Abstract
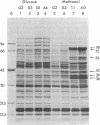
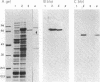
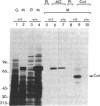
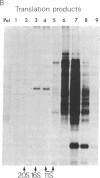
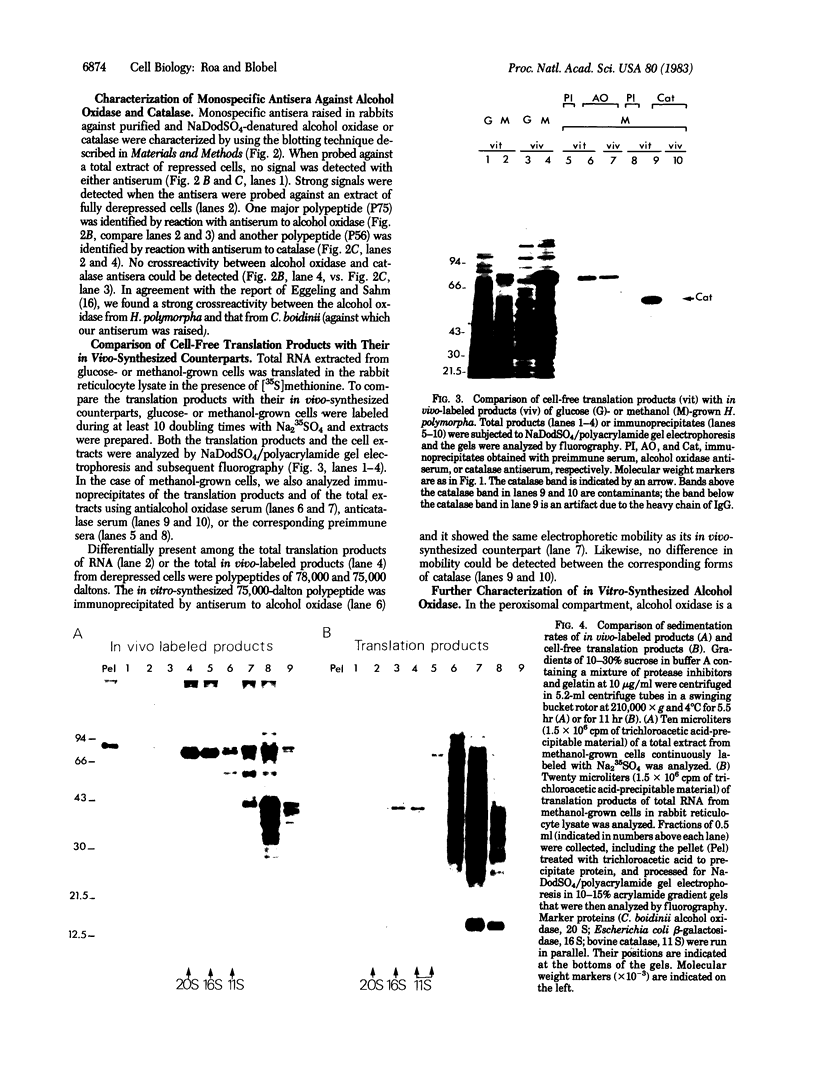
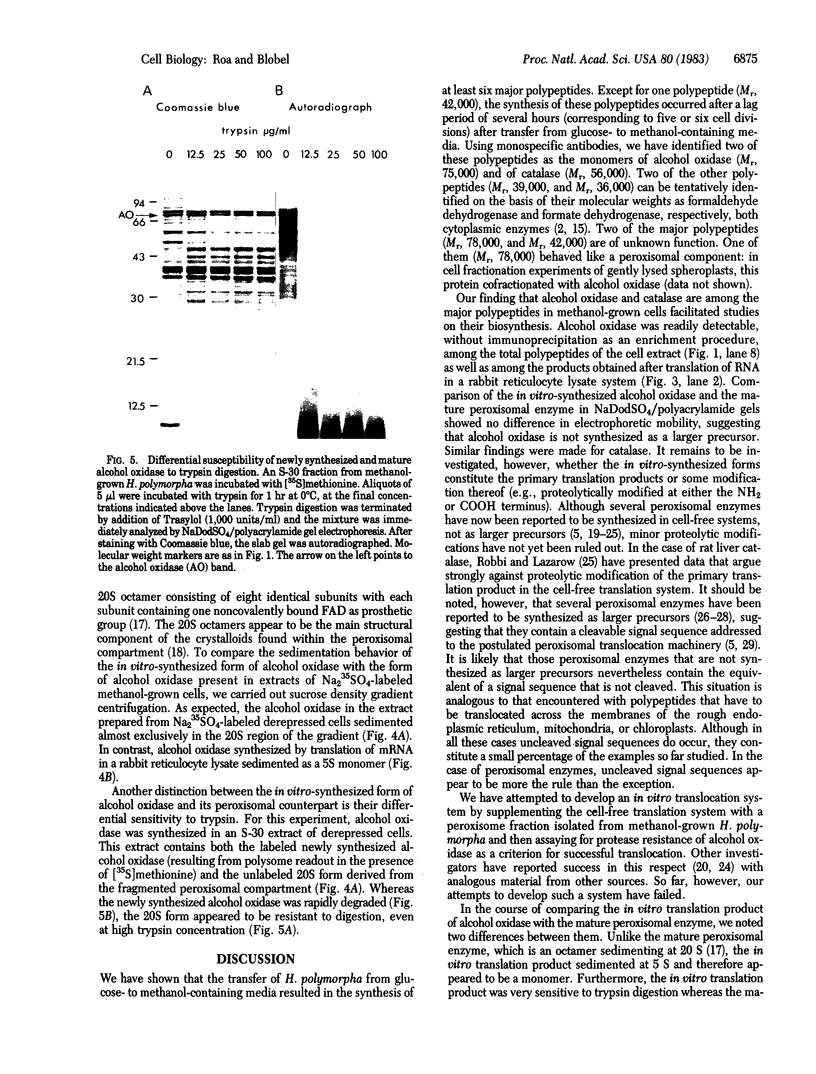
The dramatic expansion of the peroxisomal compartment known to occur in the methanol-utilizing yeast Hansenula polymorpha on transfer from glucose- to methanol-containing media was shown to be accompanied by the synthesis of at least six major polypeptides that dominate the polypeptide pattern of total cell extracts analyzed by NaDodSO4/polyacrylamide gel electrophoresis. Two of these polypeptides have been identified by immunochemical methods as the monomers of the peroxisomal enzymes alcohol oxidase and catalase. We have studied the biosynthesis of these two peroxisomal enzymes, both by in vitro translation and by in vivo labeling experiments. By the criterion of mobility in NaDodSO4/polyacrylamide gel electrophoresis, the in vitro- and in vivo-synthesized monomers were indistinguishable from each other, both in the case of alcohol oxidase and in that of catalase. Thus, neither of these peroxisomal enzymes appear to be synthesized as larger precursors. However, further analysis of in vitro-synthesized versus mature peroxisomal alcohol oxidase showed that the in vitro-synthesized form sedimented as a 5S monomer and not, like the mature peroxisomal enzyme, as a 20S octamer. Moreover, the in vitro-synthesized form was highly susceptible to trypsin digestion whereas the mature 20S octamer appeared to be resistant.
Keywords: distinct polypeptides in methanol-grown cells, alcohol oxidase, catalase, mRNA translation, differences between translation products and mature enzymes
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Djavadi-Ohaniance L., Rudin Y., Schatz G. Identification of enzymically inactive apocytochrome c peroxidase in anaerobically grown Saccharomyces cerevisiae. J Biol Chem. 1978 Jun 25;253(12):4402–4407. [PubMed] [Google Scholar]
- Eggeling L., Sahm H. Regulation of alcohol oxidase synthesis in Hansenula polymorpha: oversynthesis during growth on mixed substrates and induction by methanol. Arch Microbiol. 1980 Sep;127(2):119–124. doi: 10.1007/BF00428015. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta S., Hashimoto T., Miura S., Mori M., Tatibana M. Cell-free synthesis of the enzymes of peroxisomal beta-oxidation. Biochem Biophys Res Commun. 1982 Mar 30;105(2):639–646. doi: 10.1016/0006-291x(82)91482-6. [DOI] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer R., Fessl F., Hamilton B., Ruis H. Preparation of a mRNA-dependent cell-free translation system from whole cells of Saccharomyces cerevisiae. Eur J Biochem. 1982 Feb;122(1):199–203. doi: 10.1111/j.1432-1033.1982.tb05867.x. [DOI] [PubMed] [Google Scholar]
- Kato N., Omori Y., Tani Y., Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Catalytic properties and subunit structures. Eur J Biochem. 1976 May 1;64(2):341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang B., Burger G., Doxiadis I., Thomas D. Y., Bandlow W., Kaudewitz F. A simple method for the large-scale preparation of mitochondria from microorganisms. Anal Biochem. 1977 Jan;77(1):110–121. doi: 10.1016/0003-2697(77)90295-0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., de Duve C. The synthesis and turnover of rat liver peroxisomes. V. Intracellular pathway of catalase synthesis. J Cell Biol. 1973 Nov;59(2 Pt 1):507–524. doi: 10.1083/jcb.59.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N., Schatz G. Energy-dependent processing of cytoplasmically made precursors to mitochondrial proteins. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4365–4369. doi: 10.1073/pnas.76.9.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Peptide mapping of peroxisomal catalase and its precursor. Comparison to the primary wheat germ translation product. J Biol Chem. 1982 Jan 25;257(2):964–970. [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Synthesis of catalase in two cell-free protein-synthesizing systems and in rat liver. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4344–4348. doi: 10.1073/pnas.75.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L. M., Lord J. M. Synthesis and posttranslational segregation of glyoxysomal isocitrate lyase from castor bean endosperm. Eur J Biochem. 1981 Sep;119(1):43–49. doi: 10.1111/j.1432-1033.1981.tb05574.x. [DOI] [PubMed] [Google Scholar]
- Schüte H., Flossdorf J., Sahm H., Kula M. R. Purification and properties of formaldehyde dehydrogenase and formate dehydrogenase from Candida boidinii. Eur J Biochem. 1976 Feb 2;62(1):151–160. doi: 10.1111/j.1432-1033.1976.tb10108.x. [DOI] [PubMed] [Google Scholar]
- Veenhuis M., Harder W., van Dijken J. P., Mayer F. Substructure of crystalline peroxisomes in methanol-grown Hansenula polymorpha: evidence for an in vivo crystal of alcohol oxidase. Mol Cell Biol. 1981 Oct;1(10):949–957. doi: 10.1128/mcb.1.10.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk R. A., Hock B. Cell-free synthesis of glyoxysomal malate dehydrogenase. Biochem Biophys Res Commun. 1978 Mar 30;81(2):636–643. doi: 10.1016/0006-291x(78)91583-8. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tanaka A., Horikawa S., Numa S., Fukui S. Cell-free translation and regulation of Candida tropicalis catalase messenger RNA. Eur J Biochem. 1982 Dec 15;129(2):251–255. doi: 10.1111/j.1432-1033.1982.tb07046.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Neupert W. Biogenesis of glyoxysomes. Synthesis and intracellular transfer of isocitrate lyase. Eur J Biochem. 1980 Nov;112(2):225–233. doi: 10.1111/j.1432-1033.1980.tb07198.x. [DOI] [PubMed] [Google Scholar]
- Zimniak P., Hartter E., Woloszczuk W., Ruis H. Catalase biosynthesis in yeast: formation of catalase A and catalase T during oxygen adaptation of Saccharomyces cerevisiae. Eur J Biochem. 1976 Dec 11;71(2):393–398. doi: 10.1111/j.1432-1033.1976.tb11126.x. [DOI] [PubMed] [Google Scholar]
- van Dijkan J. P., Veenhuis M., Harder W. Peroxisomes of methanol-grown yeasts. Ann N Y Acad Sci. 1982;386:200–216. doi: 10.1111/j.1749-6632.1982.tb21417.x. [DOI] [PubMed] [Google Scholar]
- van Dijken J. P., Otto R., Harder W. Oxidation of methanol, formaldehyde and formate by catalase purified from methanol-grown Hansenula polymorpha. Arch Microbiol. 1975 Dec 31;106(3):221–226. doi: 10.1007/BF00446527. [DOI] [PubMed] [Google Scholar]