Summary
Although antiretroviral therapy has revolutionized the clinical management of AIDS, life-long treatment is required because these drugs do not eradicate HIV-infected cells. Chronic antiretroviral therapy may not protect AIDS patients from cognitive impairment, raising important quality of life issues. Because of the rise of HIV strains resistant to current drugs and uncertain vaccine prospects, an urgent need exists for the discovery and development of new therapeutic approaches. This review is focused on one such approach, which involves targeting HIV-1 Nef, a viral accessory protein essential for AIDS pathogenesis.
Introduction
Current AIDS drugs block the activity of HIV-1 enzymes critical to the viral life cycle, including reverse transcriptase and protease, as well as fusion of the virus to host cell receptors [1]. The HIV genome also encodes four accessory factors (Vpr, Vpu, Vif, and Nef) essential for viral pathogenicity that represent alternative targets for drug discovery [2–4]. HIV-1 Nef is particularly attractive in this regard, because it is critical to the HIV life cycle in vivo and also promotes immune escape of HIV-infected cells. As summarized in the next section, a large body of evidence points to HIV-1 Nef as a rational drug target in AIDS.
Discussion
A case for Nef as a drug target for AIDS
A large body of research strongly supports an essential role Nef in HIV-1 pathogenesis and AIDS progression, and many excellent reviews are available that explore this topic [5–9]. Below is a brief summary of some of the major themes that help to make the case for a drug discovery campaign targeting Nef.
HIV Nef is relatively small, polymorphic protein (27–30 kDa) that is packaged in the virion and is also expressed at high levels early in the viral life cycle. Nef is myristoylated on its N-terminus, which helps to attach it to cellular membranes essential for function [10]. Nef lacks any known biochemical activities, functioning instead through protein-protein interactions with a diverse range of host cell proteins. These interactions provide the mechanistic basis for many Nef activities, including downregulation of cell-surface immune (MHC-I) and viral receptors (CD4/CXCR4/CCR5), remodeling of the actin cytoskeleton, and stimulation of host cell signaling pathways [9]. These functions of Nef allow HIV-infected cells to avoid immune surveillance by the host, prevent viral superinfection, and enhance virion release.
Other work supports a critical role for Nef in HIV pathogenesis at the whole animal level. Early studies in non-human primates provide some of the strongest evidence that Nef is required for the development of AIDS [11]. Infection of rhesus macaques with Nef-defective SIV resulted in low viral loads and caused a substantial delay in the onset of disease. These findings are consistent with reports of rare individuals infected with Nef-defective HIV [12–14]. In these patients, viral loads remain low or undetectable and in some cases CD4+ T-cell counts remain stable for many years, even in the absence of antiretroviral therapy.
Other evidence supporting a direct role for Nef in HIV disease comes from mouse models. Because mice cannot be infected with the virus, Jolicoeur et al. developed transgenic mice in which a CD4-derived promoter was used to express Nef in HIV target cells [15]. Remarkably, expression of Nef alone in the CD4+ cell population was sufficient to cause AIDS-like disease. This Nef-dependent phenotype mimics many aspects of human AIDS, including CD4+ T-cell loss, thymic involution, splenic atrophy and subsequent kidney and lung pathology.
A more recent study has demonstrated an essential role for Nef in HIV infection using humanized ‘BLT’ (bone marrow, liver, thymus) mice [14], in which immunodeficient animals are reconstituted with the human immune system through transplantation of CD34+ stem cells from human fetal livers. BLT humanized mice display a full range of human immune cells, including B and T cells, myelomonocytic cells, and dendritic cells. Infection of these animals with wild-type HIV-1 results in rapid depletion of CD4+ T-cells from both the blood and tissue compartments. In striking contrast, infection with Nef-defective virus does not result in CD4+ T-cell loss, supporting a direct role for Nef in thymocyte killing that complements the results with Nef- transgenic mice.
Taken together, the animal and patient data described above support a dominant role for Nef in HIV pathogenesis. These studies provide a strong rationale for the discovery and development of small molecule antagonists of Nef function as a new approach to antiretroviral therapy. Furthermore, recent studies show that engineered Nef-binding proteins block its functions in cell-based studies, including CD4 and MHC-I downregulation, viral infectivity, and kinase activation [16]. These experiments provide an important proof-of-concept that Nef antagonists may be valuable weapons in the fight against AIDS. In the sections that follow, we review three examples of small molecule antagonists of HIV-1 Nef function. Each of these compounds was discovered by unique approaches, and targets a different region of the Nef structure. As a consequence, these compounds display overlapping but non-identical activity profiles against Nef functions.
Combined computational and in vitro screening yields antagonists for Nef:SH3 interaction
Nef elicits a wide range of host cell responses through a complex web of protein-protein interactions involving several conserved motifs on the protein’s surface [6,9]. One of the best studied is the Nef PxxPxR motif, which involves N-terminal proline residues 72 and 75 [17]. This motif forms a polyproline type II helix for interaction with SH3 domains in some partner proteins. Mutagenesis studies have shown that this motif is required for many Nef functions [6] including the development of AIDS-like disease in transgenic mice [18]. In addition to the PxxPxR motif, high-affinity SH3 binding by Nef also requires a unique hydrophobic pocket that engages the RT-loop of the SH3 domain (Figure 1). This hydrophobic pocket is formed in part by three highly conserved residues, and represents an attractive protein-protein interface for small molecule inhibitor binding [17].
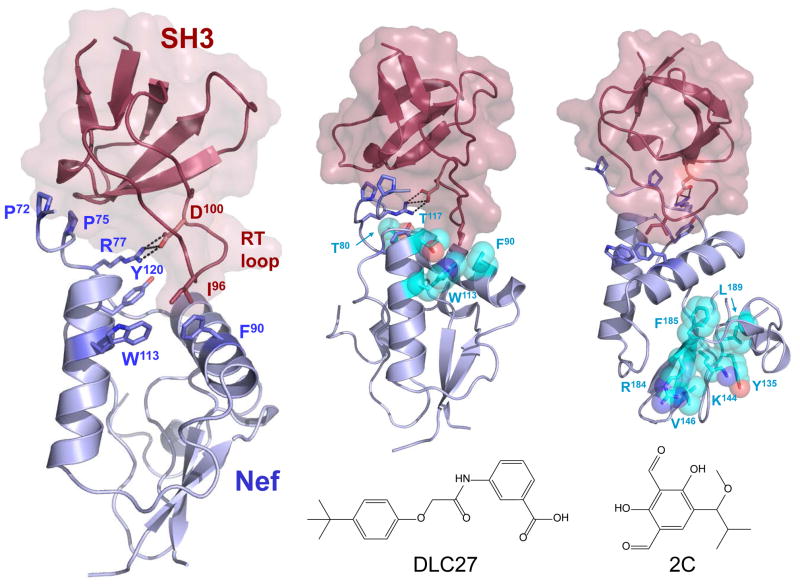
Figure 1. The Nef antagonists DLC27 and 2C bind opposite faces of the Nef protein.
Left: The X-ray crystal structure of HIV-1 Nef (blue) in complex with a Src-family kinase SH3 domain (red) is shown on the left. Key structural elements of this interaction surface include the Nef PPII helix which involves conserved residues P72 and P75, an ionic interaction between Nef R77 and SH3 D100, and a hydrophobic pocket formed in part by Nef residues F90, W113, and Y120. This pocket interacts with I96 from the RT-loop of the SH3 domain. Nef residues undergoing major NMR chemical shift changes following binding of the Nef antagonists DLC27 (center) and 2C (right) are highlighted on the structure of the Nef:SH3 complex as spheres (cyan). Note that the DLC27 binding site involves residues that interact directly with the SH3 domain RT-loop, including W113 and F90. In contrast, the 2C compound binds at a distance from the SH3 docking site, and is believed to inhibit SH3 binding and Src-family kinase activation through an allosteric mechanism. Models were rendered using the Nef:SH3 X-ray crystal structure of Lee et al. [17]. The NMR chemical shift data used to create the figure are from Betzi et al. for DLC27 [19] and Dikeakos et al. for 2C [21].
Betzi et al. [19] targeted the Nef:SH3 interface using a combination of virtual screening and a mammalian cell-based protein-protein interaction assay. Combining these approaches to screen a subset of the NCI diversity library yielded one compound that interfered with Nef:SH3 interaction in the cell based assay, albeit with low affinity. This initial hit compound, a phenoxyacetamido benzoic acid derivative termed D1, also partially inhibited Nef-dependent downregulation MHC-I without affecting cell-surface CD4. This result is consistent with previous genetic studies implicating the Nef SH3-binding function in MHC-I but not CD4 downregulation. The partial effects of D1 on MHC-I downregulation may reflect the involvement of other Nef structural motifs in this process that are not targeted by this compound (more below).
Seventy compounds based on the D1 substructure were then screened for inhibition of Nef:SH3 interaction. This study identified an analog (DLC27) which was subsequently shown to interact directly with Nef by multidimensional NMR with an apparent KD value of about 1.0 μM. Chemical shift perturbations induced by DLC27 are consistent with binding to the Nef hydrophobic pocket, and involves conserved residues Trp113 and Phe90 in this region (Figure 1). DLC27 consists of a carboxylic acid joined to a hydrophobic t-butyl-phenyl group through a modified carbamate linker. The authors suggest a mechanism of action for this compound in which the apolar moiety inserts into the Nef hydrophobic pocket and competes for binding of the SH3 domain RT loop [19]. Subsequent docking studies of DLC27 led to the development of second-generation analogs with extended linkers that interact with a groove on the Nef surface [20]. Interestingly, interaction with one of these analogs enhanced the sensitivity of Nef to proteolysis by HIV-1 protease, suggesting that compound binding to the hydrophobic pocket may destabilize the structure of the Nef core. Despite these attractive properties, solubility and toxicity issues have limited further development of this series of compounds. As a consequence, their activity against Nef in the context of viral replication and infectivity is unknown.
A chemical probe for Nef function in MHC-I downregulation
HIV-1 Nef uses a temporally regulated program to downregulate cell-surface MHC-I, allowing HIV-infected cells to escape immune surveillance. During the first two days following infection, Nef triggers MHC-I downregulation by an endocytic program called the signaling mode. By three days post-infection, Nef switches to a stoichiometric mode of downregulation that prevents delivery of newly synthesized MHC-I molecules to the cell surface [21]. Inhibition of the signaling mode blocks onset of the stoichiometric mode, demonstrating that the two modes of MHC-I downregulation are intimately linked. Nef anatgonists that block the signaling mode are therefore anticipated to prevent MHC-I downregulation by Nef, and have the potential to re-enabling immune detection and clearance of HIV-positive cells.
An early and essential step in the signaling mode is Src-family kinase (SFK) activation by Nef, which depends on the PxxPxR motif described above. In a manner analogous to T-cell receptor activation, SFK activation leads to ZAP-70 (T-cells) or Syk (monocytes) activation, followed by recruitment of a class I phosphatidylinositide 3-kinase (PI3K) [22,23]. Nef recruits different PI3K isoforms containing specific p110 catalytic subunits depending upon the cell type. In T-cells, Nef recruits p110 δ whereas in monocytes and other cell types, Nef recruits p110α or p110β. By combining with cell-type specific Src-family members, PI3Ks and ZAP-70 or Syk, Nef can assemble the multi-kinase complex required for MHC-I downregulation in a broad set of HIV-1 permissive cells.
The identification of the Nef-assembled multi-kinase complex provided an opportunity to target the signaling mode of MHC-I downregulation with kinase inhibitors. For example, Nef-dependent MHC-I downregulation in virus-infected primary CD4+ T-cells was inhibited with the class I PI3K inhibitor PI-103 and with the p110 δ-specific inhibitor PIK-23 [22,24]. By contrast, Nef-induced downregulation of CD4, which does not require the multi-kinase complex, was unaffected.
In addition to inhibiting downstream kinases, an alternative approach involves blocking association of Nef with the kinases that that form the multi-kinase complex. One compound that works via this mechanism is termed ‘2c’, which is a derivative of the Streptomyces metabolite UCS15A. This natural product was originally found to disrupt the binding of the cell-cycle regulator Sam68 to c-Src, an interaction that also involves a PxxP motif and the Src SH3 domain [25]. The 2c derivative of UCS15A blocked the ability of Nef to bind and activate Hck in vitro in a concentration-dependent manner and also inhibited the binding of Nef to Lyn and c-Src, the other two SFKs directly linked to activation by Nef [26]. Multidimensional NMR analysis indicated that 2c interacts with the Nef SH3 domain-binding site and also with the cleft formed by the central β-sheet and the C-terminal α-helices of Nef (Figure 1). These findings suggest that 2c interferes with Nef-induced SFK activation by both allosteric inhibition of SFK recruitment as well as a direct effect on SH3 domain binding.
Cellular studies showed that 2c blocked the interaction of Nef with SFKs and, in turn, prevented assembly of the SFK/ZAP-70/PI3K multi-kinase complex. Control experiments showed that 2c had no effect on Nef trafficking steps required for interaction with SFKs nor did 2c block PI3K activity directly. Consistent with an essential role for the Nef-assembled multi-kinase complex in MHC-I downregulation, treatment of primary CD4+ T-cells with 2c repressed the ability of HIV-1 to downregulate MHC-I. As expected, HIV-1-induced CD4 downregulation was unaffected [21]. In addition to its effects on MHC-I downregulation, 2c was also observed to interfere with HIV infectivity in a Nef-dependent manner [27]. This observation supports a role for the Nef-assembled multi-kinase complex in both immune evasion and viral infectivity. However, both of these effects required 2c concentrations in the double-digit μM range, raising the possibility of off-target effects. Nevertheless, these studies provide an important proof-of-concept that inhibition of Nef-dependent kinase signaling is a viable approach to the development of Nef antagonists.
Identification of Nef antagonists by effector kinase coupling
The Nef protein lacks intrinsic biochemical activity, making development of high-throughput screening assays for Nef function challenging. One approach to this problem involves coupling of Nef to activation of the SFK Hck, one of the best-characterized Nef binding partners [6]. Hck is strongly expressed in macrophages and other HIV target cells [28], and Nef-induced activation of Hck has been implicated in several Nef functions including enhancement of viral replication as well as downregulation of MHC-I [21,22,24,29] as described above.
Like other members of the Src kinase family, Hck activity is downregulated in part by intramolecular docking of its SH3 domain onto the back of the kinase domain, which helps to stabilize the inactive conformation of the active site [30]. Nef binds to the Hck SH3 domain and displaces it from the back of the kinase domain, leading to constitutive kinase activation [31,32]. Using purified downregulated Hck and HIV-1 Nef, Emert-Sedlak et al. identified in vitro kinase assay conditions where Hck activation is completely dependent upon its interaction with Nef [33]. Screening a relatively small kinase-biased library with this assay, they identified a series of kinase inhibitors based on a 4-amino-diphenylfuranopyrimidine (DFP) scaffold. These compounds were shown to preferentially inhibit Hck in the presence of Nef, suggesting that binding of Nef to the Hck SH3 domain allosterically influences the active site to favor compound binding. This concept has been confirmed more recently in independent studies [34]. The DFP-based compounds blocked Nef-dependent enhancement of HIV-1 replication in the low micromolar range. Subsequent work showed that the DFP compounds inhibit HIV-1 replication supported by a wide range of Nef subtypes, and that the block to viral replication correlated with inhibition of endogenous SFK activity in the HIV-infected cells [35]. The mechanism by which inhibition of Nef-mediated SFK activation compromises viral replication is not clear. However, Nef-induced activation of tyrosine kinase signaling pathways mimics cytokine or antigen-mediated host cell activation [6], which favors HIV replication. Blocking this effect of Nef may explain, at least in part, the inhibitory activity of these kinase inhibitors. Nevertheless, these studies provide important evidence that Nef-dependent activation of SFKs is important for efficient HIV-1 replication. These studies, together with those using the compound 2C, support the idea that inhibition of host cell kinase pathways linked to HIV infection may represent a new therapeutic strategy for HIV disease.
In a subsequent study, the Nef/Hck assay was used in a fully automated format to screen a much larger, chemically diverse library of more than 220,000 compounds [36]. Following secondary assays to evaluate Nef dependence, about 60 compounds were identified with at least a three-fold inhibitory preference for Hck in the presence of Nef vs. Hck alone. Each of these compounds was then screened for inhibition of Nef-dependent HIV replication and infectivity in several different cell lines, yielding six compounds with antiretroviral activity.
The most active compound to emerge from the large-scale Nef/Hck screen was a diphenylpyrazolo compound, termed ‘B9’ (Figure 2). This compound is a remarkably selective inhibitor of Nef-dependent Hck activity, displaying more than a 10-fold preference for the Nef/Hck complex vs. Hck alone. B9 potently inhibits wild-type HIV-1 replication, with an IC50 value in the triple-digit nanomolar range in two different cell lines. In contrast, B9 did not affect the replication of Nef-defective HIV even at concentrations as high as 3.0 μM, supporting a Nef-dependent mechanism of action. Like the DFP compounds described above, B9 was broadly active against HIV replication supported by a wide range of HIV-1 Nef subtypes and inhibited endogenous SFK activation. In addition, B9 blocked HIV-1 infectivity in the TZM-bl cell model in a Nef-dependent manner [36].
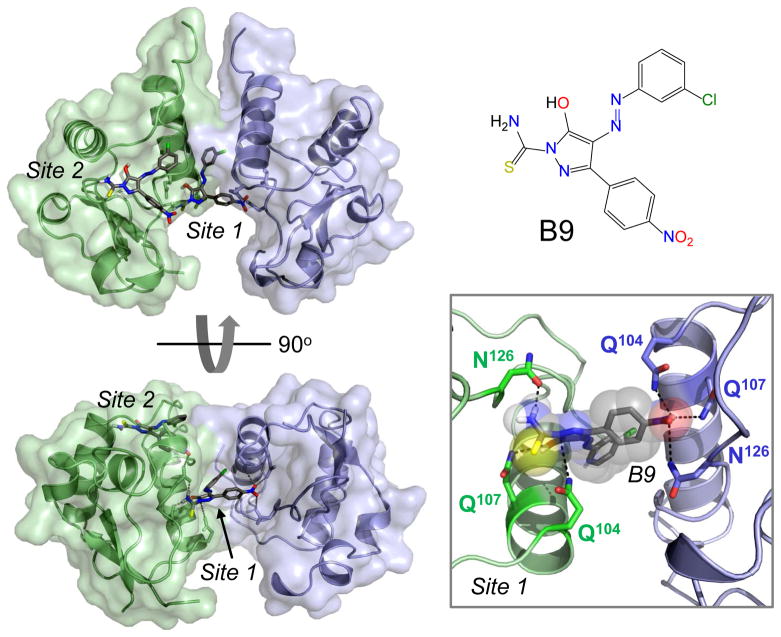
Figure 2. The diphenylpyrazolo Nef antagonist B9 is predicted to bind to the Nef dimerization interface.
Possible binding sites for B9 were explored using AutoDock Vina [43] and the X-ray crystal structure of the HIV-1 Nef dimer (PDB: 1EFN) [17]. Two energetically favorable binding sites were predicted, with the most favorable site located at the Nef-dimerization interface (Site 1) and a less favorable site on the surface of each monomer (Site 2; only a single B9 molecule is shown in this site for clarity). Two views of the overall structure of the Nef dimer are shown on the left, with the individual Nef subunits colored blue and green. A close-up view of Site 1 is shown on the lower right, where B9 is predicted to make polar contacts with conserved Nef residues Q104, Q107, and N126. A role for N126 in B9 binding to Nef has been demonstrated experimentally, and B9 has been shown to disrupt Nef dimers in a cell-based assay [36]. The structure of B9 is shown at the top right.
Unlike the DFP compounds, however, B9 most likely works through direct interaction with HIV-1 Nef rather than as a kinase inhibitor. Docking studies predicted two energetically favorable binding sites for B9 on the surface of Nef. The most intriguing of these putative binding sites maps to the dimer interface formed between two Nef molecules in the X-ray crystal structure of Nef in complex with a SFK SH3 domain. In this site, B9 is predicted to form multiple polar contacts with three conserved Nef residues, Gln104, Gln107, and Asn126, which extend from the αB helices of each Nef subunit in the dimer (Figure 2). Direct interaction of Nef with B9 was demonstrated by surface plasmon resonance, and the predicted binding site was validated in part through mutagenesis of Asn126, one of the key residues anticipated to contact B9. Substitution of this Nef position with Leu, Gln or Ala completely inhibited binding to the compound. Complete elucidation of the binding site will require X-ray crystallography or NMR spectroscopy of Nef dimers with B9 bound.
Evidence to date supports direct interaction of B9 with Nef through a pocket in the dimer interface. How could this binding event explain the antiretroviral actions of this compound as well as its ability to inhibit Nef-dependent SFK activation? One possibility is that B9 binding may affect Nef dimerization, which has been independently linked to HIV-1 function [37–40]. Using a cell-based bimolecular fluorescence complementation assay, Poe and Smithgall demonstrated that HIV Nef forms dimers both at the plasma membrane and the trans-Golgi network [40]. Mutagenesis studies showed that perturbations to the dimer interface that reduced Nef dimerization also interfered with Nef functions in terms of CD4 downregulation and HIV replication. Using the BiFC assay, B9 was found to block Nef dimerization in cells as a potential mechanism of action [36]. If the Nef dimer interface is indeed the critical binding site for B9, then inhibition of Nef-dependent Hck activation by this compound must involve an allosteric mechanism of action. One possibility is that Nef dimers recruit two Hck proteins, resulting in juxtaposition of their kinase domains and subsequent activation via trans-phosphorylation. Disruption of Nef dimer formation by B9 would therefore block this critical first step in the kinase activation mechanism. Support for this idea comes from kinase assays with a dimerization-defective mutant of Nef, which fails to activate Hck in the kinase assay [36]. Conversely, chemically enforced dimerization of Nef enhances Hck activation in cells [38]. On the other hand, X-ray crystallographic studies of Nef in complex with an MHC-I peptide fused to the μ1 subunit of the AP-1 adaptor involved in a late step in MHC-I downregulation, revealed Nef as a monomer [41]. While this structure may represent one active conformation required for this critical step in Nef action, understanding how Nef transits between its monomeric and dimeric forms and whether one or both forms interact with other cellular proteins essential for Nef function requires further investigation [6,21,42]. Small molecules, such as those described here, represent valuable tools to probe the dynamic structural transitions associated with MHC-I downregulation and other critical Nef functions.
Conclusion
A large body of research on the HIV-1 Nef protein strongly supports the discovery and development of pharmacological antagonists of this virulence factor as a new approach to HIV/AIDS therapy. Work described in the preceding sections shows that small molecules binding different regions of the HIV-1 Nef protein compromise many of its functions. These proof-of-concept studies justify a larger effort to develop early-stage lead compounds into analogs that can be tested in animal models of AIDS and ultimately move towards clinical trials. Nef inhibitors may work synergistically with existing antiretroviral drugs by suppressing Nef-mediated enhancement of viral infectivity and replication in vivo. In addition, rescue of MHC-I downregulation may help in the clearance of HIV-infected cells by cytotoxic T cells.
Acknowledgments
Research in the authors’ laboratories was supported by the National Institutes of Health (R01 AI057083 and R01 AI102724 to T.E.S. and R01 CA151564 to G.T.).
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Temesgen Z, Warnke D, Kasten MJ. Current status of antiretroviral therapy. Expert Opin Pharmacother. 2006;7:1541–1554. doi: 10.1517/14656566.7.12.1541. [DOI] [PubMed] [Google Scholar]
- 2.Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–398. doi: 10.1016/j.chom.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Ali A, Wang J, Nathans RS, Cao H, Sharova N, et al. Synthesis and structure-activity relationship studies of HIV-1 virion infectivity factor (Vif) inhibitors that block viral replication. Chem Med Chem. 2012;7:1217–1229. doi: 10.1002/cmdc.201200079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W, Zuo T, Luo X, Jin H, Liu Z, et al. Indolizine Derivatives as HIV-1 VIF-ElonginC Interaction Inhibitors. Chem Biol Drug Des. 2013;81:730–741. doi: 10.1111/cbdd.12119. [DOI] [PubMed] [Google Scholar]
- 5.Arora VK, Fredericksen BL, Garcia JV. Nef: agent of cell subversion. Microbes Infect. 2002;4:189–199. doi: 10.1016/s1286-4579(01)01527-1. [DOI] [PubMed] [Google Scholar]
- 6.Saksela K. Interactions of the HIV/SIV pathogenicity factor Nef with SH3 domain-containing host cell proteins. Curr HIV Res. 2011;9:531–542. doi: 10.2174/157016211798842107. [DOI] [PubMed] [Google Scholar]
- 7.Joseph AM, Kumar M, Mitra D. Nef: “necessary and enforcing factor” in HIV infection. Curr HIV Res. 2005;3:87–94. doi: 10.2174/1570162052773013. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill E, Kuo LS, Krisko JF, Tomchick DR, Garcia JV, et al. Dynamic evolution of the human immunodeficiency virus type 1 pathogenic factor, Nef. J Virol. 2006;80:1311–1320. doi: 10.1128/JVI.80.3.1311-1320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 11.Kestler H, Ringler DJ, Mori K, Panicali DL, Sehgal PK, et al. Importance of the nef gene for maintenance of high viral loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 13.Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. doi: 10.1126/science.270.5238.988. [DOI] [PubMed] [Google Scholar]
- 14.Zou W, Denton PW, Watkins RL, Krisko JF, Nochi T, et al. Nef functions in BLT mice to enhance HIV-1 replication and deplete CD4+CD8+ thymocytes. Retrovirology. 2012;9:44. doi: 10.1186/1742-4690-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanna Z, Kay DG, Rebai N, Guimond A, Jothy S, et al. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 16.Bouchet J, Herate C, Guenzel CA, Verollet C, Jarviluoma A, et al. Single-domain antibody-SH3 fusions for efficient neutralization of HIV-1 Nef functions. J Virol. 2012;86:4856–4867. doi: 10.1128/JVI.06329-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C-H, Saksela K, Mirza UA, Chait BT, Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 18.Hanna Z, Weng X, Kay DG, Poudrier J, Lowell C, et al. The pathogenicity of human immunodeficiency virus (HIV) type 1 Nef in CD4C/HIV transgenic mice is abolished by mutation of its SH3-binding domain, and disease development is delayed in the absence of Hck. J Virol. 2001;75:9378–9392. doi: 10.1128/JVI.75.19.9378-9392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betzi S, Restouin A, Opi S, Arold ST, Parrot I, et al. Protein protein interaction inhibition (2P2I) combining high throughput and virtual screening: Application to the HIV-1 Nef protein. Proc Natl Acad Sci U S A. 2007;104:19256–19261. doi: 10.1073/pnas.0707130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lugari A, Breuer S, Coursindel T, Opi S, Restouin A, et al. A specific protein disorder catalyzer of HIV-1 Nef. Bioorg Med Chem. 2011;19:7401–7406. doi: 10.1016/j.bmc.2011.10.051. [DOI] [PubMed] [Google Scholar]
- 21.Dikeakos JD, Atkins KM, Thomas L, Emert-Sedlak L, Byeon IJ, et al. Small molecule inhibition of HIV-1-induced MHC-I down-regulation identifies a temporally regulated switch in Nef action. Mol Biol Cell. 2010;21:3279–3292. doi: 10.1091/mbc.E10-05-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung CH, Thomas L, Ruby CE, Atkins KM, Morris NP, et al. HIV-1 Nef assembles a Src family kinase-ZAP-70/Syk-PI3K cascade to downregulate cell-surface MHC-I. Cell Host & Microbe. 2007;1:121–133. doi: 10.1016/j.chom.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell. 2002;111:853–866. doi: 10.1016/s0092-8674(02)01162-5. [DOI] [PubMed] [Google Scholar]
- 24.Atkins KM, Thomas L, Youker RT, Harriff MJ, Pissani F, et al. HIV-1 Nef binds PACS-2 to assemble a multikinase cascade that triggers major histocompatibility complex class I (MHC-I) down-regulation: analysis using short interfering RNA and knock-out mice. J Biol Chem. 2008;283:11772–11784. doi: 10.1074/jbc.M707572200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oneyama C, Nakano H, Sharma SV. UCS15A, a novel small molecule, SH3 domain-mediated protein-protein interaction blocking drug. Oncogene. 2002;21:2037–2050. doi: 10.1038/sj.onc.1205271. [DOI] [PubMed] [Google Scholar]
- 26.Trible RP, Emert-Sedlak L, Smithgall TE. HIV-1 Nef selectively activates SRC family kinases HCK, LYN, and c-SRC through direct SH3 domain interaction. J Biol Chem. 2006;281:27029–27038. doi: 10.1074/jbc.M601128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chutiwitoonchai N, Hiyoshi M, Mwimanzi P, Ueno T, Adachi A, et al. The identification of a small molecule compound that reduces HIV-1 Nef-mediated viral infectivity enhancement. PLoS One. 2011;6:e27696. doi: 10.1371/journal.pone.0027696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guiet R, Poincloux R, Castandet J, Marois L, Labrousse A, et al. Hematopoietic cell kinase (Hck) isoforms and phagocyte duties - from signaling and actin reorganization to migration and phagocytosis. Eur J Cell Biol. 2008;87:527–542. doi: 10.1016/j.ejcb.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Saksela K, Cheng G, Baltimore D. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 1995;14:484–491. doi: 10.1002/j.1460-2075.1995.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engen JR, Wales TE, Hochrein JM, Meyn MA, III, Banu OS, et al. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008;65:3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee C-H, et al. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 32.Briggs SD, Sharkey M, Stevenson M, Smithgall TE. SH3-mediated Hck tyrosine kinase activation and fibroblast transformation by the Nef protein of HIV-1. J Biol Chem. 1997;272:17899–17902. doi: 10.1074/jbc.272.29.17899. [DOI] [PubMed] [Google Scholar]
- 33.Emert-Sedlak L, Kodama T, Lerner EC, Dai W, Foster C, et al. Chemical library screens targeting an HIV-1 accessory factor/host cell kinase complex identify novel antiretroviral compounds. ACS Chem Biol. 2009;4:939–947. doi: 10.1021/cb900195c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pene-Dumitrescu T, Shu ST, Wales TE, Alvarado JJ, Shi H, et al. HIV-1 Nef interaction influences the ATP-binding site of the Src-family kinase, Hck. BMC Chem Biol. 2012;12:1. doi: 10.1186/1472-6769-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narute PS, Smithgall TE. Nef alleles from all major HIV-1 clades activate Src-family kinases and enhance HIV-1 replication in an inhibitor-sensitive manner. PLoS One. 2012;7:e32561. doi: 10.1371/journal.pone.0032561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emert-Sedlak LA, Narute P, Shu ST, Poe JA, Shi H, et al. Effector Kinase Coupling Enables High-Throughput Screens for Direct HIV-1 Nef Antagonists with Antiretroviral Activity. Chem Biol. 2013;20:82–91. doi: 10.1016/j.chembiol.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walk SF, Alexander M, Maier B, Hammarskjold ML, Rekosh DM, et al. Design and use of an inducibly activated human immunodeficiency virus type 1 Nef to study immune modulation. J Virol. 2001;75:834–843. doi: 10.1128/JVI.75.2.834-843.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye H, Choi HJ, Poe J, Smithgall TE. Oligomerization is required for HIV-1 Nef-induced activation of the Src family protein-tyrosine kinase, Hck. Biochemistry. 2004;43:15775–15784. doi: 10.1021/bi048712f. [DOI] [PubMed] [Google Scholar]
- 39.Liu LX, Heveker N, Fackler OT, Arold S, Le Gall S, et al. Mutation of a conserved residue (D123) required for oligomerization of human immunodeficiency virus type 1 Nef protein abolishes interaction with human thioesterase and results in impairment of Nef biological functions. J Virol. 2000;74:5310–5319. doi: 10.1128/jvi.74.11.5310-5319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poe JA, Smithgall TE. HIV-1 Nef dimerization is required for Nef-mediated receptor downregulation and viral replication. J Mol Biol. 2009;394:329–342. doi: 10.1016/j.jmb.2009.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jia X, Singh R, Homann S, Yang H, Guatelli J, et al. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol. 2012;19:701–706. doi: 10.1038/nsmb.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dikeakos JD, Thomas L, Kwon G, Elferich J, Shinde U, et al. An interdomain binding site on HIV-1 Nef interacts with PACS-1 and PACS-2 on endosomes to down-regulate MHC-I. Mol Biol Cell. 2012;23:2184–2197. doi: 10.1091/mbc.E11-11-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]