Abstract
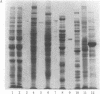
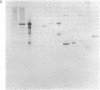
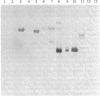
Antiserum to yeast C1-tetrahydrofolate (C1-H4folate) synthase reacts with other eukaryotic C1-H4folate synthases and prokaryotic 10-formyltetrahydrofolate (10-CHO-H4folate) synthetases [formate:tetrahydrofolate ligase (ADP-forming), EC 6.3.4.3] even though these enzymes vary in subunit size and function and probably vary widely in sequence. The comigration of the purified enzymes with the immunoreactive material establishes the specificity of the reaction for C1-H4folate synthase proteins. Reciprocal crossreaction of the antibody to Clostridium acidiurici 10-CHO-H4folate synthetase with the eukaryotic proteins indicates that such broad cross-species reactions are not specific to the antisera elicited in response to the yeast C1-H4folate synthase. These specific crossreactions among divergent species have been observed only on an electrophoretic transfer blot of a denaturing polyacrylamide gel. These observations may have been possible because of the sensitivity and specificity of the technique, which differ from more conventional immunochemical methods.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N. Immunochemical resemblance between human leukemia and hen egg-ehite lysozyme and their reduced carboxymethyl derivatives. J Mol Biol. 1971 Oct 14;61(1):237–250. doi: 10.1016/0022-2836(71)90220-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Champion A. B., Rabinowitz J. C. Ferredoxin and formyltetrahydrofolate synthetase: comparative studies with Clostridium acidiurici, Clostridium cylindrosporum, and newly isolated anaerobic uric acid-fermenting strains. J Bacteriol. 1977 Dec;132(3):1003–1020. doi: 10.1128/jb.132.3.1003-1020.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev I. K., Harvey R. J. A complex of N5,N10-methylenetetrahydrofolate dehydrogenase and N5,N10-methenyltetrahydrofolate cyclohydrolase in Escherichia coli. Purification, subunit structure, and allosteric inhibition by N10-formyltetrahydrofolate. J Biol Chem. 1978 Jun 25;253(12):4245–4253. [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hough C. A., Edwardson J. A. Antibodies to thaumatin as a model of the sweet taste receptor. Nature. 1978 Jan 26;271(5643):381–383. doi: 10.1038/271381a0. [DOI] [PubMed] [Google Scholar]
- Katiyar S. S., Porter J. W. Antibodies specific for NADPH-binding region of enzymes possessing dehydrogenase activities. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1221–1223. doi: 10.1073/pnas.80.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McGuire J. J., Rabinowitz J. C. Studies on the mechanism of formyltetrahydrofolate synthetase. The Peptococcus aerogenes enzyme. J Biol Chem. 1978 Feb 25;253(4):1079–1085. [PubMed] [Google Scholar]
- Paukert J. L., Straus L. D., Rabinowitz J. C. Formyl-methyl-methylenetetrahydrofolate synthetase-(combined). An ovine protein with multiple catalytic activities. J Biol Chem. 1976 Aug 25;251(16):5104–5111. [PubMed] [Google Scholar]
- Paukert J. L., Williams G. R., Rabinowitz J. C. Formyl-methenyl-methylenetetrahydrofolate synthetase (combined); correlation of enzymic activities with limited proteolytic degradation of the protein from yeast. Biochem Biophys Res Commun. 1977 Jul 11;77(1):147–154. doi: 10.1016/s0006-291x(77)80176-9. [DOI] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- RABINOWITZ J. C., PRICER W. E., Jr Formyltetrahydrofolate synthetase. I. Isolation and crystallization of the enzyme. J Biol Chem. 1962 Sep;237:2898–2902. [PubMed] [Google Scholar]
- Rossmann M. G., Moras D., Olsen K. W. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974 Jul 19;250(463):194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Schirch L. Formyl-methenyl-methylenetetrahydrofolate synthetase from rabbit liver (combined). Evidence for a single site in the conversion of 5,10-methylenetetrahydrofolate to 10-formyltetrahydrofolate. Arch Biochem Biophys. 1978 Aug;189(2):283–290. doi: 10.1016/0003-9861(78)90214-x. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Smith G. K., Mueller W. T., Wasserman G. F., Taylor W. D., Benkovic S. J. Characterization of the enzyme complex involving the folate-requiring enzymes of de novo purine biosynthesis. Biochemistry. 1980 Sep 2;19(18):4313–4321. doi: 10.1021/bi00559a026. [DOI] [PubMed] [Google Scholar]
- Tan L. U., Drury E. J., MacKenzie R. E. Methylenetetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase. A multifunctional protein from porcine liver. J Biol Chem. 1977 Feb 10;252(3):1117–1122. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Enzymes of clostridial purine fermentation. Methylenetetrahydrofolate dehydrogenase. J Biol Chem. 1967 Oct 10;242(19):4378–4385. [PubMed] [Google Scholar]
- de Mata Z. S., Rabinowitz J. C. Formyl-methenyl-methylenetetrahydrofolate synthetase(combined) from yeast. Biochemical characterization of the protein from an ade3 mutant lacking the formyltetrahydrofolate synthetase function. J Biol Chem. 1980 Mar 25;255(6):2569–2577. [PubMed] [Google Scholar]