have at least ten lobules in the vermis—lobules I through X—and four lobules in the hemispheres—the simple lobule, crus I of the ansiform lobule, crus II of the ansiform lobule and the paramedian lobule—along the anterior-posterior (AP) axis [1]–[3], [11].
Foliation of the principal fissures initiates in the vermis area around E17.5. By E18.5, the five cardinal lobes (anterobasal, anterodorsal, central, posterior, and inferior lobes) and the four principal fissures (preculminate, primary, secondary, and posterolateral fissures) can be identified [1], [12]. After birth, the cardinal lobes are gradually divided by additional fissures. The anterobasal lobe is the first folia to be divided at P0 and forms lobules I through III [3], [13]. Lobules VII and VIII are developed from the central lobe by P1. Lobule VI is demarcated from lobule VII by P3. By P5, the anterodorsal lobe is transformed into lobules IV and V. The posterior lobe becomes lobule IX and the inferior lobe corresponds to lobule X [12]. Development of all ten folia along the AP axis of the vermis is completed around P7 in most mouse strains.
Two dynamic cellular events, the proliferation of the granule cell precursors (GCPs) and the anchoring of Purkinje cells, drive folial development [1], [14]–[17]. At E17.5, the differential proliferation rate of GCPs induces thickening of the external granule cell layer (EGL) at sites that will ultimately form the bases of the principal fissures. This thickening is accompanied by invagination of the EGL and folding of the Purkinje cell layer (PCL). Sudarov and Joyner defined the base of each developing fissure as an anchoring center [12]. Anchoring centers acquire a distinct cytoarchitecture with GCPs located at anchoring centers becoming distinctly elongated along the axis of the forming fissure. Bergmann glia cells located at the base of the immature fissures radiate their fibers towards the anchoring center. This is in contrast to most areas of the cerebellum, where fibers from Bergmann glial are oriented parallel to each other and project perpendicular to the surface of the EGL [12]. In addition, Ma et al. showed that Ric-8a, a guanine nucleotide exchange factor in the G-protein-coupled receptor pathway, is required to maintain adhesion between Bergmann glia and the basement membrane and that this adhesion is required for complete fissure formation during cerebellar development [18].
It is well established that sonic hedgehog (SHH) signaling regulates the expansion of GCPs during cerebellar development [15], [17]. Since GCP proliferation is a critical factor in folial development, it is not surprising that alterations in SHH signaling pathway genes cause cerebellar hypoplasia and abnormal cerebellar foliation [14], [16], [19], [20]. SHH is expressed in the Purkinje cells of the cerebellum [15], [17]. Thus, crosstalk between GCPs and Purkinje cells plays a critical role in normal foliation.
The arginine-glutamic acid dipeptide (RE) repeats gene (Rere) is named after the dipeptide repeats found in RERE's carboxyl terminal. RERE was previously known as Atrophin 2 (ATR2) based on the similarities seen between RERE and Atrophin 1 (ATR1). In particular, the C-terminal region of RERE is highly homologous to that of ATR1. However, RERE has unique BAH, ELM2, SANT, and GATA domains in its N-terminal region which are thought to play a role in protein/protein interactions, chromatin remodeling and DNA binding [21]. It has been proposed that RERE acts as a nuclear receptor coregulator through physical interaction with members of nuclear receptor subfamily 2 (NR2) such as NR2F2 (COUP-TFII) and NR2E1 (TLX) [22], [23]. In addition, RERE also has been shown to recruit histone deacetylase 1/2 (HDAC1/2) in vitro and in mouse embryos [23], [24]. This function requires expression of RERE's N-terminal region.
The role of RERE during embryonic development has been explored using mouse models. Zoltewicz et al. identified a mouse line carrying an N-ethyl-N-nitrosourea (ENU) induced splice junction mutation in Rere (c.396+2T>A) which they named openmind (om) based on the open neural tube defects seen in embryos that were homozygous for this mutation [24]. This mutation disrupts the splice donor site of Rere's second coding exon causing skipping of this exon, a shift in the reading frame and induction of a premature stop codon. Whole-mount in situ hybridization revealed that Rere mRNA was virtually undetectable in Rere om/om embryos, suggesting that this mutation was a null allele [24]. We subsequently demonstrated that Rere om/om embryos also have undetectable levels of RERE protein by western blot [25].
Mice that are heterozygous for the om allele (Rere +/om) are viable and fertile. In contrast, Rere om/om embryos have heart looping defects and die with signs of cardiac failure between E9.5 and E11.5 [24]. These embryos also have fusion of the telencephalic vesicles, defects of the optic vesicles and failure of anterior neural tube closure [24]. These data suggest that RERE plays a critical role in early development of the central nervous system. However, Rere om/om embryos cannot be effectively used to determine the role of RERE in the development of the cerebellum whose patterning and maturation begin during the latter stages of embryonic development and are not completed until the postnatal period.
In this study we used RERE-deficient embryos and mice bearing the om null allele and a hypomorphic Rere allele, eyes3 (c.578T>C, p.Val193Ala) to study the role of RERE during cerebellar development. The eyes3 allele was identified in an ENU-based screen and causes its deleterious effects on development by reducing RERE function rather than decreasing RERE protein expression [25]. Unlike Rere om/om embryos which die around E9.5, Rere om/eyes3 mice can be recovered in Mendelian ratios at birth [25]. Although the majority of these mice die in the perinatal period due to cardiac and renal defects, some survive into adulthood making it possible to determine the effect of RERE depletion on all stages of cerebellar development.
Materials and Methods
Ethics statement
All experiments using mouse models were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The associated protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine (Animal Welfare Assurance #A3832-01).
All efforts were made to minimize suffering. Experiments that were likely to induce discomfort, distress, pain, or injury, or required the use of restraining devices were performed on mice that had been properly anesthetized by inhalation of isoflurane in an appropriate enclosure. Euthanasia was carried out using methods consistent with the recommendations of the Panel of Euthanasia of the American Veterinary Medical Association and included inhalation of carbon dioxide or an overdose of an inhaled anesthetic, such as isoflurane, in an appropriate enclosure.
Mouse strains
C57BL/6 embryos and mice were used to define the expression pattern of RERE in the cerebellum. The generation of the om and eyes3 alleles of Rere were described previously [24], [25]. Experiments using these alleles were conducted on a mixed B6/129S6 background.
Preparation of paraffin embedded tissue sections and tissue staining
Tissues were removed by dissection and fixed with Buffered Formalde-Fresh solution (Fisher Scientific, Pittsburgh, PA, USA) for 1 day at 4°C. After washing with phosphate buffered saline solution (PBS), tissues were dehydrated in ethanol and embedded in paraffin. Paraffin embedded tissue blocks were sectioned at 6 µm with an RM2155 microtome (Fisher Scientific). Nissl staining was used for histological analyses of the cerebellum.
Immunohistochemical staining
After deparaffinization, cerebellar tissue sections were blocked with 1X PBS containing 1% bovine serum albumin and 5% normal donkey serum for 1 hour at room temperature. Sections were then incubated with anti-RERE (sc-98415, 1∶100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-calbindin (AB1778, 1∶200; Millipore, Billerica, MA, USA), mouse monoclonal anti-calbindin (C9848, 1∶100; Sigma, St. Louis, MO, USA)—used only for double labeling experiments with anti-RERE—anti-Phospho-Histone H3 (pHH3) (#9701, 1∶200; Cell Signaling, Danvers, MA, USA), anti-Pax6 (PAX6, 1∶200; Developmental Studies Hybridoma Bank (DSHB), Iowa, IO, USA), anti-NR2F2 (ab41859, 1∶1000; abcam, Cambridge, MA, USA), or anti-Cleaved Caspase-3 (#9664, 1∶200; Cell Signaling, Danvers, MA, USA) antibodies diluted in the same blocking solution (1% BSA and 5% normal donkey serum in 1X PBS) overnight at 4°C. Information about the specificity and previous use of each of the primary antibodies used in this study is summarized in Table S1.
After washing with 1X PBS, the sections were incubated with biotin conjugated anti-rabbit IgG or biotin conjugated anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA, USA). Immunoreactivity of each antibody was visualized using either a 3,3′-diaminobenzidine (DAB) substrate kit (Vector Laboratories, Burlingame, CA, USA) or a tyramide signal amplification (TSA) kit (Invitrogen, Grand Island, NY, USA) containing Alexa Fluor 488 dye or Alexa Fluor 568 dye for fluorescent labeling per manufacturer's instructions. Images were acquired on a Zeiss Axioplan microscope equipped with an AxioCam digital camera and imaging system.
Assays for proliferation and apoptosis
Sagittal sections obtained from the vermis region were exclusively used for each assay. To quantify proliferative GCPs, Phospho-Histone H3-positive cells were counted only in the EGL and normalized to the area of the EGL using Image J software (http://rsbweb.nih.gov/ij/). For quantification, data from at least three independent littermates were used. Apoptotic cells were labeled with anti-Cleaved Caspase-3 antibodies. Caspase-3 positive cells were examined in the EGL and in the entire cerebellum.
Western blot analysis
The cerebellums of embryos were removed by dissection and were homogenized with lysis buffer containing 20 mM Tris-HCl (pH7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% SDS, 2.5 mM sodium pyrophosphate, 1 mM Na2VO4, and Complete Protease Inhibitor Cocktail (Roche Applied Bioscience, Mannheim, Germany) per manufacturer's instructions. Protein extracts were resolved by SDS-PAGE and transferred to nitro cellulose membranes. These membranes were probed with anti-SHH (sc-9024, 1∶500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-HSP70 (#4872, 1∶1000; Cell Signaling, Danvers, MA, USA) antibodies. Each blot was visualized using a SuperSignal West Pico Chemiluminescent detection kit (Thermo Scientific, Rockford, IL, USA) per manufacturer's instructions and quantified using ImageJ software (http://rsbweb.nih.gov/ij/). The expression level of SHH was normalized by the intensity of the HSP70 band in the same blot. Data from three cerebellums of each genotype were used for quantification.
Results
Expression of RERE in the developing mouse cerebellum
Although Zoltewicz et al. demonstrated that Rere is broadly expressed between E8.5 and E11.5—with expression being detected in the notochord, the ventral diencephalon, the spinal cord, and the optic vesicles—the expression of RERE has not been described in the developing cerebellum [24]. To evaluate the expression pattern of RERE, we performed immunohistochemical analyses on embryos and mice harvested at E15.5, E17.5, P0 and P14 using an antibody whose specificity was previously tested by western blot [25].
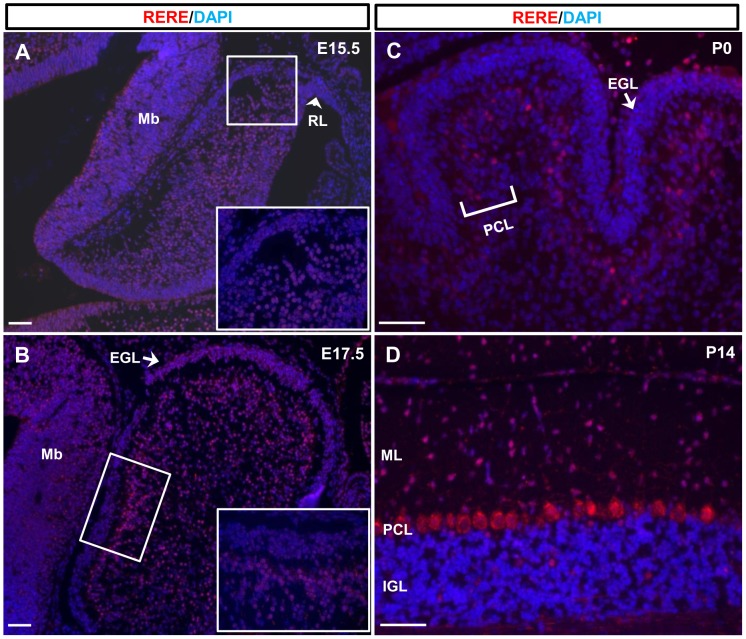
At E15.5, RERE-positive cells were broadly detected in the cerebellum but not in the rhombic lip where GCPs originate (Fig. 1A). At E17.5, RERE expression was still maintained in the entire cerebellum (Fig. 1B, Fig. S1A). Even though RERE-expressing cells were seen in the entire cerebellar region after birth, immunoreactivity of RERE was particularly strong in the PCL at P0, P7 and P14 (Figs. 1C and D, Fig. 2C, and Fig. S1A). At P14, RERE-positive cells were also located in the molecular layer (ML) and the internal granule cell layer (IGL) (Figs. 1D and 2B).
Figure 1. RERE is expressed in the developing cerebellum.
A–D. Mid-sagittal sections of the vermis region were prepared from wild-type embryonic and postnatal mouse cerebellums at E15.5 (A), E17.5 (B), P0 (C), and P14 (D) and were stained with an anti-RERE antibody. RERE-positive cells were labeled with a red color and DAPI was used for counter staining. Inserts represent high magnification views of the boxed areas. A. RERE-positive cells are detected in the cortex of the cerebellum but not in the rhombic lip at E15.5. B. RERE expression is maintained in the entire cerebellum at E17.5. C–D. RERE immunoreactivity is particularly strong in the Purkinje cell layer at P0 (C) and P14 (D). EGL, external granule cell layer; IGL, internal granule cell layer; Mb, midbrain; ML, molecular layer; PCL, Purkinje cell layer; RL, rhombi lip. Scale bar = 100 µm.
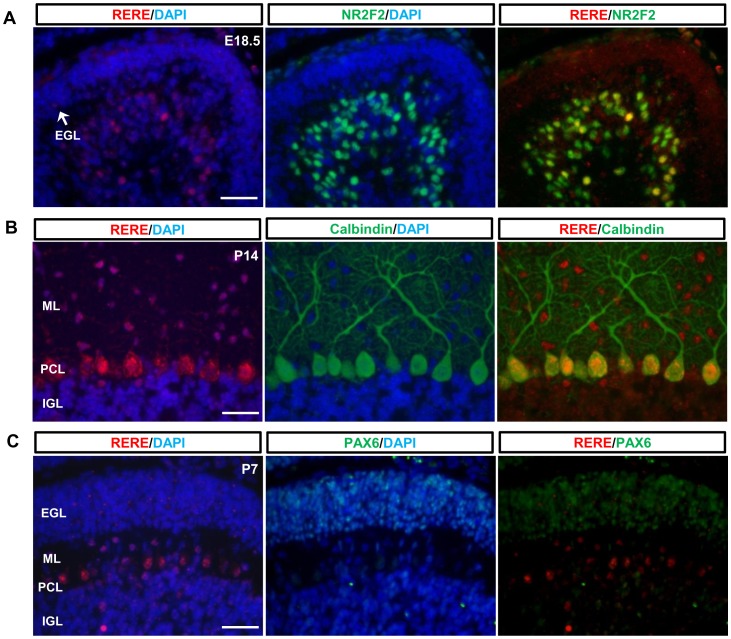
Figure 2. RERE is not expressed in granule cell precursors (GPCs) but is expressed in Purkinje cells.
A–C. Mid-sagittal sections of the vermis region were prepared from wild-type mouse cerebellums at E18.5 (A), P14 (B) and P7 (C). A. Mid-sagittal sections of the vermis were stained with anti-RERE antibody (red color) and anti-NR2F2 antibody (green color). DAPI was used for counter staining. A subset of Purkinje cells labeled with NR2F2 is positive for RERE at E18.5. B. Purkinje cells labeled with mouse monoclonal anti-calbindin antibodies (green color) were also stained with anti-RERE antibodies at P14. RERE expressing cells were also identified in the molecular layer and in the internal granule cell layer at P14. C. RERE immunoreactivity was not detected in PAX6-positive cells in the vermis regions of wild-type cerebellums at P7. EGL, external granule cell layer; IGL, internal granule cell layer; ML, molecular layer; PCL, Purkinje cell layer. Scale bar = 200 µm.
To determine if RERE was expressed in Purkinje cells at various times during cerebellar development, we used two different Purkinje cells markers: NR2F2, which is also known to be expressed in Purkinje cells during the early stages of cerebellar development [26], [27], [44] and calbindin which is a well-known marker for post-mitotic Purkinje cells [28]. At E18.5, a subset of Purkinje cells was positive for both RERE and NR2F2 antibodies but expression of RERE was not detected in all NR2F2-expressing cells (Fig. 2A). In contrast, all Purkinje cells were positive for both RERE and calbindin at P14 (Fig. 2B).
RERE immunoreactivity was seen in the EGL at E17.5 and P7 (Fig. 1B, Fig. 2C, and Fig. S1A) but was less intense than seen in other regions of the cerebellum, had a punctate pattern and did not always counterstain with DAPI. To determine if RERE was expressed in GCPs at these time points, we looked for co-expression of RERE and PAX6—a marker GCPs [29]. However, no RERE, PAX6 double positive cells were identified at E17.5 and P7 suggesting that RERE is not expressed in GCPs during development (Fig. 2C, Fig. S1B).
Formation of the principal fissures is delayed in Rere om/eyes3 embryos
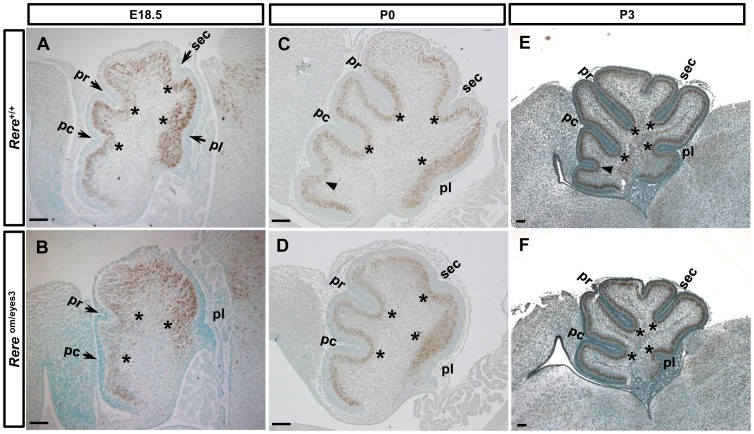
Since RERE is expressed in the cerebellum at E17.5 when foliation begins, we hypothesized that cerebellar foliation may be affected by RERE deficiency. To determine if this was true, we analyzed mid-sagittal sections of the cerebellar vermes of Rere om/eyes3 and wild-type embryos and pups harvested at E18.5, P0 and P3. To visualize the anchoring of fissures, the PCL was stained using an anti-calbindin antibody. In wild-type embryos, the principal fissures were present at E18.5 with indentations of the EGL and PCL marking the locations of the preculminate, primary, secondary and posterolateral fissures (Fig. 3A). In the cerebellums from Rere om/eyes3 embryos at the same time point, the secondary fissure was not visible (Fig. 3B). Invaginations corresponding to the preculminate, primary and posterolateral fissures were visible but their length was shorter than those of wild-type embryos (Fig. 3B).
Figure 3. Foliation of the principal fissures is delayed in the cerebellums of Rere om/eyes3 embryos.
A–F. Mid-sagittal sections of the vermis were prepared from cerebellums of wild-type and Rere om/eyes3 embryos and mice at E18.5 (A–B), P0 (B–C) and P3 (E–F). The Purkinje cell layer was marked using a rabbit polyclonal anti-calbindin antibody. Asterisks indicate the base of each principal fissure. A–B. At E18.5, all of the principal fissure were formed in the cerebellum of wild-type embryos (A) but the preculminate fissure, the primary fissure and the posterolateral fissure were barely appreciable in the cerebellums of Rere om/eyes3 embryos (B). The secondary fissure was not seen in the cerebellums of Rere om/eyes3 embryos (B). Arrows mark each principal fissure. C–D. Delayed development of principal fissures was restored in the cerebellums of Rere om/eyes3 embryos at P0 (D). However, the precentral fissure was found in wild-type cerebellums (arrow head, C) but was not seen in the cerebellums of Rere om/eyes3 mice (D) at P0. E–F. At P3, invagination of the precentral fissure (arrow head, E) demarcates the division of lobule III from lobule 1/II in the cerebellums of wild-type mice but the precentral fissure was not visible in the cerebellums of Rere om/eyes3 mice (F). pc, preculminate fissure; pl, posterolateral fissure; pr, primary fissure; sec, secondary fissure. Scale bar = 100 µm.
At P0, the secondary fissure was formed in the cerebellums from Rere om/eyes3 mice (Fig. 3D). Furthermore, development of principal fissures in Rere om/eyes3 mice was comparable with the cerebellums of wild-type mice (Figs. 3C and D). However, the anchoring center of the precentral fissure was present in the cerebellums from wild-type mice but was absent in cerebellums from Rere om/eyes3 mice (Figs. 3C and D). While indentation of the precentral fissure became deeper in the cerebellums of wild-type mice, the precentral fissure was still missing in the cerebellums of Rere om/eyes3 mice at P3 (Figs. 3E and F). This suggests that the formation of the principal fissures and their corresponding anchoring centers is delayed in Rere om/eyes3 embryos and the precentral fissure is not formed in Rere om/eyes3 mice by P3.
Reduction in proliferation of granule cell precursors and expression of SHH
Expansion of the number of GCPs is known to be a key driving force in cerebellar foliation [14]–[17], [30]. Mice lacking Atoh1 (Math1) and NeuroD1/β2 have foliation defects caused by loss of GCPs [30]–[32]. This led us to determine if the delayed foliation seen in Rere om/eyes3 embryos and mice was accompanied by an increased level of GCP apoptosis. This was accomplished by staining for apoptotic cells in the EGL of Rere om/eyes3 and wild-type embryos at E18.5 using an anti-Cleaved Caspase-3 antibody. However, no Cleaved Caspase-3-positive cells were detected in the EGLs of embryos of either genotype at E18.5 (Figs. S2A and B). In addition, apoptotic GCPs were not identified in the EGL of Rere om/eyes3 mice and wild-type mice at P3 (Figs. S2C and D).
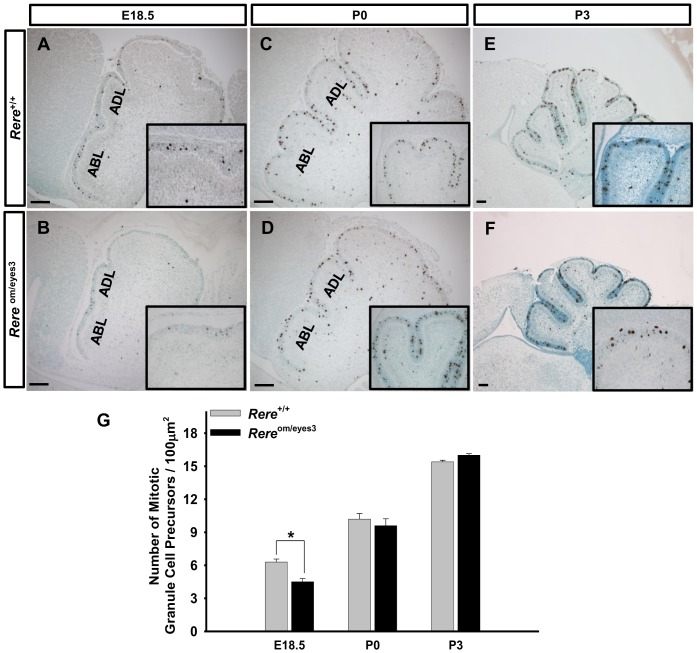
Next, we determined if the proliferative activity of the GCPs is affected in Rere om/eyes3 embryos by labeling mitotic GCPs using Phospho-Histone H3 (pHH3) as a marker for cells that are in the mitotic phase (M phase) of the cell cycle. We found that the number of mitotic GCPs per µm2 was decreased in the EGLs of Rere om/eyes3 embryos when compared with that of wild-type embryos at E18.5 (Figs. 4A, B and G). However, the number of mitotic GCPs per µm2 in the EGLs of Rere om/eyes3 and wild-type mice at P0 and P3 was not statistically different (Figs. 4C–G).
Figure 4. Granule cell precursor proliferation was decreased in the cerebellums of Rere om/eyes3 embryos at E18.5.
A-F. Proliferation assays were performed on cerebellar vermis sections prepared from the cerebellums of wild-type and Rere om/eyes3 embryos and mice at E18.5 (A–B), P0 (C–D) and P3 (E–F) using an anti-Phospho Histone H3 antibody. A-B. The number of mitotic granule cell precursors in Rere om/eyes3 embryos appeared to be lower than that of their wild-type littermates at E18.5. C-F. Mitotic activity of GCPs was comparable between Rere om/eyes3 embryo and wild-type littermate at P0 and P3. G. Phospho-Histone H3-positive cells in the entire external granule cell layer (EGL) were counted and normalized by the area of the EGL (n≥3 with twenty slides containing three sections for each genotype). The number of Phospho-Histone H3-positive GCPs per µm2 was reduced in Rere om/eyes3 embryos at E18.5 when compared to wild-type littermates (* = p<0.03) but was indistinguishable between Rere om/eyes3 embryos and wild-type littermates at P0 and P3. ABL, anterobasal lobe; ADL, anterodorsal lobe. Scale bar = 200 µm.
The precentral fissure, in mice, is developed from the anterobasal lobe (ABL) around the time of birth. Since the precentral fissure is not identified in the cerebellums of Rere om/eyes3 mice at P3, we questioned whether GCP proliferation in the ABL was reduced when compared to other areas of the cerebellum. To address this question, we compared the proliferative activity of GCPs in the ABL with the proliferative activity of GCPs in the adjacent anterodorsal lobe (ADL). Significant reductions in GCP proliferative activity were identified in both the ABLs and ADLs of Rere om/eyes3 embryos at E18.5 when compared with those of their wild-type littermates (Fig. S3). However, the proliferative activity of GCPs was comparable between the ABL and ADL at this time point. At P0, the proliferative activity of GCPs was not affected in either the ABLs or the ADLs of Rere om/eyes3 mice when compared with those of their wild-type littermates. The proliferative activity of GCPs was also comparable between the ABL and ADL at this time point (Fig. S3). This suggests that absence of the precentral fissure in Rere om/eyes3 mice is not due to a specific reduction in GCP proliferation in the ABL.
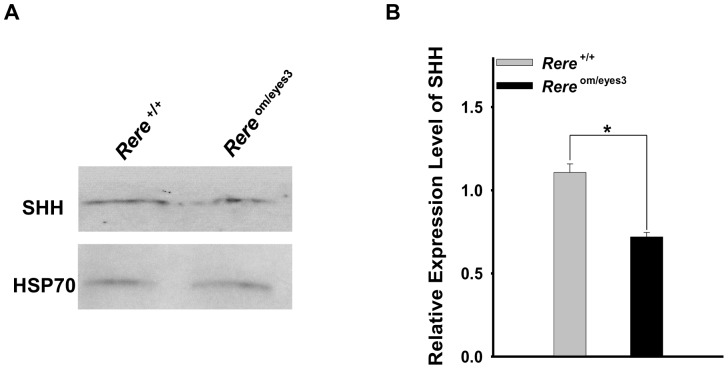
It has been shown that SHH is produced from Purkinje cells and acts as a mitogen to increase GCP proliferation in the mouse cerebellum [14], [15], [17], [19]–[20]. Since RERE was expressed in a subset of Purkinje cells at E18.5, and proliferation of GCPs was decreased in Rere om/eyes3 embryos at that time point, we examined the expression level of SHH in the cerebellum of Rere om/eyes3 embryos at E18.5 by western blot. We found that the expression of SHH was significantly lower in extracts from cerebellums obtained from Rere om/eyes3 embryos when compared to extracts from cerebellums obtained from wild-type embryos (Figs. 5A and B).
Figure 5. The level of SHH expression was reduced in the cerebellums of Rere om/eyes3 embryos at E18.5.
A. Protein extracts prepared from the cerebellums of Rere om/eyes3 embryos and wild-type littermates at E18.5 were subjected to western blot analysis using an anti-SHH antibody and an anti-HSP70 antibody. Expression of SHH in the cerebellum of Rere om/eyes3 embryos was lower than that of their wild-type littermates. Expression of HSP70 was comparable between Rere om/eyes3 embryos and their wild-type littermates. B. To quantify the level of SHH expression, the density of the SHH band was normalized by the density of HSP 70 band in same blot. The expression level of SHH was significantly decreased in the cerebellums of Rere om/eyes3 embryos when compared to the level of SHH in the cerebellums of their wild-type littermates at E18.5 (three independent experiments were performed for quantification; * = p<0.01).
Migration and maturation of Purkinje cells is delayed in the developing cerebellums of Rere om/eyes3 embryos
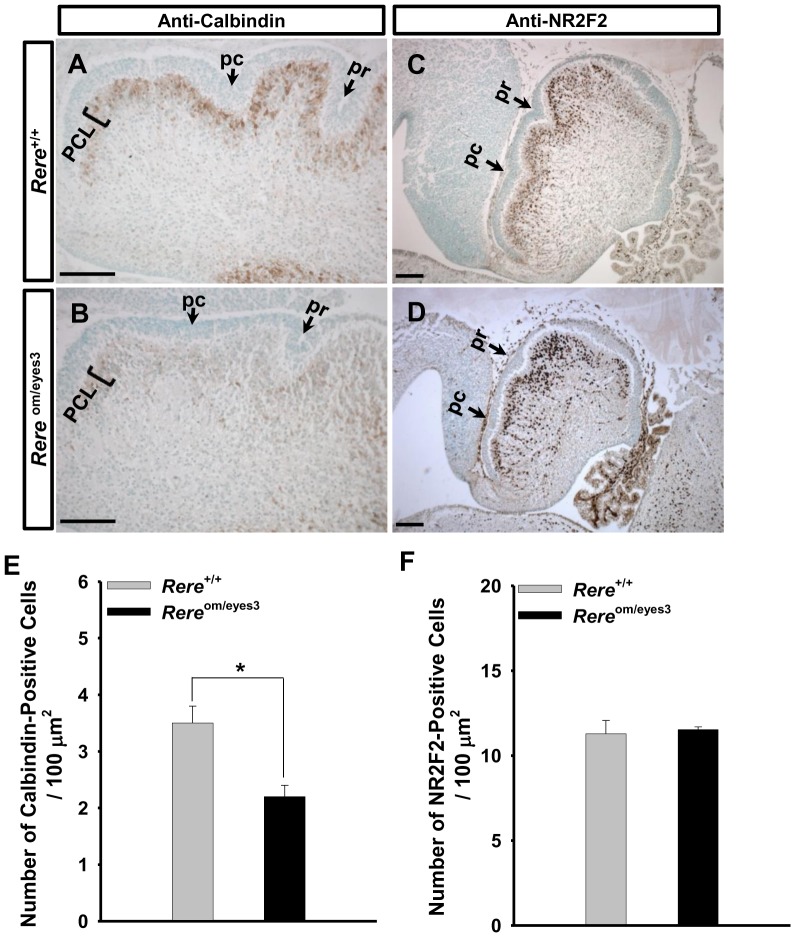
In addition to a delay in foliation, we found that the number of calbindin-positive cells in the cerebellums of Rere om/eyes3 embryos appeared to be less than that seen in wild-type littermates at E18.5 (Figs. 6A and B). To the quantify number of calbindin-positive cells, we counted calbindin-positive cells in the cerebellums of both genotypes and normalized by area. The number of calbindin-positive cells per µm2 was significantly decreased in the cerebellums of Rere om/eyes3 embryos when compared with cerebellums obtained from their wild-type littermates at E18.5 (Fig. 6 E). Since calbindin is a marker of post-mitotic Purkinje cells, this suggested that the number of these cells is reduced in the cerebellums of Rere om/eyes3 embryos.
Figure 6. The migration and maturation of Purkinje cells was altered in Rere om/eyes3 embryos at E18.5.
A–D. Mid-sagittal sections of the vermis were probed with rabbit polyclonal anti-calbindin antibodies (A and B) or anti-NR2F2 antibodies (C and D). A–B. Number of calbindin-positive cells appeared to be lower in the cerebellums of Rere om/eyes3 embryos (B) when compared with those of their wild-type littermates (A). C–D. In the cerebellums of wild-type embryos, most of the NR2F2-positive cells were located in the Purkinje cell layer underneath the external granule cell layer at E18.5 (C). In contrast, the number of NR2F2-positve cells was increased in the center of cerebellar cortex of Rere om/eyes3 embryos at same time point (D). E-F. Calbindin-positive cells (E) and NR2F2-positive cells (F) were counted in the cerebellums of 18.5 embryos of both genotypes and normalized by the area of the cerebellum. The number of calbindin-positive cells/µm2 was significantly lower in the cerebellums of Rere om/eyes3 embryos when compared with wild-type littermates (* = p<0.03). However, number of NR2F2-positive cells/µm2 was comparable between Rere om/eyes3 embryos and their wild-type littermates. Quantification was performed using fifteen slides containing at least three sections from three independent littermates. pc, preculminate fissure; pr, primary fissure; PCL, Purkinje cell layer. Scale bar = 100 µm.
To determine why the number of post-mitotic Purkinje cells was less in the Rere om/eyes3 cerebellum, we first looked for evidence of increased Purkinje cell apoptosis by marking apoptotic cells using an anti-Cleaved Caspase-3 antibody. However, no Caspase-3-positive cells were detectable in the PCL of Rere om/eyes3 embryos or their wild-type embryo littermates, suggesting that an increase in Purkinje cell apoptosis was not the cause of reduced post-mitotic Purkinje cell counts at E18.5 (Figs. S2A and B).
Since apoptosis was not associated with reduced number of Purkinje cells in the cerebellums of Rere om/eyes3 embryos, we looked for evidence that Purkinje cells are not properly recruited in the cerebellums of Rere om/eyes3 embryos. To do so, we used NR2F2 as a marker for early Purkinje cells since it is known to be expressed in the cerebellar analge as early as E13.0 and in migrating Purkinje cells starting at E15.5 [27]. In the cerebellums of wild-type embryos at E18.5, most NR2F2-positive cells were located in the PCL and only a few NR2F2-positive cells were identified in the center of cerebellar cortex (Fig. 6C). This pattern is similar to the distribution of calbindin-positive cells in wild-type embryos at this time point (Fig. 6A). In comparison to their wild-type littermates, Rere om/eyes3 embryos had more NR2F2-positive cells located in the center of cerebellar cortex at E18.5 (Figs. 6C and D) and the distribution pattern of NR2F2-positive cells was wider than that of calbindin-positive cells in Rere om/eyes3 embryos at the same time point (Fig. 6B and D).
Although the number of calbindin positive cells per µm2 of cerebellar cortex was decreased in Rere om/eyes3 embryos compared to their wild-type littermates, we found that the number of NR2F2-positive cells per µm2 of cerebellar cortex was comparable between embryos of both genotypes at E18.5 (Fig. 6F).
Taken together, our results suggest that although the total number of Purkinje cells is comparable between Rere om/eyes3 and wild-type embryos, the migration and maturation of Purkinje cells in the cerebellums of Rere om/eyes3 embryos is delayed.
RERE deficiency results in abnormal foliation in the cerebellums of Rere om/eyes3 mice
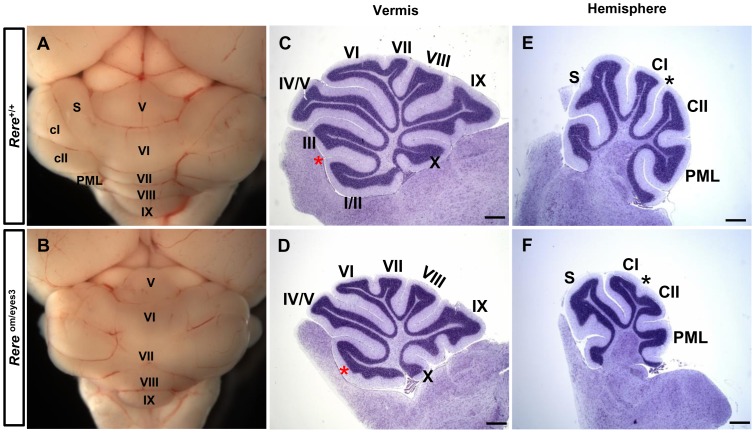
The majority of Rere om/eyes3 mice die in the perinatal period with cardiac and renal defects [25]. However, a subset survives into adulthood allowing us to determine the effects of RERE-depletion on adult cerebellar morphology. Along the medial-lateral axis, the external morphological land marks of the cerebellum—including the vermis and hemispheres—were identified in both Rere om/eyes3 mice and wild-type mice after gross dissection at P21 (Figs. 7A and B). However the cerebellar vermes and cerebellar hemispheres of Rere om/eyes3 mice were smaller than those of their wild-type littermates (Figs. 7A and B). These size differences may be due, in part, to the postnatal growth retardation that has been previously documented in Rere om/eyes3 mice [25].
Figure 7. Rere om/eyes3 mice have cerebellar foliation defects at P21.
A–B. The cerebellar vermis and hemispheres were evident in wild-type and Rere om/eyes3 mice. C–D. Mid-sagittal sections of the vermis region were stained with cresyl violet. In the cerebellums of Rere om/eyes3 mice, the precentral fissure was severely attenuated and failed to divide lobule I/II from lobule III. Red asterisks indicate the location of the precentral fissure in wild-type mice (C) and marks the abnormal lobule in Rere om/eyes3 mice (D). E–F. Sagittal sections of the cerebellar hemispheres were stained with cresyl violet. The intercrural fissure was fully developed in wild-type mice (E), but was severely attenuated in the cerebellums of Rere om/eyes3 mice (F). The black asterisks indicate the intercrural fissure. Roman letters mark corresponding lobules in the vermis of the cerebellum. CI, lobule crus I; CII, lobule crus II; PML, paramedian lobe; S, simple lobule. Scale bar = 500 µm.
In sagittal sections prepared from the vermes of wild-type mice, the anterobasal lobe was completely divided into lobule I/II and lobule III by the precentral fissure at P21 (Fig 7C). In contrast, lobule I/II and lobule III failed to separate at P21 in Rere om/eyes3 mice due to severe attenuation of the precentral fissure (Fig. 7D).
Abnormalities in cerebellar foliation were also evident in the cerebellar hemispheres of Rere om/eyes3 mice. In sagittal sections of the cerebellar hemispheres of wild-type mice, lobule crus I and lobule crus II were fully divided by the intercrural fissure at P21 (Fig. 7E). In Rere om/eyes3 mice, development of the intercrural fissure was severely attenuated and failed to separate lobule crus I from lobule crus II at P21 (Fig. 7F).
Decreased branching of Purkinje cell dendrites in the cerebellums of Rere om/eyes3 mice
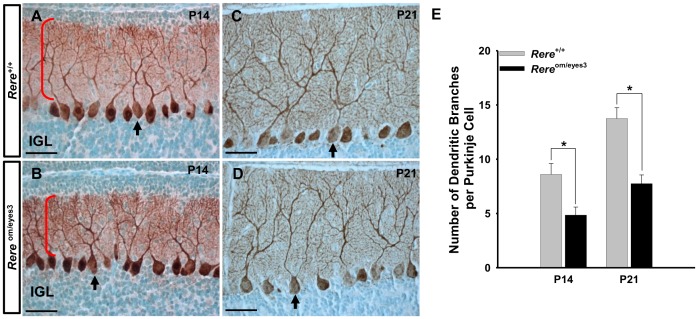
Since RERE is expressed in Purkinje cells during the postnatal period (Figs. 1D and 2B–C), we questioned whether postnatal Purkinje cells maturation was affected by RERE deficiency. Cerebellar sections from Rere om/eyes3 mice and wild-type littermates at P14 and P21 were stained with an anti-calbindin antibody. We found a monolayer of Purkinje cells in cerebellums harvested from mice of both genotypes at P14 and P21 (Figs. 8A–D). However, the Purkinje cell dendrites of the Rere om/eyes3 mice appeared to have decreased arborization when compared to those of wild-type mice (Figs. 8A vs. B and 8C vs. D). When the number of high order dendritic branches was counted, we found that the average number of Purkinje cell dendrites in Rere om/eyes3 mice was significantly decreased when compared to their wild-type littermates at P14 and P21 (Fig. 8E).
Figure 8. Branching of Purkinje cell dendrites was reduced in the cerebellums of Rere om/eyes3 mice.
A–D. Sagittal sections of the vermis of Rere om/eyes3 mice and their wild-type littermates were probed with rabbit polyclonal anti-calbindin antibodies at P14 (A, B) and P21 (C, D). A–D. A monolayer of Purkinje cells was found in the cerebellums of mice of both genotypes. Arrows point to individual Purkinje cells. Red brackets mark the dendrites of the Purkinje cells at P14. The Purkinje cell dendrites branching of Rere om/eyes3 mice (B, D) appeared to be decreased when compared that of their wild-type littermates (A, C). E. Quantification of Purkinje cells branches revealed that the Purkinje cells of Rere om/eyes3 mice at both P14 and P21 have significantly fewer branches compared to the Purkinje cells of wild-type mice at the same time points (* = p<0.03). Purkinje cells containing at least two secondary dendrites were exclusively used for quantification of dendritic branching. Quantification was performed using ten slides containing at least three sections from three independent littermates. IGL, internal granule cell layer. Scale bar = 50 µm.
Discussion
RERE is required for normal prenatal and postnatal cerebellar development
A number of nuclear receptors and their associated coregulators have been shown to play key roles in the temporospatial regulation of gene expression which is required for proper development of the central nervous system. RERE is a nuclear receptor coregulator that is widely expressed in the developing central nervous system and is required for normal brain development. Zoltewicz et al. demonstrated that a complete lack of RERE causes severe defects in CNS development with Rere om/om embryos displaying fusion of the telencephalic vesicles, defects of the optic vesicles, and failure of the anterior neural tube closure at E9.5 [24]. Subsequently, we used RERE-deficient Rere om/eyes3 embryos to shown that RERE deficiency causes brain hypoplasia and decreased numbers of neuronal nuclear antigen (NeuN)-positive hippocampal neurons [25].
To determine if RERE plays a specific role in cerebellar development, we first determined the expression pattern of RERE in the cerebellum at various time points in its development. Zoltewicz et al. had previously shown that RERE is expressed in the isthmus [24], a signaling center at the junction of the mesencephalon and the metencephalon which plays a critical role in regulating cerebellar development [33]–[36]. In this study, we determined that RERE was broadly expressed in the cortex of the cerebellar primordium from E15.5 to E17.5. We also found that RERE was expressed in the postnatal cerebellum with particularly strong expression in Purkinje cells at P0, P7 and P14.
Even though RERE-positive cells were identified in the internal granule cell layer, these cells did not colocalize with PAX6 immunostaining at P7. This suggests that RERE is not expressed in the granule cells of the IGL but may, instead, be expressed in Golgi cells, Lugaro cells and/or the unipolar brush cells at this time point.
Based on these findings, we performed a detailed analysis of cerebellar development using Rere om/eyes3 embryos and mice. This analysis revealed that RERE plays a role in both prenatal and postnatal cerebellar development and that RERE depletion leads to abnormal cerebellar foliation, decreased GCP proliferation associated with altered levels of SHH, delay of Purkinje cell maturation and migration, and reduced branching of Purkinje cell dendrites. These findings are discussed in greater detail in the subsections that follow.
Abnormal foliation of the cerebellums of Rere om/eyes3 mice
Although the principal fissures and cardinal lobes were seen in the cerebellums of Rere om/eyes3 mice after birth, we discovered that RERE deficiency causes a delay in the foliation process during the prenatal period. In wild-type cerebellums, invagination of the EGL and the PCL mark the anchoring centers of the principal fissures at E18.5. These areas will ultimately form the bases of the principal fissures. In contrast, invagination of the EGL and the PCL was limited in the cerebellums of Rere om/eyes3 embryos at E18.5 and the secondary fissure was not anchored. Furthermore, the principal fissures of E18.5 Rere om/eyes3 embryos were shorter than the principal fissures of wild-type embryos at the same time point. At P0 and P3, all of the principal fissures, including the secondary fissure, were identified in the cerebellums of Rere om/eyes3 mice.
Foliation of non-principal fissures begins after birth and undergoes maturation during postnatal development in the mouse cerebellum [1], [12]. In wild-type mice, the anterobasal lobe undergoes further foliation by invagination of the precentral fissure at P0 and lobule III is fully separated from lobule I/II by the precentral fissure at P3. In contrast, in Rere om/eyes3 mice, there was no evidence of precentral fissure anchoring at P3 and lobule I/II and lobule III failed to separate at P21 due to severe attenuation of the precentral fissure.
In the cerebral hemispheres of wild-type mice, the intercrural fissure divides the ansiform lobule into lobule Crus I and lobule Crus II. In Rere om/eyes3 mice, development of the intercrural fissure was limited with failure of the ansiform lobule to subdivide into lobule crus I and lobule crus II at P21.
Based on these data we conclude that RERE deficiency causes a delay in principal fissure initiation during prenatal cerebellar development and that RERE is required for normal postnatal foliation of the cerebellar vermis and hemispheres.
Decreased proliferation of GCPs and expression of SHH in Rere om/eyes3 embryo cerebellum
Expansion in the number of GCP is known to be a driving force in the foliation of the cerebellum [14], [16]–[18], [30]. Our studies did not find evidence of increased GCP apoptosis in Rere om/eyes3 embryos. Instead, we found evidence that RERE deficiency causes a decrease in the proliferative activity of GCPs in the cerebellums of Rere om/eyes3 embryos at E18.5 when compared to wild-type littermates. In contrast, at P0 and P3, GCP proliferation was comparable between Rere om/eyes3 mice and their wild-type littermates. From E18.5 to P3, GCP proliferation was comparable between the anterobasal lobe and the anterodorsal lobe, suggesting that absence of the precentral fissure in Rere om/eyes3 mice is not due to a specific reduction in GCP proliferation in the anterobasal lobe. Since RERE expression was not detected in the rhombic lip at E15.5 or in PAX6-positive GCPs at E18.5, we conclude that RERE deficiency affects GCP proliferation prior to birth in non-cell-autonomous manner.
In mice, Purkinje cell depletion has been shown to cause defects in foliation associated with reduced rates of GCPs proliferation, emphasizing the importance of cross talk between these cell types during cerebellar development [37], [38]. It is well established that SHH is expressed in Purkinje cells and that the SHH signaling pathway is critical for GCP proliferation [16], [18]–[20]. Since RERE is expressed in a subset of Purkinje cells at E18.5, and the mitotic activity of GCPs was decreased in Rere om/eyes3 embryos at the same time, we hypothesized that RERE could be regulating GCP proliferation by modulating the expression of SHH. When we tested this hypothesis, we found that expression of SHH was significantly decreased at E18.5 in the cerebellums of Rere om/eyes3 embryos.
Taken together, these results suggest that RERE deficiency contributes to delay of principal fissure formation through decreased proliferation of GCPs which, in turn, is caused by reduced expression of SHH in the prenatal cerebellum. Whether this reduction in prenatal SHH expression is due to RERE's cell autonomous effects in Purkinje cells remains to be determined.
Purkinje cell abnormalities in Rere om/eyes3 embryos
In mice, Purkinje cell precursors migrate out along the radial-glial-fiber system after E13 and settle in the cortex area of the cerebellar primordium forming a thick and dense zone of Purkinje cells known as the Purkinje cell plate around E15.5 [39], [40]. The Purkinje cells within the Purkinje cell plate form multiple layers along the principal fissures at E18.5, which are maintained until a few days after birth [39], [40]. Rere om/eyes3 embryos at E18.5 had decreased numbers of post-mitotic Purkinje cells per area in the cerebellum when compared to their wild-type littermates. At the same time point, the number of NR2F2-positive Purkinje cells was comparable between Rere om/eyes3 embryos and their littermates but a greater number of migrating NR2F2-positive Purkinje cells were detected in Rere om/eyes3 embryos. These data suggest that migration of Purkinje cells is delayed in Rere om/eyes3 embryos. In addition, the delayed maturation of Purkinje cells in Rere om/eyes3 embryos—evidenced by a decreased number of calbindin-positive Purkinje cells/per area compared to wild-type mice at E18.5—may be responsible for the reduced expression of SHH detected in Rere om/eyes3 embryos at the same time point.
Since RERE is expressed in Purkinje cells at P14, we hypothesized that RERE may also have effects on postnatal Purkinje cell maturation. When we examined cerebellums at P14 and P21, we found that Purkinje cells were organized in a monolayer in both wild-type and Rere om/eyes3 mice. However, closer examination revealed that the level of Purkinje cell dendrite branching was significantly decreased in Rere om/eyes3 mice compared to their wild-type littermate controls at these time points. This suggests that RERE depletion also leads to abnormal postnatal maturation of Purkinje cells.
It is possible that RERE's effects on Purkinje cells migration and maturation are due to its cell-autonomous role within these cells. Testing this hypothesis, and dissecting out RERE's role in other cell types within the cerebellum, will require the use of Rere conditional knockout mice. These mice will also be useful in determining if RERE depletion in the cerebellum leads to abnormalities in motor coordination—something which cannot be easily determined in Rere om/eyes3 mice due to the potential confounding effects of systemic RERE depletion [25].
Nuclear receptors in cerebellar development
RERE's role as a nuclear receptor coregulator suggests that it functions during cerebellar development to mediate the effects of one or more nuclear receptors. RERE has been shown to interact with members of nuclear receptor subfamily 2 (NR2) such as NR2E1 and NR2F2 [22], [23]. NR2E1-deficient mice can have hypoplasia of rhinencephalic and limbic structures—including the olfactory, infra-rhinal and entorhinal cortex, amygdala and dentate gyrus—enlarged ventricles, hydrocephalus, retinal dystrophy, blindness and aggression. However, cerebellar defects have not been described as a consequence of decreased NR2E1 expression, even though NR2E1 has been shown to be expressed in the human cerebellum [41]–[43].
NR2F2 has been documented to play a critical role in cerebellum development, but the expression pattern of NR2F2 in the cerebellum, and many of its effects on cerebellar development, are distinct from those of RERE [44]. Specifically, NR2F2 is expressed exclusively in the Purkinje cells of the cerebellum. In the prenatal stage, delay of foliation was found in Rere om/eyes3 embryos but was not seen in embryos in which Nr2f2 expression was conditionally ablated in neurons using a transgenic Nse-Cre (Nr2f2 flox/flox;Nse-Cre) [44]. Postnatally, foliation defects are seen in both Rere om/eyes3 and Nr2f2 flox/flox; Nse-Cre mice but truncated development of the precentral fissure leads to incomplete division of lobules I/II and III in RERE-deficient mice while NR2F2 depletion led to failure of lobules VIa and VIb to be properly divided. This makes it unlikely that RERE and NR2F2 participate together in regulating these aspects of cerebellar development.
Depletion of both RERE and NR2F2 have a detrimental effect on Purkinje cell maturation with the Purkinje cells of both Rere om/eyes3 and Nr2f2 flox/flox;Nse-Cre mice having decreased dendrite branching. Future studies will be needed to determine if RERE interacts with NR2F2 to affect Purkinje cell maturations and to identify the other nuclear receptors that interact with RERE during cerebellar development.
Supporting Information
RERE is expressed in the developing cerebellum but not in granule cell precursors. A–B. Mid-sagittal sections of the vermis region were prepared from wild-type embryonic and postnatal mouse cerebellums at E17.5 (A, B) and P7 (A) and were stained with an anti-RERE antibody. RERE-positive cells were labeled with a red color (A and B; Alexa 568) and PAX6-positive cells were visualized with a green color (B; Alexa 488). A. Immunoreactivity for anti-RERE antibodies is detected in the external granule cell layer (EGL) and other areas of the cerebellum at E17.5 and P7. B. RERE, PAX6 double positive cells are not identified in the cerebellum at E17.5 suggesting that RERE is not expressed in granule cell precursors. Scale bar = 100 µm. IGL, internal granule cell layer.
(DOCX)
Apoptotic activity in the cerebellum was comparable between Rere om/eyes3 embryos and controls. A–D Mid-sagittal sections from embryos and mice of both genotypes were probed with anti-Cleaved Caspase-3 antibodies to label apoptotic cells at E18.5 (A, B) and P3 (C, D). A-B. A few Cleaved Caspase-3-positive cells were identified in the cerebellar cortexes of Rere om/eyes3 embryos and their wild-type littermates at E18.5. In contrast, apoptotic cells were undetectable in the EGLs and PCLs of Rere om/eyes3 embryos and their wild-type littermates. C–D. Apoptotic activity of the cerebellums between Rere om/eyes3 mice and their wild-type littermates was comparable at P3. Scale bar = 100 µm. EGL, external granule cell layer; Mb, midbrain; PCL, Purkinje cell layer.
(DOCX)
Mitotic activity of granule cell precursors was comparable between the anterobasal and anterodorsal lobes. At E18.5 and P0, proliferation assays were performed on cerebellar vermis sections prepared from wild-type and Rere om/eyes3 embryos and mice using an anti-Phospho Histone H3 antibody. The number of Phospho-Histone H3-positive granule cell precursors (GCPs) in the anterobasal lobe (ABL) and the anterodorsal lobe (ADL) was reduced in Rere om/eyes3 embryos at E18.5 when compared to wild-type littermates (* = p<0.03). However, the proliferative activity of GCPs was comparable between the ABLs and ADLs of Rere om/eyes3 embryos at same time point. At P0, the mitotic activity of GCPs in the ABL and ADL was indistinguishable between Rere om/eyes3 embryos and wild-type littermates. In addition, proliferative activity of GCPs was similar between the ABLs and ADLs of Rere om/eyes3 mice at P0. Phospho-Histone H3-positive cells in the external granule cell layer (EGL) of the ABL and ADL were counted and normalized by the corresponding area of the EGL of each lobe (n≥3 with twenty slides containing three sections for each genotype).
(DOCX)
Additional information about the antibodies used in this study.
(DOCX)
Funding Statement
This research was funded, in part, by the Carloine Wiess Law Fund for Molecular Medicine. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Altman J, Bayer SA (1997) Development of the cerebellar system in relation to its evolution, structure and functions. New York: CRC Press.
- 2. Larsell O (1952) The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol 97: 281–356. [DOI] [PubMed] [Google Scholar]
- 3.Larsell O (1970) The comparative anatomy and histology of the cerebellum from monotremes through apes. Minneapolis: University of Minnesota Press.
- 4. Welker WI (1990) The significance of foliation and fissuration of cerebellar cortex. The cerebellar folium as a fundamental unit of sensorimotor integration. Arch Ital Biol 128: 87–109. [PubMed] [Google Scholar]
- 5. Sillitoe RV, Joyner AL (2007) Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu Rev Cell Dev Biol 23: 549–577. [DOI] [PubMed] [Google Scholar]
- 6. Sotelo C (2004) Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol 72: 295–339. [DOI] [PubMed] [Google Scholar]
- 7. Vogel MW, Ji Z, Millen K, Joyner AL (1996) The Engrailed-2 homeobox gene and patterning of spinocerebellar mossy fiber afferents. Brain Res Dev Brain Res 96: 210–218. [DOI] [PubMed] [Google Scholar]
- 8. Chen YT, Collins LL, Uno H, Chang C (2005) Deficits in motor coordination with aberrant cerebellar development in mice lacking testicular orphan nuclear receptor 4. Mol Cell Biol 25: 2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McNeill EM, Klockner-Bormann M, Roesler EC, Talton LE, Moechars D, et al. (2001) Nav2 hypomorphic mutant mice are ataxic and exhibit abnormalities in cerebellar development. Dev Biol 353: 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sgaier SK, Millet S, Villanueva MP, Berenshteyn F, Song C, et al. (2005) Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron 45: 27–40. [DOI] [PubMed] [Google Scholar]
- 11. Ullmann JF, Keller MD, Watson C, Janke AL, Kurniawan ND, et al. (2012) Segmentation of the C57BL/6J mouse cerebellum in magnetic resonance images. Neuroimage 62: 1408–1414. [DOI] [PubMed] [Google Scholar]
- 12. Sudarov A, Joyner AL (2007) Cerebellum morphogenesis: the foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inouye M, Oda SI (1980) Strain-specific variations in the folial pattern of the mouse cerebellum. J Comp Neurol 190: 357–362. [DOI] [PubMed] [Google Scholar]
- 14. Corrales JD, Blaess S, Mahoney EM, Joyner AL (2006) The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development 133: 1811–1821. [DOI] [PubMed] [Google Scholar]
- 15. Lewis PM, Gritli-Linde A, Smeyne R, Kottmann A, McMahon AP (2004) Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev Biol 270: 393–410. [DOI] [PubMed] [Google Scholar]
- 16. Mares V, Lodin Z (1970) The cellular kinetics of the developing mouse cerebellum. II. The function of the external granular layer in the process of gyrification. Brain Res 23: 343–352. [DOI] [PubMed] [Google Scholar]
- 17. Wechsler-Reya RJ, Scott MP (1999) Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22: 103–114. [DOI] [PubMed] [Google Scholar]
- 18. Ma S, Kwon HJ, Huang Z (2012) Ric-8a, a gunanine nucleotide exchange factor for heterotrimeric G proteins, regulates Bergmann glia-basement membrane adhesion during cerebellar foliation. J. Neurosci 32: 14979–14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corrales JD, Rocco GL, Blaess S, Guo Q, Joyner AL (2004) Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development 131: 5581–5590. [DOI] [PubMed] [Google Scholar]
- 20. Dahmane N, Ruiz i Altaba A (1999) Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126: 3089–3100. [DOI] [PubMed] [Google Scholar]
- 21. Yanagisawa H, Bundo M, Miyashita T, Okamura-Oho Y, Tadokoro K, et al. (2000) Protein binding of a DRPLA family through arginine-glutamic acid dipeptide repeats is enhanced by extended polyglutamine. Hum Mol Genet 9: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 22. Vilhais-Neto GC, Maruhashi M, Smith KT, Vasseur-Cognet M, Peterson AS, et al. (2010) Rere controls retinoic acid signalling and somite bilateral symmetry. Nature 463: 953–957. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Rajan H, Pitman JL, McKeown M, Tsai CC (2006) Histone deacetylase-associating Atrophin proteins are nuclear receptor corepressors. Genes Dev 20: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zoltewicz JS, Stewart NJ, Leung R, Peterson AS (2004) Atrophin 2 recruits histone deacetylase and is required for the function of multiple signaling centers during mouse embryogenesis. Development 131: 3–14. [DOI] [PubMed] [Google Scholar]
- 25. Kim BJ, Zaveri HP, Shchelochkov OA, Yu Z, Hernandez-Garcia A, et al. (2013) An Allelic Series of Mice Reveals a Role for RERE in the Development of Multiple Organs Affected in Chromosome 1p36 Deletions. PLoS One 8: e57460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qin J, Suh JM, Kim BJ, Yu CT, Tanaka T, et al. (2007) The expression pattern of nuclear receptors during cerebellar development. Dev Dyn 236: 810–820. [DOI] [PubMed] [Google Scholar]
- 27. Yamamoto M, Fujinuma M, Tanaka M, Drager UC, McCaffery P (1999) Sagittal band expression of COUP-TF2 gene in the developing cerebellum. Mech Dev 84: 143–146. [DOI] [PubMed] [Google Scholar]
- 28. Wassef M, Zanetta JP, Brehier A, Sotelo C (1985) Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev Biol 111: 129–137. [DOI] [PubMed] [Google Scholar]
- 29. Stoykova A, Gruss P (1994) Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci 14: 1395–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, et al. (1997) Math1 is essential for genesis of cerebellar granule neurons. Nature 390: 169–172. [DOI] [PubMed] [Google Scholar]
- 31. Cho JH, Tsai MJ (2006) Preferential posterior cerebellum defect in BETA2/NeuroD1 knockout mice is the result of differential expression of BETA2/NeuroD1 along anterior-posterior axis. Dev Biol 290: 125–138. [DOI] [PubMed] [Google Scholar]
- 32. Jensen P, Zoghbi HY, Goldowitz D (2002) Dissection of the cellular and molecular events that position cerebellar Purkinje cells: a study of the math1 null-mutant mouse. J Neurosci 22: 8110–8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hollyday M, McMahon JA, McMahon AP (1995) Wnt expression patterns in chick embryo nervous system. Mech Dev 52: 9–25. [DOI] [PubMed] [Google Scholar]
- 34. Meyers EN, Lewandoski M, Martin GR (1998) An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet 18: 136–141. [DOI] [PubMed] [Google Scholar]
- 35. Millen KJ, Hui CC, Joyner AL (1995) A role for En-2 and other murine homologues of Drosophila segment polarity genes in regulating positional information in the developing cerebellum. Development 121: 3935–3945. [DOI] [PubMed] [Google Scholar]
- 36. Wang VY, Zoghbi HY (2001) Genetic regulation of cerebellar development. Nat Rev Neurosci 2: 484–491. [DOI] [PubMed] [Google Scholar]
- 37. Dussault I, Fawcett D, Matthyssen A, Bader JA, Giguere V (1998) Orphan nuclear receptor ROR alpha-deficient mice display the cerebellar defects of staggerer. Mech Dev 70: 147–153. [DOI] [PubMed] [Google Scholar]
- 38. Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, et al. (1998) staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proc Natl Acad Sci U S A 95: 3960–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Armstrong CL, Hawkes R (2000) Pattern formation in the cerebellar cortex. Biochem Cell Biol 78: 551–562. [PubMed] [Google Scholar]
- 40. Hatten ME (1999) Central nervous system neuronal migration. Annu Rev Neurosci 22: 511–539. [DOI] [PubMed] [Google Scholar]
- 41. Monaghan AP, Bock D, Gass P, Schwager A, Wolfer DP, et al. (1997) Defective limbic system in mice lacking the tailless gene. Nature 390: 515–517. [DOI] [PubMed] [Google Scholar]
- 42. Nishimura M, Naito S, Yokoi T (2004) Tissue-specific mRNA expression profiles of human nuclear receptor subfamilies. Drug Metab Pharmacokinet 19: 135–149. [DOI] [PubMed] [Google Scholar]
- 43. Young KA, Berry ML, Mahaffey CL, Saionz JR, Hawes NL, et al. (2002) Fierce: a new mouse deletion of Nr2e1; violent behaviour and ocular abnormalities are background-dependent. Behav Brain Res 132: 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim BJ, Takamoto N, Yan J, Tsai SY, Tsai MJ (2009) Chicken Ovalbumin Upstream Promoter-Transcription Factor II (COUP-TFII) regulates growth and patterning of the postnatal mouse cerebellum. Dev Biol 326: 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RERE is expressed in the developing cerebellum but not in granule cell precursors. A–B. Mid-sagittal sections of the vermis region were prepared from wild-type embryonic and postnatal mouse cerebellums at E17.5 (A, B) and P7 (A) and were stained with an anti-RERE antibody. RERE-positive cells were labeled with a red color (A and B; Alexa 568) and PAX6-positive cells were visualized with a green color (B; Alexa 488). A. Immunoreactivity for anti-RERE antibodies is detected in the external granule cell layer (EGL) and other areas of the cerebellum at E17.5 and P7. B. RERE, PAX6 double positive cells are not identified in the cerebellum at E17.5 suggesting that RERE is not expressed in granule cell precursors. Scale bar = 100 µm. IGL, internal granule cell layer.
(DOCX)
Apoptotic activity in the cerebellum was comparable between Rere om/eyes3 embryos and controls. A–D Mid-sagittal sections from embryos and mice of both genotypes were probed with anti-Cleaved Caspase-3 antibodies to label apoptotic cells at E18.5 (A, B) and P3 (C, D). A-B. A few Cleaved Caspase-3-positive cells were identified in the cerebellar cortexes of Rere om/eyes3 embryos and their wild-type littermates at E18.5. In contrast, apoptotic cells were undetectable in the EGLs and PCLs of Rere om/eyes3 embryos and their wild-type littermates. C–D. Apoptotic activity of the cerebellums between Rere om/eyes3 mice and their wild-type littermates was comparable at P3. Scale bar = 100 µm. EGL, external granule cell layer; Mb, midbrain; PCL, Purkinje cell layer.
(DOCX)
Mitotic activity of granule cell precursors was comparable between the anterobasal and anterodorsal lobes. At E18.5 and P0, proliferation assays were performed on cerebellar vermis sections prepared from wild-type and Rere om/eyes3 embryos and mice using an anti-Phospho Histone H3 antibody. The number of Phospho-Histone H3-positive granule cell precursors (GCPs) in the anterobasal lobe (ABL) and the anterodorsal lobe (ADL) was reduced in Rere om/eyes3 embryos at E18.5 when compared to wild-type littermates (* = p<0.03). However, the proliferative activity of GCPs was comparable between the ABLs and ADLs of Rere om/eyes3 embryos at same time point. At P0, the mitotic activity of GCPs in the ABL and ADL was indistinguishable between Rere om/eyes3 embryos and wild-type littermates. In addition, proliferative activity of GCPs was similar between the ABLs and ADLs of Rere om/eyes3 mice at P0. Phospho-Histone H3-positive cells in the external granule cell layer (EGL) of the ABL and ADL were counted and normalized by the corresponding area of the EGL of each lobe (n≥3 with twenty slides containing three sections for each genotype).
(DOCX)
Additional information about the antibodies used in this study.
(DOCX)