Abstract
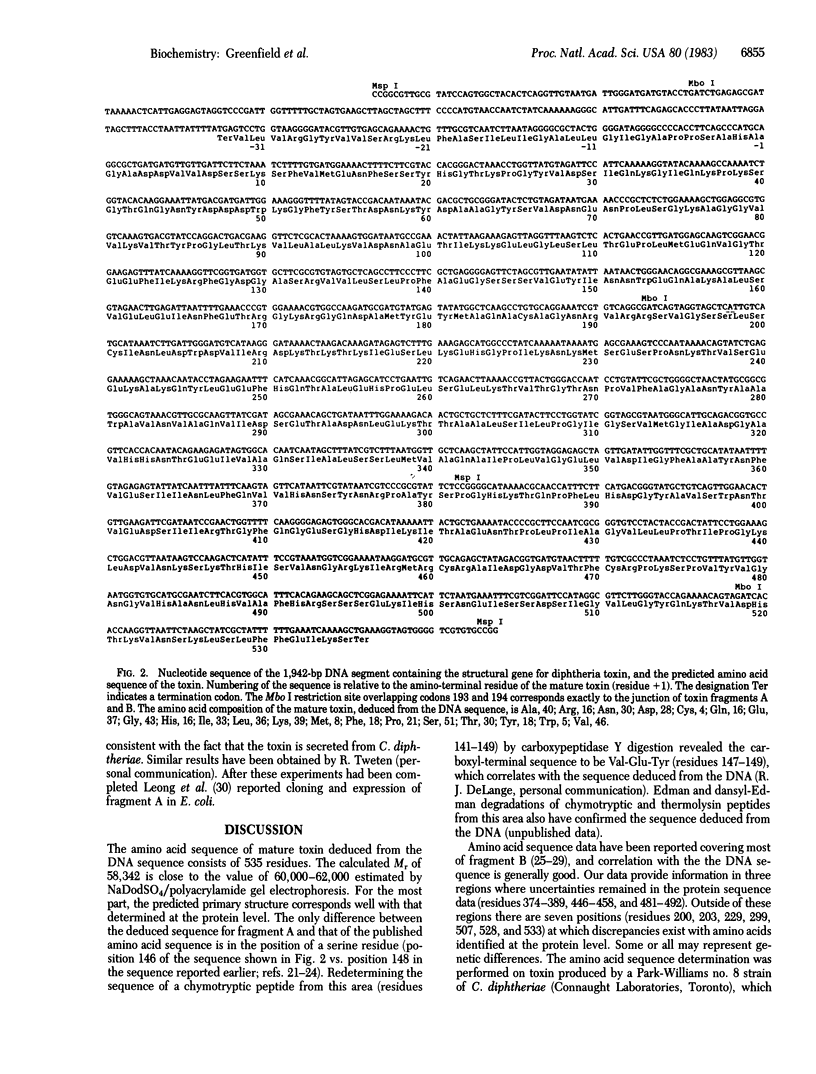
A 1,942-base-pair DNA segment encoding the structural gene for diphtheria toxin was sequenced, and the primary structure of the toxin was deduced. Restriction enzyme fragments corresponding to nontoxic or hypotoxic peptides of the toxin were isolated from corynebacteriophage beta and cloned into Escherichia coli on plasmid pBR322, and the sequence was determined. The mature toxin molecule deduced from the sequence has 535 amino acid residues and a molecular weight of 58,342. The deduced sequence for the fragment A moiety was the same as that determined at the protein level, except for a single serine residue, which had been mispositioned in the earlier study. Several differences were noted with respect to the partial sequence data available on the fragment B moiety, some or all of which may reflect genetic variations among populations of corynephages carrying the toxin gene. The DNA sequence predicts a 25-residue leader peptide preceding the mature protein, which is presumably involved in secretion of the toxin from lysogenized Corynebacterium diphtheriae. We infer that initiation of translation probably occurs at a GTG codon (codon -25). Cloned restriction fragments containing sequences for the amino-terminal region of toxin, together with 5' flanking regions, were expressed in E. coli. Toxin-related peptides were synthesized and secreted into the periplasmic space. These results provide a basis for applying recombinant DNA methods to the study of diphtheria toxin and for producing novel, genetically altered forms of the toxin suited to the construction of new classes of immunotoxins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck G. A., Groman N. B. Identification of deoxyribonucleic acid restriction fragments of beta-converting corynebacteriophages that carry the gene for diphtheria toxin. J Bacteriol. 1981 Oct;148(1):153–162. doi: 10.1128/jb.148.1.153-162.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck G. A., Groman N. B. Physical mapping of beta-converting and gamma-nonconverting corynebacteriophage genomes. J Bacteriol. 1981 Oct;148(1):131–142. doi: 10.1128/jb.148.1.131-142.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Weiss J., Konrad M., White T., Bahl C., Yu S. D., Marks D., Steiner D. F. Biosynthesis and periplasmic segregation of human proinsulin in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5401–5405. doi: 10.1073/pnas.78.9.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D. W., Collier R. J. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. doi: 10.1128/iai.16.3.832-841.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. J., Michel J. L., Rappuoli R., Murphy J. R. Restriction map of corynebacteriophages beta c and beta vir and physical localization of the diphtheria tox operon. J Bacteriol. 1981 Oct;148(1):124–130. doi: 10.1128/jb.148.1.124-130.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Drazin R. E., Collier R. J. Amino-acid sequence of fragment A, an enzymically active fragment from diphtheria toxin. Proc Natl Acad Sci U S A. 1976 Jan;73(1):69–72. doi: 10.1073/pnas.73.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Williams L. C., Collier R. J. The amino acid sequence of fragment A, an enzymically active fragment of diphtheria toxin. I. The tryptic peptides from the maleylated protein. J Biol Chem. 1979 Jul 10;254(13):5827–5831. [PubMed] [Google Scholar]
- DeLange R. J., Williams L. C., Drazin R. E., Collier R. J. The amino acid sequence of fragment A, an enzymically active fragment of diphtheria toxin. III. The chymotryptic peptides, the peptides derived by cleavage at tryptophan residues, and the complete sequence of the protein. J Biol Chem. 1979 Jul 10;254(13):5838–5842. [PubMed] [Google Scholar]
- Donovan J. J., Simon M. I., Draper R. K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc Natl Acad Sci U S A. 1981 Jan;78(1):172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazin R. E., Collier R. J., Williams L. C., DeLange R. J. The amino acid sequence of fragment A, an enzymically active fragment of diphtheria toxin. II. The cyanogen bromide peptides. J Biol Chem. 1979 Jul 10;254(13):5832–5837. [PubMed] [Google Scholar]
- Falmagne P., Capiau C., Zanen J., Kayser G., Ruysschaert J. M. Structure-activity relationships of the B fragment of diphtheria toxin: the lipid-binding domains. Toxicon. 1982;20(1):243–246. doi: 10.1016/0041-0101(82)90209-4. [DOI] [PubMed] [Google Scholar]
- Falmagne P., Lambotte P., Dirkx J. Isolation and characterization of the cyanogen bromide peptides from the B fragment of diphtheria toxin. Biochim Biophys Acta. 1978 Jul 21;535(1):54–65. doi: 10.1016/0005-2795(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Gilliland D. G., Steplewski Z., Collier R. J., Mitchell K. F., Chang T. H., Koprowski H. Antibody-directed cytotoxic agents: use of monoclonal antibody to direct the action of toxin A chains to colorectal carcinoma cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4539–4543. doi: 10.1073/pnas.77.8.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. H., Wu R. Exonuclease III: use for DNA sequence analysis and in specific deletions of nucleotides. Methods Enzymol. 1983;100:60–96. doi: 10.1016/0076-6879(83)00046-4. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorek M., Delpeyroux F., Chenciner N., Streeck R. E., Murphy J. R., Boquet P., Tiollais P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science. 1983 Aug 26;221(4613):855–858. doi: 10.1126/science.6348945. [DOI] [PubMed] [Google Scholar]
- Kagan B. L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser G., Lambotte P., Falmagne P., Capiau C., Zanen J., Ruysschaert J. M. A CNBR peptide located in the middle region of diphtheria toxin fragment B induces conductance change in lipid bilayers. Possible role of an amphipathic helical segment. Biochem Biophys Res Commun. 1981 Mar 31;99(2):358–363. doi: 10.1016/0006-291x(81)91753-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lambotte P., Falmagne P., Capiau C., Zanen J., Ruysschaert J. M., Dirkx J. Primary structure of diphtheria toxin fragment B: structural similarities with lipid-binding domains. J Cell Biol. 1980 Dec;87(3 Pt 1):837–840. doi: 10.1083/jcb.87.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D., Coleman K. D., Murphy J. R. Cloned fragment A of diphtheria toxin is expressed and secreted into the periplasmic space of Escherichia coli K12. Science. 1983 Apr 29;220(4596):515–517. doi: 10.1126/science.6403984. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Pihl A. Chimeric toxins. Pharmacol Ther. 1981;15(3):355–381. doi: 10.1016/0163-7258(81)90050-4. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Gilberg W. Construction of plasmid vectors with unique PstI cloning sites in a signal sequence coding region. Gene. 1980 Dec;12(3-4):235–241. doi: 10.1016/0378-1119(80)90105-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M., Mekada E., Uchida T., Okada Y. One molecule of diphtheria toxin fragment A introduced into a cell can kill the cell. Cell. 1978 Sep;15(1):245–250. doi: 10.1016/0092-8674(78)90099-5. [DOI] [PubMed] [Google Scholar]


