Abstract
The aim of this study was to evaluate the immunomodulatory effects of supplementing intravenous omega-3 fatty acids in fish oil (IVFO) in elderly patients undergoing hip surgery. This was a single centre, randomized, controlled, comparative, phase IV study in elderly patients undergoing hip surgery. The subjects, within the age range of 50–90 years, were assigned randomly to the group receiving intravenous omega-3 fatty acids in fish oil (IVFO, Omegaven®) or the control group not receiving intravenous fish oil (n = 20 in each group). IL-6, IL-8, IL-10, and HS-CRP levels were the inflammatory markers assessed in this study. The within-group comparison was done by paired t-test and between-group comparison by unpaired t-test. At day 4, IL-6 values in the IVFO group decreased as compared to day 0. At day 4, IL-8 mean values increased for both IVFO and control groups. This increase was highly significant in the control group (P = 0.0182). IL 10 values decreased at day 4 and increased at day 8 in the IVFO group. Increase in HS-CRP levels was nonsignificant at day 4 in the IVFO group (P = 0.60) and significant at day 8 for the control group (P = 0.0084) as compared to day 0. Various biochemical parameters including albumin, protein, SGOT, SGPT, blood glucose, and urea values generated evidence regarding the safety profile of IVFO. This study suggests a role for IVFO in the short-term suppression of inflammatory mediators for patients undergoing hip surgery. However, further, larger trials may be needed to establish its definitive role in this patient population.
Keywords: Omega-3 fatty acids, Omegaven, Interleukins, Inflammation, Hip surgery
Introduction
Patients who have undergone a major operation or severe trauma may develop malfunctioning of their host defense mechanism, leading to suppression of specific and nonspecific immune functions and an enhanced susceptibility toward microbial infections. This further results from a multitude of metabolic or immunologic imbalances due to trauma, tissue ischemia and operation injury, length of surgery and anesthesia, loss of blood, and associated illness [1]. However, the mechanisms of the pathophysiological alterations are quite complex. The interaction of various factors such as the traumatic insult, microbial pathogenicity factors, or mediators of the neuroendocrine axis leads to adverse host reactions, which are driven by excessive production of inflammatory mediators (e.g., proinflammatory cytokines or proinflammatory lipid mediators) and may result finally in systemic inflammatory reactions [2].
It has been demonstrated that lipid-derived fatty acids are not only used as energy-providing substrates but possess additional “pharmacological” functions, which may beneficially influence healing processes and patient outcome. This consideration appears to be particularly true for the polyunsaturated omega-3 fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [3]. The decreased ratio of omega-6 to omega-3 in membrane phospholipids has been shown to be associated with an altered cytokine production. Generation of proinflammatory cytokines has been shown to be markedly suppressed with administration of omega-3 fatty acids as compared to omega 6 fatty acids [4-6].
The proinflammatory cytokines such as interleukin, IL-6, and chemokines such as IL-8 and IL-1, are involved in the induction and perpetuation of inflammation [7, 8]. High intake of omega-3 fatty acids in fish oils containing eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) or the vegetable origin α-linolenic acid (ALA) may decrease the production of these proinflammatory cytokines [9]. Omega-3 fatty acids act by decreasing the formation of arachidonic acid (AA), which belongs to the group of omega-6 fatty acids. AA is converted from linoleic acid (LA), which originates from a diet rich in grains and vegetable oils. AA causes activation of inflammatory mediators such as prostaglandins (PGE2), leukotrienes (LTB4, LTC4, LTD4), and related metabolites that are potent inflammatory mediators leading to production of cytokines [10].
IL-8, a proinflammatory chemokine, is important in inducing inflammation. Interleukin-10 (IL-10), on the other hand, is an anti-inflammatory cytokine secreted by white adipose tissue [11]. It is generally accepted that IL-10 confers protection against an overwhelming inflammatory response. C-reactive protein (CRP) is a protein found in the blood, whose levels rise in response to inflammation (i.e., C-reactive protein is an acute-phase protein). CRP is a member of the class of acute-phase reactants as its levels rise dramatically during inflammatory processes occurring in the body. It is believed that it plays an important role in innate immunity as an early defense system against infections.
There is a scarcity of literature evidence in terms of preoperative administration of IV omega-3 fatty acids as a supplementation to demonstrate its effects on inflammatory and immune responses. Hip surgery is a consequence of musculoskeletal trauma that mainly affects the older population with comorbid conditions. This study was planned to judge the inflammatory response of preoperative supplementation with intravenous omega-3 fatty acids in elderly patients undergoing hip surgery, considering the hyperinflammation associated with this type of surgery in elderly patients. The objective of this study was to study and evaluate the anti-inflammatory effects of intravenous omega-3 fatty acid supplementation in elderly patients undergoing hip surgery.
Materials and Methods
This was a single centre, randomized, controlled, comparative, phase IV study in elderly patients undergoing hip surgery. The study included 40 subjects, 20 in each treatment group. The subjects were assigned randomly to IVFO (receiving intravenous omega-3 fatty acids in fish oil) or control treatment (did not receive intravenous omega-3 fatty acids), with equal size of the two treatment groups. Randomization was performed by a responsible person with the method of closed envelopes. Forty elderly patients (age 50–90 years) admitted for undergoing hip surgery were recruited and randomly divided into two groups to receive or not to receive the study drug. The study was conducted as per the ICH GCP standards.
Elderly patients, of either sex, undergoing hip surgeries, aged more than 50 years were included in the study. Patients with allergy to any of the constituents of nutritional products, primary diagnosis of hypertriglyceridemia, long-term steroid therapy and cycloxygenase inhibitors (more than 3 months), severe cardiac disease, hepatic disorders (total bilirubin >1.5 times the upper limit of normal), psychiatric disorders likely to affect compliance, severe hemorrhagic disorders, stroke, embolism or uncontrolled severe renal failure (serum creatinine >2 mg/dl) without dialysis/hemofiltration, and serum HIV positivity were excluded. Patients were also excluded if they had participated in any other clinical trials within the past 2 months.
The IVFO group (20 patients) received intravenous omega-3 fatty acids in fish oil (Omegaven®) for 3 preoperative days. The control group II (20 patients) did not receive omega-3 fatty acids for 3 preoperative days. IVFO was administrated at the dosage of 2 ml/kg BW/day (i.e., 0.2 g/kg/day) of 10 % fish oil at the rate of less than 0.05 g/kg/h which was about eight drops per minute. Total duration of infusion of 50 ml IVFO was 3–4 h. The time points for recording the data were baseline (D0), day of surgery (D4), and fourth postoperative day (D8). The variables evaluated were as follows:
Inflammation: interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), and plasma high end C-reactive protein levels (HS-CRP)
General: serum total proteins, serum albumin, liver, kidney, and metabolic functions
The approval for this study was taken from the independent ethics committee of Nizam’s Institute of Medical Sciences, Hyderabad.
Statistical Analysis
Forty patients were recruited in the study to be randomized in two treatment groups of 20 patients each. For 90 % statistical power, required sample size was 18 patients in each group.
All the results of the study were analyzed by using standard statistical methods. All recorded and derived variables were presented using standard procedures (e.g., means, standard deviation, minimum, maximum, median and quartiles for continuous variables, and frequency tables for categorical data). Laboratory values were categorized into high, low, and normal using the reference ranges of the laboratory and presented in shift tables. Assessment of all these biochemical parameters, within-group comparison, was done by paired t-test and between-group comparisons by unpaired t-test. All data were documented in raw data listings. The analysis was performed for the intent-to-treat population, defined as all patients for whom therapy with study medication was initiated.
Results
Patients within the age range of 50–90 years were included. Maximum patients were in the age range of 61–70 years. Ratio of male–female was 12:8 for both the groups.
Inflammatory Markers
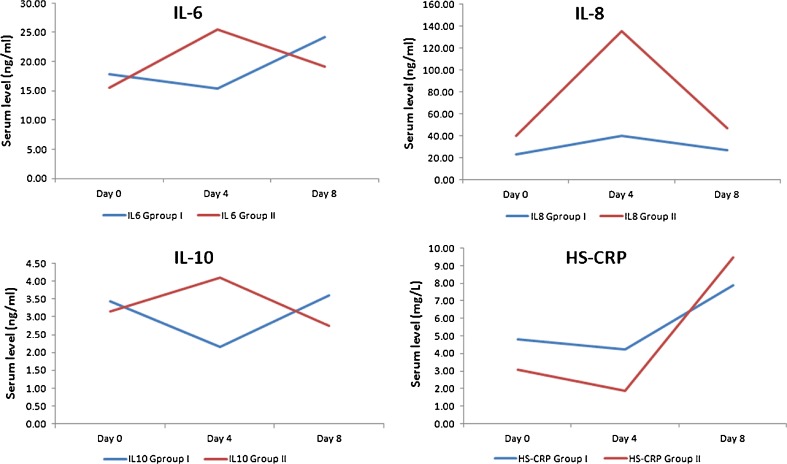
Inflammatory markers including IL-6, IL-8, IL-10, and HS-CRP levels were analyzed in this study, which are reported in Table 1. It was observed that at day 4, IL-6 levels in the IVFO group decreased as compared to D0 (D4/D0 = 0.86) though the decrease was nonsignificant (P = 0.3395). On the contrary, in the control group, IL-6 values increased at day 4 (D4/D0 = 1.64) (P = 0.0440) with a slight reduction at day 8 (D8/D0=1.23). The increase at day 4 was statistically significant (P = 0.0440).
Table 1.
Levels of various inflammatory markers from day 0 through day 8
| Group | Time | IL-6 | IL-8 | IL-10 | HS-CRP |
|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | ||
| IVFO | D0 | 17.81 | 23.21 | 3.43 | 4.80 |
| D4 | 15.37 | 40.23 | 2.15 | 4.23 | |
| Ratio (D4/D0) | 0.86 (P = 0.34) | 1.73 (P = 0.069) | 0.63 (P = 0.101) | 0.88 (P = 0.602) | |
| D8 | 24.18 | 26.69 | 3.60 | 7.91 | |
| Ratio (D8/D0) | 1.36 (P = 0.086) | 1.15 (P = 0.806) | 1.05 (P = 0.876) | 1.65 (P = 0.168) | |
| Control | D0 | 15.56 | 40.22 | 3.15 | 3.08 |
| D4 | 25.53 | 135.89 | 4.09 | 1.89 | |
| Ratio (D4/D0) | 1.64 (P = 0.044) | 3.38 (P = 0.018) | 1.30 (P = 0.224) | 0.61 (P = 0.021) | |
| D8 | 19.11 | 46.99 | 2.76 | 9.48 | |
| Ratio (D8/D0) | 1.23(P = 0.517) | 1.17 (P = 0.759) | 0.87 (P = 0.702) | 3.07 (P = 0.008) | |
| Between-group comparison | D4/D0 | P = 0.0245 | P = 0.223 | P = 0.039 | P = 0.246 |
| D8/D0 | P = 0.778 | P = 0.983 | P = 0.695 | P = 0.233 |
Within-group comparison was done by paired t-test and between-group comparison by unpaired t–test
Between-group comparisons reported significant results at day 4 but nonsignificant at day 8. At day 4, IL-8 mean values increased for the both IVFO group and control group (D4/D0=1.73, 3.38, respectively). The increase was highly significant for the control group (P = 0.0182). Further, a nonsignificant decrease in IL-8 levels was reported at day 8 for both the groups (D8/D0=1.15, 1.17; P = 0.080, 0.753, respectively).
IL-10 values decreased at day 4 (D4/D0=0.63) followed by an increase at day 8 (D8/D0=1.05) in the IVFO group, while it increased at day 4 in the control group (D4/D0=1.3) and decreased at day 8 (D8/D0=0.87) in the control group. For within-group comparison, a non-significant decrease was reported for HS-CRP levels at day 4, and while it increased on day 8 for the IVFO group (D4/D0=0.88, D8/D0=1.65, P = 0.6019, 0.1675, respectively). For the control group, HS-CRP increased significantly on day 8 (D8/D0=3.07, P = 0.0210, 0.0084). Figure 1 presents the trend line for all inflammatory markers assessed in this study from day 0 through day 8.
Fig. 1.
Comparison of inflammatory markers in both groups
Biochemical Parameters
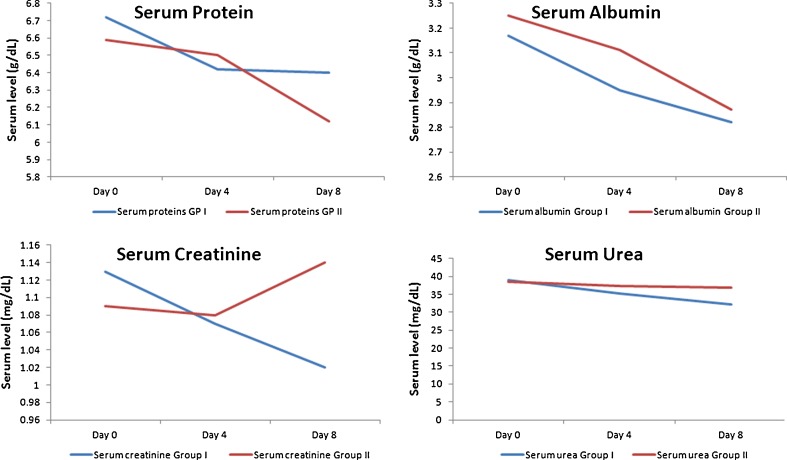
Biochemical tests were performed to evaluate the safety profile of intravenous omega-3 fatty acids in fish oil (Omegaven) (Table 2). The level of serum proteins decreased from day 0 to day 4 and was also lower at day 8 compared to day 0 (D4–D0 = −0.29; D8–D0 = −0.32). Although the protein levels decreased for the control group too, the difference was nonsignificant (D4–D0 = −0.0899; D8–D0 = −0.4699). For serum albumin values, a decrease was observed for both day 4 and day 8, for the IVFO group (D4–D0 = −0.2201; D8–D0 = −0.3550) and for the control group (D4–D0 = −0.1450; D8–D0 = −0.3800).
Table 2.
Levels of various biochemical parameters from day 0 through day 8
| Days | Group I | Group II | Between-Group P Value | Days | Group I | Group II | Between-Group P Value |
|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | ||||
| Serum protein values | Serum albumin values | ||||||
| Day 0 | 6.72 | 6.59 | — | Day 0 | 3.18 | 3.26 | — |
| Day 4 | 6.43 | 6.50 | 0.2507 | Day 4 | 2.95 | 3.11 | 0.4726 |
| Day 8 | 6.40 | 6.12 | 0.5751 | Day 8 | 2.82 | 2.88 | 0.8646 |
| Serum creatinine values | Serum urea values | ||||||
| Day 0 | 1.13 | 1.09 | — | Day 0 | 38.90 | 38.6 | — |
| Day 4 | 1.07 | 1.08 | 0.3954 | Day 4 | 35.25 | 37.35 | 0.5489 |
| Day 8 | 1.02 | 1.14 | 0.0262 | Day 8 | 32.15 | 36.95 | 0.1982 |
| Serum bilirubin values | Serum SGOT values | ||||||
| Day 0 | 0.77 | 0.77 | – | Day 0 | 31.95 | 31.50 | — |
| Day 4 | 0.83 | 0.76 | 0.4220 | Day 4 | 31.20 | 31.30 | 0.8597 |
| Day 8 | 0.70 | 0.82 | 0.3590 | Day 8 | 28.80 | 30.90 | 0.5422 |
| Serum SGPT values | Blood glucose values | ||||||
| Day 0 | 31.25 | 30.25 | — | Day 0 | 119.7 | 129.50 | — |
| Day 4 | 31.35 | 33.70 | 0.1776 | Day 4 | 122.2 | 105.20 | 0.1229 |
| Day 8 | 31.1 | 35.90 | 0.1254 | Day 8 | 129.30 | 132.35 | 0.5954 |
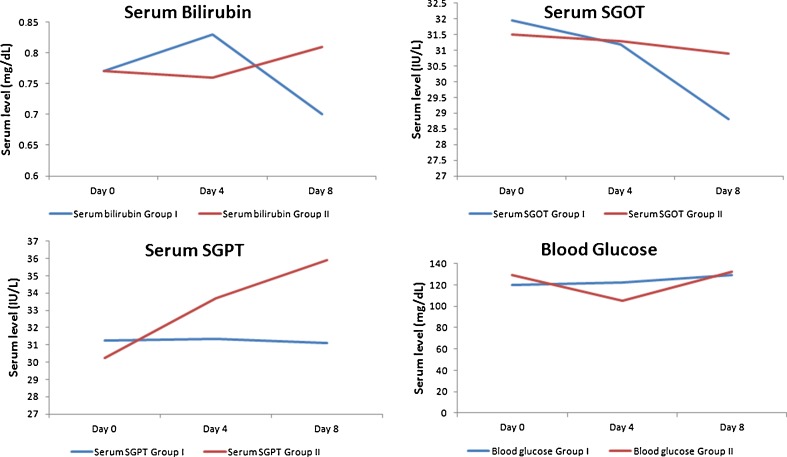
A nonsignificant decrease in creatinine values was reported for both the groups at day 4 (IVFO group D4–D0=–0.0599 and control group –0.0099). At day 8, a slight decrease was reported for the IVFO group (D8–D0=–0.1099) contrary to a slight increase in control group (D8–D0=0.0450), but both were nonsignificant. Serum urea values decreased at both day 4 and day 8 and for both the groups; however, the decrease was nonsignificant. A nonsignificant increase and decrease in bilirubin values was also reported at day 4 for the IVFO group (D4–D0=0.0599) and the control group (D4–D0 = −0.0050). At day 8, both the groups reported nonsignificant increase in serum bilirubin values.
A nonsignificant decrease was reported at day 4 and day 8 for both the groups for SGOT values, for the IVFO group (D4–D0 = −0.75 and D8–D0 = −3.15) and the control group (D4–D0 = −0.20 and D8–D0 = −0.60). At day 4, a nonsignificant increase was reported for serum SGPT values. At day 8, a non-significant decrease was reported for the IVFO group and a nonsignificant increase was reported for the control group. At day 4, a nonsignificant increase and decrease in blood glucose levels was reported for the IVFO group (D4–D0=2.50) and the control group (D4–D0 = −24.30), respectively. However, at day 8 a non-significant increase was reported for both the groups, D8–D0=9.60 and 2.85, respectively. Figures 2 and 3 present the trend line for all biochemical parameters assessed in this study from day 0 through day 8.
Fig. 2.
Comparison of key biochemical parameters in both groups
Fig. 3.
Comparison of key biochemical parameters in both groups
Hospitalization
Patients in the control group spent more number of days in hospital as compared to those in the IVFO group (17.30 vs. 16.85). However, the difference between the two groups was nonsignificant.
Discussion
Evidence that the type of dietary fat may influence the inflammatory markers in surgery has been well documented [12]. A wide variety of doses and formulations of fish oil/omega-3 fatty acids have been evaluated for anti-inflammatory effects in healthy subjects and for therapeutic efficacy in various diseases mediated by inflammatory processes. Clinical trials of fish oil/omega-3 supplements suggest that higher doses might prove more efficacious in alleviating symptoms, particularly with regard to changes in inflammatory biomarkers [13].
In a double-blind controlled study by Koller et al. [14], it was demonstrated that the omega-3 fatty acid was appropriate to significantly increase the release of five-series leukotriene B from stimulated leukocytes after 5 days of administration to patients undergoing major intestinal surgery. In a retrospective evaluation by Tsekos et al. [15], it was found that perioperative parenteral administration of approximately 10 g fish oil per day improved outcome to surgical patients in a routine clinical setting probably by modulating endogenous production of cytokines. The data support the hypothesis that a “preloading” of cell membrane phospholipids with active precursors of desirable immune modulators is beneficial for patient recovery independent of the general nutritional status.
Omega-3 fatty acid deficiency has been recognized and appreciated in recent times [16]. After intravenous administration, EPA and DHA promptly get incorporated into the cell membrane, compete with arachidonic acid (AA) in the cyclo-oxygenase and 5-lipoxygenase pathways, resulting in a reduced generation of diene prostanoids (e.g., PGE2, PGI2, and TXA2) and tetraene leukotrienes (e.g., LTB4), derived from AA in favor of the corresponding triene prostanoids (e.g., PGE3, PGI3, TXA3) and pentaene leukotriene (LTB5) derived from EPA [17].
In this study, the inflammatory markers assessed were IL-6, IL-8, IL-10, and HS-CRP; the anti-inflammatory effect of intravenous omega-3 fatty acids in fish oil appeared to be short-lived. At day 4, IL-6, IL-10, and HS-CRP values in the IVFO group decreased as compared to day 0; however, these values again increased on day 8. In a randomized-controlled study, Wachtler et al. [18] showed that the systemic levels of IL-6 and IL-10 decreased significantly in surgical patients 5 days after administration of TPN enriched with omega-3 fatty acids. In another clinical trial, Weiss et al. [19] reported that IL6 levels were significantly decreased in patients receiving fish oil perioperatively. In our study, serum IL-6 and IL-10 levels were lower in the IVFO group at day 4 as compared to group II. These findings suggest that supplementation with omega-3 fatty acids may restrain inflammatory response, modulate lymphocyte proliferation, and maintain the function of immunocompetent cells under inflammatory conditions such as surgical trauma.
Supplementation with omega-3 fatty acids also results in the generation of three-series prostanoids and reduces release of PGE2 [20]. The omega-3 fatty acids compete with AA for their phospholipase-mediated release from membranes [21] and for metabolism of the free fatty acids by cyclo-oxygenase and lipoxygenase [22]. Synthesis of a range of less inflammatory eicosanoids could thus contribute to downregulation of the skin inflammatory response. It has been reported that PGE2 increases IL-8 production by posttranslational mechanisms involving stabilization of mRNA [23]. IL-8 is well-known for its proinflammatory properties. It causes an increase in oxidative stress leading to inflammation. In our study, IL-8 mean values increased for the both IVFO group and group II at day 4. The increase was highly significant for group II (p = 0.0182). Further, a nonsignificant decrease in IL-8 levels was reported at day 8 for both the groups. The significant increase in IL-8 values can be attributed to strong proinflammatory activity of IL-8. Rise was also seen in the IVFO group, but combined with IVFO increase in this inflammatory marker was less. Between-group comparisons at D4, a nonsignificant decrease and at D8 a nonsignificant increase were reported.
The relevance of the inflammatory and endothelial activation biomarkers in the atherogenic process has been suggested by several studies. HS-CRP and IL-6 are markers of systemic inflammation and independent predictors of cardiovascular disease in healthy women. Recent data suggest that HS-CRP plays an active role in atherogenesis [24]. A study by Burns et al. [25] has reported non-significant effect of omega-3 fatty acids on HS-CRP levels. In our study, the HS-CRP levels decreased initially on IVFO administration but the decrease was nonsignificant. This effect was short-lived as the levels again increased at day 8, and this increase was nonsignificant compared to day 0 levels. These findings suggest that omega-3 fatty acids may restrain inflammatory response for a shorter duration postsurgery.
Omega-3 fatty acids hold very little interaction with various biochemical markers such as serum proteins, albumin, SGOT, SGPT, blood glucose with a nonsignificant effect on these and can be considered safe to administer. The literature evidence in relation to support this fact was reported by Kooshki et al. [26]. However, omega-3 fatty acids should be used cautiously in people who bruise easily, have a bleeding disorder, or take blood-thinning medications.
A shorter postoperative hospital stay was noted in our study for patients receiving IVFO. However, the difference was nonsignificant as compared to the control group. Various factors may influence the outcomes of surgical patients. A short, single nutritional intervention is unlikely to produce extensive effects on the outcomes of postoperative patients.
This study suggests the role of IVFO for short-term suppression of inflammatory markers for elderly patients undergoing hip surgery. Intravenous supplementation with omega-3 fatty acids in fish oil may have a more favorable effect on the outcomes of such patients. However, further studies are required to establish its definitive role in patients undergoing hip surgery.
Acknowledgments
The funding for this study was through an unrestricted grant from Fresenius Kabi. The authors are grateful for the assistance of Dr. Sadanand Kulkarni during the preparation of this article.
References
- 1.Abraham E. Physiologic stress and cellular ischemia: relationship to immunosuppression and susceptibility to sepsis. Crit Care Med. 1991;19:613–618. doi: 10.1097/00003246-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Fry DE. Sepsis syndrome. Am Surg. 2000;66:126–132. [PubMed] [Google Scholar]
- 3.Stevens JA, Olson S. Reducing falls and resulting hip fractures among older women. MMWR Recomm Rep. 2000;49:3–12. [PubMed] [Google Scholar]
- 4.Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18:S57–S63. doi: 10.1016/8756-3282(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 5.Singer BR, McLauchlan GJ, Robinson CM. Epidemiology of fractures in 15000 adults: the influence of age and gender. J Bone Joint Surg Br. 1998;80:243–248. doi: 10.1302/0301-620X.80B2.7762. [DOI] [PubMed] [Google Scholar]
- 6.Kanis JA, Johnell O, Sembo I, et al. Long term risk of osteoporotic fracture in Malmo. Osteoporos Int. 2000;11:669–674. doi: 10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- 7.Kmiec Z. Cytokines in inflammatory bowel disease. Arch Immunol Ther Exp. 1998;46:143–155. [PubMed] [Google Scholar]
- 8.O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 9.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. doi: 10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 10.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juge-Aubry CE, Somm E, Pernin A, Alizadeh N, Giusti V, Dayer JM, et al. Adipose tissue is a regulated source of interleukin-10. Cytokine. 2005;29:270–274. doi: 10.1016/j.cyto.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Kim JB, Jung MH, Cho JY, Park JW, Suh JY, Lee JM. The influence of type 2 diabetes mellitus on the expression of inflammatory mediators and tissue inhibitor of metalloproteinases-2 in human chronic periodontitis. J Periodontal Implant Sci. 2011;41:109–116. doi: 10.5051/jpis.2011.41.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91:1185–1194. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koller M, Senkal M, Kemen M, Konig W, Zumtobel V, Muhr G. Impact of omega-3 fatty acid enriched TPN on leukotriene synthesis in leukocytes after major surgery. Clin Nutr. 2002;22:59–64. doi: 10.1054/clnu.2002.0592. [DOI] [PubMed] [Google Scholar]
- 15.Tsekos E, Reuter C, Stehle P, Boeden G. Perioperative administration of parenteral fish oil supplements in a routine clinical setting improves patient outcome after major abdominal surgery. Clin Nutr. 2004;23:325–330. doi: 10.1016/j.clnu.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Furst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clin Nutr. 2000;19:7–14. doi: 10.1054/clnu.1999.0072. [DOI] [PubMed] [Google Scholar]
- 17.Mayer K, Fegbeutel C, Hattar K, Sibelius U, Krämer HJ, Heuer KU, et al. Omega-3 vs. omega-6 lipid emulsions exert differential influence on neutrophils in septic shock patients: impact on plasma fatty acids and lipid mediator generation. Intensive. Care Med. 2003;29:1472–1481. doi: 10.1007/s00134-003-1900-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachtler P, Konig W, Senkal M, Kemen M, Koller M. Influence of a total parenteral nutrition enriched with omega-3 fatty acids on leukotriene synthesis of peripheral leukocytes and systemic cytokine levels in patients with major surgery. J Trauma. 1997;42:191–198. doi: 10.1097/00005373-199702000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Weiss G, Meyer F, Matthies B, Pross M, Koenig W, Lippert H. Immunomodulation by perioperative administration of n-3 fatty acids. Br J Nutr. 2002;87(Suppl 1):S89–S94. doi: 10.1079/BJN2001461. [DOI] [PubMed] [Google Scholar]
- 20.Pupe A, Moison R, De Haes P, van Henegouwen GB, Rhodes L, Degreef H, et al. Eicosapentaenoic acid, a n-3 polyunsaturated fatty acid differentially modulates TNF-a, IL-1b, IL-6 and PGE2 expression in UVB-irradiated human keratinocytes. J Invest Dermatol. 2002;118:692–698. doi: 10.1046/j.1523-1747.2002.01615.x. [DOI] [PubMed] [Google Scholar]
- 21.Punnonen K, Puustinen T, Jansen CT. Ultraviolet B irradiation induces changes in the distribution and release of arachidonic acid, dihomo-g-linoleic acid, and eicosapentaenoic acid in human keratinocytes in culture. J Invest Dermatol. 1987;88:611–614. doi: 10.1111/1523-1747.ep12470217. [DOI] [PubMed] [Google Scholar]
- 22.Lands WEM. Biochemistry and physiology of n-3 fatty acids. FASEB J. 1992;6:2530–2536. doi: 10.1096/fasebj.6.8.1592205. [DOI] [PubMed] [Google Scholar]
- 23.Storey A, McArdle F, Friedmann PS, Jackson MJ, Rhodes LE. Eicosapentaenoic acid and docosahexaenoic acid reduce UVB- and TNF-α-induced IL-8 secretion in keratinocytes and UVB-induced IL-8 in fibroblasts. J Invest Dermatol. 2005;124:248–255. doi: 10.1111/j.0022-202X.2004.23543.x. [DOI] [PubMed] [Google Scholar]
- 24.Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41(4 Suppl):37S–42S. doi: 10.1016/S0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 25.Burns T, Maciejewski SR, Hamilton WR, Zheng M, Mooss AN, Hilleman DE. Effect of omega-3 fatty acid supplementation on the arachidonic acid:eicosapentaenoic acid ratio. Pharmacotherapy. 2007;27:633–638. doi: 10.1592/phco.27.5.633. [DOI] [PubMed] [Google Scholar]
- 26.Kooshki A, Taleban FA, Tabibi H, Hedayati M. Effects of marine omega-3 fatty acids on serum systemic and vascular inflammation markers and oxidative stress in hemodialysis patients. Ann Nutr Metab. 2011;58:197–202. doi: 10.1159/000329727. [DOI] [PubMed] [Google Scholar]