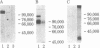Abstract
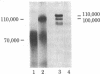
Conditioned medium from cultures of human umbilical vein endothelial cells was analyzed for the presence of tissue plasminogen activator (tPA) and urokinase. Immunoprecipitation studies using metabolically labeled conditioned medium and anti-tPA IgG revealed a single band on autoradiographs corresponding to a Mr of 100,000. No bands were observed after immunoprecipitation with anti-urokinase IgG. The Mr 100,000 tPA was found to be inactive and did not bind to fibrin clots. However, exposure of this tPA to 1% NaDodSO4 resulted in the appearance of plasminogen activator activity with no apparent change in its Mr. Treatment with 10 mM diisopropylfluorophosphate prior to NaDodSO4 activation did not inhibit the NaDodSO4-induced appearance of plasminogen activator activity, indicating that the active site was not available for diisopropylfluorophosphate binding. The possibility that the properties of this Mr 100,000 tPA reflected a tPA-inhibitor complex was examined. Attempts to dissociate such a complex by denaturation, reduction, or extremes of temperature were not successful. However, after treatment of conditioned medium with 1 M hydroxylamine in the presence of 0.1% NaDodSO4, the Mr of the anti-tPA immunoprecipitable material declined by 40,000 to Mr 60,000, a Mr consistent with that of other human tPAs. Hydroxylamine has been shown previously to dissociate covalently coupled serine protease-inhibitor complexes. Furthermore, incubation of purified human melanoma cell tPA with conditioned medium resulted in an increase in its Mr by 40,000 with a concomitant decline in tPA activity. The data suggest that the latent tPA present in the conditioned medium of endothelial cells is composed of a Mr 60,000 tPA associated with an inhibitor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasted B. Purification and characterization of human vascular plasminogen activator. Biochim Biophys Acta. 1980 Feb 27;621(2):241–254. doi: 10.1016/0005-2795(80)90176-2. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Binder B. R., Spragg J., Austen K. F. Purification and characterization of human vascular plasminogen activator derived from blood vessel perfusates. J Biol Chem. 1979 Mar 25;254(6):1998–2003. [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Björk I., Jackson C. M., Jörnvall H., Lavine K. K., Nordling K., Salsgiver W. J. The active site of antithrombin. Release of the same proteolytically cleaved form of the inhibitor from complexes with factor IXa, factor Xa, and thrombin. J Biol Chem. 1982 Mar 10;257(5):2406–2411. [PubMed] [Google Scholar]
- Bornstein P., Balian G. Cleavage at Asn-Gly bonds with hydroxylamine. Methods Enzymol. 1977;47:132–145. doi: 10.1016/0076-6879(77)47016-2. [DOI] [PubMed] [Google Scholar]
- Cohen A. B., Geczy D., James H. L. Interaction of human alpha-1-antitrypsin with porcine trypsin. Biochemistry. 1978 Feb 7;17(3):392–400. doi: 10.1021/bi00596a002. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Björk I. Release of a two-chain form of antithrombin from the antithrombin-thrombin complex. Eur J Biochem. 1979 Nov 1;101(1):31–38. doi: 10.1111/j.1432-1033.1979.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Korninger C., Collen D. Studies on the specific fibrinolytic effect of human extrinsic (tissue-type) plasminogen activator in human blood and in various animal species in vitro. Thromb Haemost. 1981 Aug 28;46(2):561–565. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Levin E. G., Loskutoff D. J. Comparative studies of the fibrinolytic activity of cultured vascular cells. Thromb Res. 1979;15(5-6):869–878. doi: 10.1016/0049-3848(79)90195-6. [DOI] [PubMed] [Google Scholar]
- Levin E. G., Loskutoff D. J. Cultured bovine endothelial cells produce both urokinase and tissue-type plasminogen activators. J Cell Biol. 1982 Sep;94(3):631–636. doi: 10.1083/jcb.94.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin E. G., Loskutoff D. J. Serum-mediated suppression of cell-associated plasminogen activator activity in cultured endothelial cells. Cell. 1980 Dec;22(3):701–707. doi: 10.1016/0092-8674(80)90546-2. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., van Mourik J. A., Erickson L. A., Lawrence D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2956–2960. doi: 10.1073/pnas.80.10.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSESSON M. W. The preparation of human fibrinogen free of plasminogen. Biochim Biophys Acta. 1962 Feb 26;57:204–213. doi: 10.1016/0006-3002(62)91112-5. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Collen D. Purification and characterization of the plasminogen activator secreted by human melanoma cells in culture. J Biol Chem. 1981 Jul 10;256(13):7035–7041. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Welbergen J. Immunological characterization of plasminogen activator activities in human tissues and body fluids. J Lab Clin Med. 1981 Apr;97(4):477–486. [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Welbergen J. Relationship between tissue plasminogen activator and the activators in blood and vascular wall. Thromb Res. 1980 Jun 15;18(6):815–830. doi: 10.1016/0049-3848(80)90204-2. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Summaria L., Hsieh B., Shah R. J. The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 May 25;242(10):2333–2342. [PubMed] [Google Scholar]
- Scott R. W., Eaton D. L., Duran N., Baker J. B. Regulation of extracellular plasminogen activator by human fibroblasts. The role of protease nexin. J Biol Chem. 1983 Apr 10;258(7):4397–4403. [PubMed] [Google Scholar]
- Sweet R. M., Wright H. T., Janin J., Chothia C. H., Blow D. M. Crystal structure of the complex of porcine trypsin with soybean trypsin inhibitor (Kunitz) at 2.6-A resolution. Biochemistry. 1974 Sep 24;13(20):4212–4228. doi: 10.1021/bi00717a024. [DOI] [PubMed] [Google Scholar]
- Wall R. T., Harker L. A., Quadracci L. J., Striker G. E. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol. 1978 Aug;96(2):203–213. doi: 10.1002/jcp.1040960209. [DOI] [PubMed] [Google Scholar]
- Wiman B., Collen D. On the mechanism of the reaction between human alpha 2-antiplasmin and plasmin. J Biol Chem. 1979 Sep 25;254(18):9291–9297. [PubMed] [Google Scholar]
- Wun T. C., Schleuning W. D., Reich E. Isolation and characterization of urokinase from human plasma. J Biol Chem. 1982 Mar 25;257(6):3276–3283. [PubMed] [Google Scholar]