Abstract
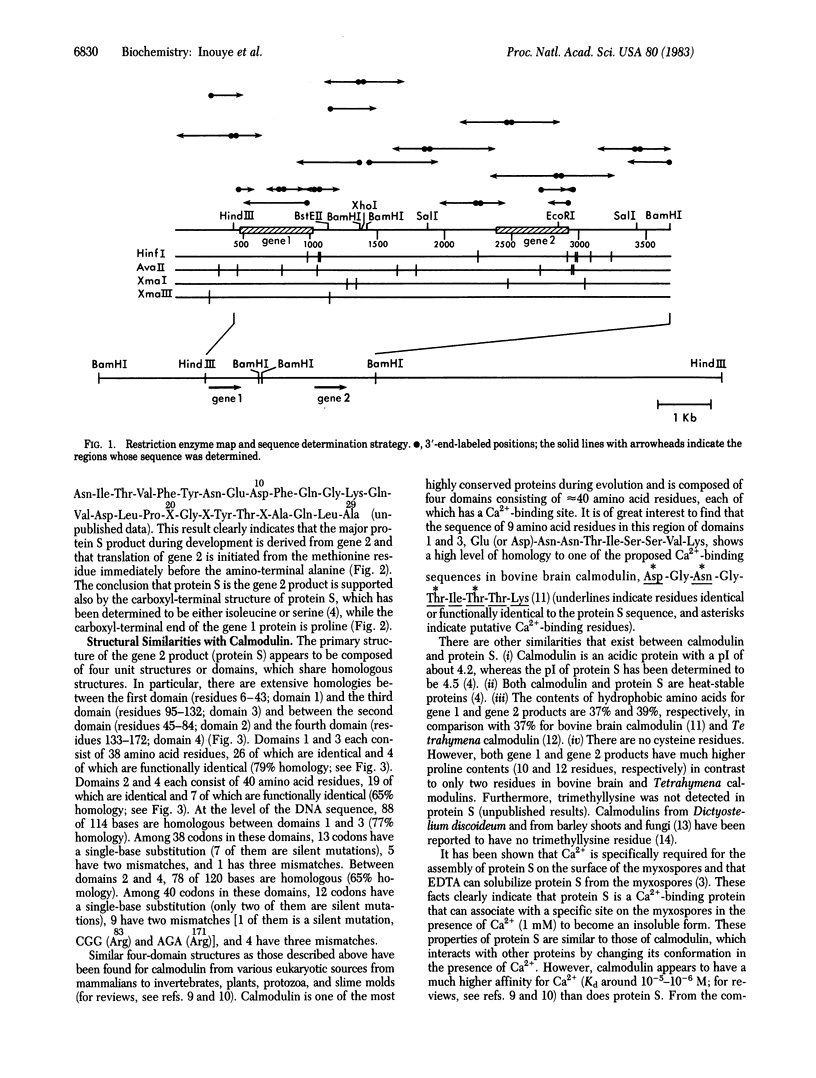
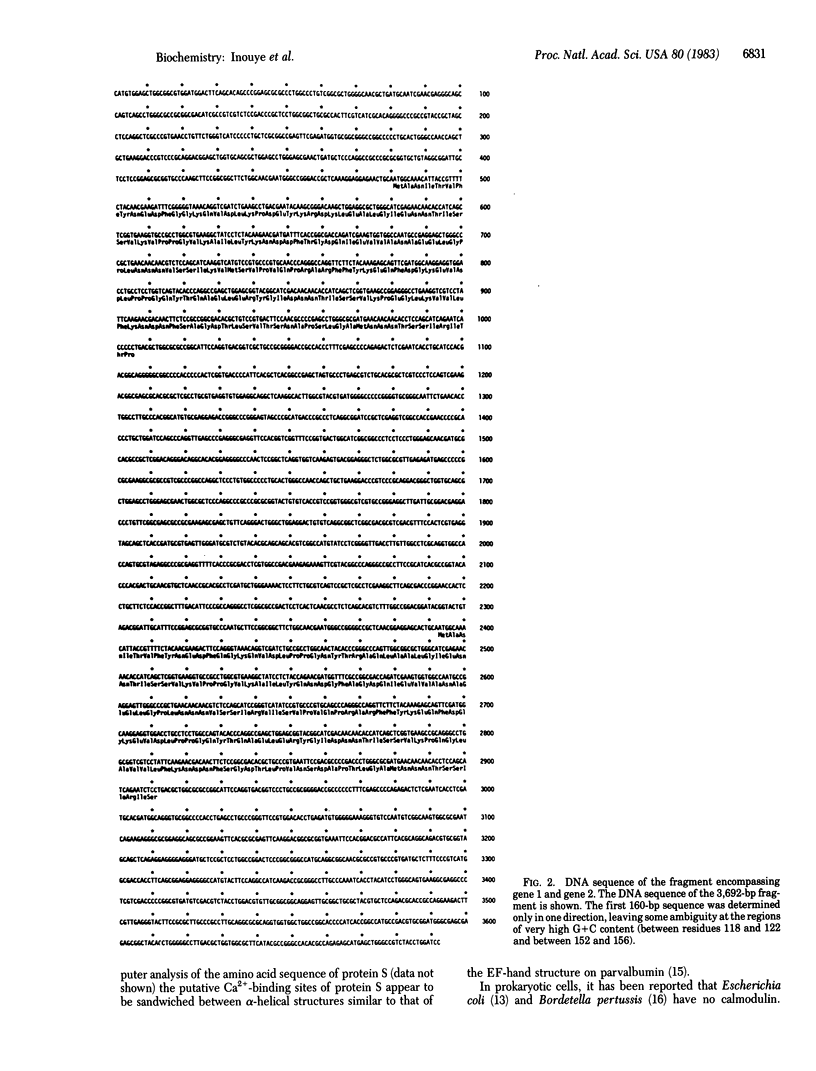
During differentiation of Myxococcus xanthus, a large amount of protein S is produced and assembled on the surface of the myxospore by a process that specifically requires Ca2+. The gene for protein S has been cloned, and two tandemly repeated homologous genes have been found to be within a short distance of each other in the M. xanthus chromosome. We determined the DNA sequence of 3,692 bp encompassing both genes and deduced the amino acid sequences of the two gene products. The gene 1 (upstream) product and the gene 2 (downstream) product show extensive amino acid sequence homology (88%). However, from their structures, protein S was found to be produced from gene 2, indicating that gene 2 is specifically turned on during differentiation. The structure of protein S shows striking similarities with calmodulin: protein S is composed of four internally homologous domains. In particular, the first and the third domains, consisting of 38 residues each, show a high level of homology (79%), and the second and the fourth domains, consisting of 40 residues each, show homology of 65%. In the first and the third domains, there is a common sequence of nine residues, Glu (or Asp)-Asn-Asn-Thr-Ile-Ser-Ser-Val-Lys, which is highly homologous to one of the proposed Ca2+-binding sequences in bovine brain calmodulin, Asp-Gly-Asn-Gly-Thr-Ile-Thr-Thr-Lys.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazari W. L., Clarke M. Characterization of a novel calmodulin from Dictyostelium discoideum. J Biol Chem. 1981 Apr 10;256(7):3598–3603. [PubMed] [Google Scholar]
- Geisselsoder J., Campos J. M., Zusman D. R. Physical characterization of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978 Feb 25;119(2):179–189. doi: 10.1016/0022-2836(78)90432-1. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Nairn A. C., Perry S. V. The preparation of calmodulins from barley (Hordeum sp.) and basidiomycete fungi. Biochem J. 1980 Mar 1;185(3):755–760. doi: 10.1042/bj1850755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Biosynthesis and self-assembly of protein S, a development-specific protein of Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):209–213. doi: 10.1073/pnas.76.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Inouye S., Zusman D. R. Gene expression during development of Myxococcus xanthus: pattern of protein synthesis. Dev Biol. 1979 Feb;68(2):579–591. doi: 10.1016/0012-1606(79)90228-8. [DOI] [PubMed] [Google Scholar]
- Inouye S., Harada W., Zusman D., Inouye M. Development-specific protein S of Myxococcus xanthus: purification and characterization. J Bacteriol. 1981 Nov;148(2):678–683. doi: 10.1128/jb.148.2.678-683.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Ike Y., Inouye M. Tandem repeat of the genes for protein S, a development-specific protein of Myxococcus xanthus. J Biol Chem. 1983 Jan 10;258(1):38–40. [PubMed] [Google Scholar]
- Inouye S., Inouye M., McKeever B., Sarma R. Preliminary crystallographic data for protein S, a development-specific protein of Myxococcus xanthus. J Biol Chem. 1980 Apr 25;255(8):3713–3714. [PubMed] [Google Scholar]
- Iwasa Y., Yonemitsu K., Matsui K., Fukunaga K., Miyamoto E. Calmodulin-like activity in the soluble fraction of Escherichia coli. Biochem Biophys Res Commun. 1981 Feb 12;98(3):656–660. doi: 10.1016/0006-291x(81)91164-5. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- Lin Y. M. Calmodulin. Mol Cell Biochem. 1982 Jun 11;45(2):101–112. doi: 10.1007/BF00223504. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979 Dec;18(4):1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Waisman D. M. Calmodulin and its role in the second-messenger system. Curr Top Cell Regul. 1979;15:47–107. doi: 10.1016/b978-0-12-152815-7.50006-5. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- Wolff J., Cook G. H., Goldhammer A. R., Berkowitz S. A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M., Yagi K., Toda H., Kondo K., Narita K., Yamazaki R., Sobue K., Kakiuchi S., Nagao S., Nozawa Y. The amino acid sequence of the Tetrahymena calmodulin which specifically interacts with guanylate cyclase. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1051–1057. doi: 10.1016/0006-291x(81)90725-7. [DOI] [PubMed] [Google Scholar]



