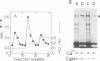Abstract
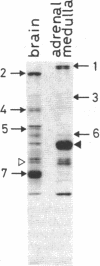
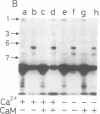
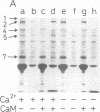
Regulatory actions of calmodulin on the contractile apparatus and cytoskeleton of smooth muscle and nonmuscle tissue are mediated by a number of specific calmodulin-binding proteins that bind to F-actin in a flip-flop manner--i.e., they bind to calmodulin or F-actin depending on the presence or absence, respectively, of Ca2+. A survey for such proteins in brain, adrenal gland, and pituitary gland identified six polypeptides on polyacrylamide gels--Mr 340,000 (band 1), Mr 240,000/235,000 doublet (band 2), Mr 150,000 (band 3), Mr 129,000 (band 4), Mr 105,000 (band 5), and Mr 94,000 (band 6)--as flip-flop-regulated calmodulin- and F-actin-binding polypeptides. In addition to these polypeptides, a Mr 58,000 non-flip-flop calmodulin-binding actin-binding polypeptide (band 7) was found in all tissues examined. Band 2 was identified as calspectin (spectrin-related protein; fodrin). The flip-flop regulation of calspectin required the presence of a heat-labile nondialyzable factor contained in a supernatant fraction of brain homogenates. Band 1 was distinct from microtubule-associated proteins (MAPs) 1 and 2. However, when band 1 polypeptide was kept on ice 3 days, it converted to a lower molecular weight doublet that migrated with MAP2 on NaDodSO4 gel electrophoresis. Bands 1 and 2 were found in all tissues examined.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines A. J. The spread of spectrin. Nature. 1983 Feb 3;301(5899):377–378. doi: 10.1038/301377b0. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. A., Katagiri J., Binder H. K., Williams R. C., Jr Separation and characterization of microtubule proteins from calf brain. Biochemistry. 1977 Dec 13;16(25):5610–5617. doi: 10.1021/bi00644a035. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlin R. K., Grab D. J., Siekevitz P. Function of a calmodulin in postsynaptic densities. III. Calmodulin-binding proteins of the postsynaptic density. J Cell Biol. 1981 Jun;89(3):449–455. doi: 10.1083/jcb.89.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisow M. J., Burgoyne R. D. Recruitment of cytosolic proteins to a secretory granule membrane depends on Ca2+-calmodulin. Nature. 1983 Feb 3;301(5899):432–435. doi: 10.1038/301432a0. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V. Calmodulin-binding proteins from brain and other tissues. Biochem J. 1979 Nov 1;183(2):285–295. doi: 10.1042/bj1830285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982 Aug 10;257(15):9143–9151. [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Itano T., Itano R., Penniston J. T. Interactions of basic polypeptides and proteins with calmodulin. Biochem J. 1980 Sep 1;189(3):455–459. doi: 10.1042/bj1890455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K. Ca2+-and calmodulin-dependent flip-flop mechanism in microtubule assembly-disassembly. FEBS Lett. 1981 Sep 14;132(1):141–143. doi: 10.1016/0014-5793(81)80448-6. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Kambayashi J., Sakon M., Kosaki G. Lack of tissue specificity of calmodulin: a rapid and high-yield purification method. FEBS Lett. 1981 Apr 20;126(2):203–207. doi: 10.1016/0014-5793(81)80242-6. [DOI] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Krinks M. H. Calcineurin: a calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B., Krinks M. H. Purification of cyclic 3',5'-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to Sepharose. Biochemistry. 1978 Jan 10;17(1):120–126. doi: 10.1021/bi00594a017. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Expression of spectrin in nonerythroid cells. Cell. 1982 Dec;31(3 Pt 2):505–508. doi: 10.1016/0092-8674(82)90306-3. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Dedman J. R., Chafouleas J. G., Means A. R., Roth T. F. Interactions of calmodulin with coated vesicles from brain. Proc Natl Acad Sci U S A. 1981 Jan;78(1):308–312. doi: 10.1073/pnas.78.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maékawa S., Abe T. Isolation of a new calmodulin-binding protein from rat brain. Biochem Biophys Res Commun. 1980 Nov 28;97(2):621–627. doi: 10.1016/0006-291x(80)90309-5. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Schiebler W., Greengard P. A major calmodulin-binding protein common to various vertebrate tissues. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3780–3784. doi: 10.1073/pnas.79.12.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. K., Desai R., Thompson T. R., Wang J. H. Purification of the heat-stable inhibitor protein of the Ca2+-activated cyclic nucleotide phosphodiesterase by affinity chromatography. Can J Biochem. 1978 Jun;56(6):598–604. doi: 10.1139/o78-090. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Desai R., Waisman D. M., Wang J. H. Purification and subunit structure of bovine brain modulator binding protein. J Biol Chem. 1979 May 25;254(10):4276–4282. [PubMed] [Google Scholar]
- Sobue K., Fujita M., Muramoto Y., Kakiuchi S. The calmodulin-binding protein in microtubules is tau factor. FEBS Lett. 1981 Sep 14;132(1):137–140. doi: 10.1016/0014-5793(81)80447-4. [DOI] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Kakiuchi S. Solubilization and partial purification of protein kinase systems from brain membranes that phosphorylate calspectin. A spectrin-like calmodulin-binding protein (fodrin). FEBS Lett. 1982 Dec 13;150(1):185–190. doi: 10.1016/0014-5793(82)81331-8. [DOI] [PubMed] [Google Scholar]
- Sobue K., Morimoto K., Kanda K., Maruyama K., Kakiuchi S. Reconstitution of Ca2+-sensitive gelation of actin filaments with filamin, caldesmon and calmodulin. FEBS Lett. 1982 Feb 22;138(2):289–292. doi: 10.1016/0014-5793(82)80463-8. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelecka-Gołaszewska H., Próchniewicz E., Nowak E., Zmorzyński S., Drabikowski W. Chicken-gizzard actin: polymerization and stability. Eur J Biochem. 1980 Feb;104(1):41–52. doi: 10.1111/j.1432-1033.1980.tb04397.x. [DOI] [PubMed] [Google Scholar]
- Trifaró J. M. Common mechanisms of hormone secretion. Annu Rev Pharmacol Toxicol. 1977;17:27–47. doi: 10.1146/annurev.pa.17.040177.000331. [DOI] [PubMed] [Google Scholar]