Abstract
Animal studies have highlighted the role of the ventral hippocampus-prefrontal cortex pathway in the acquisition of mature cortical function through refinement of GABAergic circuits during adolescence. Inhibitory GABAergic responses are mediated by highly specialized interneurons, which have distinct functional properties and are characterized by the expression of calcium binding proteins. Among these, we recently found that parvalbumin (PV)- and calretinin (CR)-positive interneurons in the prefrontal cortex follow opposite developmental trajectories during the periadolescent transition period. In the present study, we asked whether interneurons expressing PV and CR in the ventral hippocampus follow similar periadolescent trajectories as seen in the prefrontal cortex. By measuring the relative abundance of these interneurons in three age groups (postnatal days -PD- 25-40, 45-55, and 60-85), we found that regions within the dorso-ventral axis of the ventral hippocampus undergo distinct developmental trajectories in PV expression during the periadolescent transition. Specifically, the ventral subiculum displayed a dramatic increase in PV-positive interneurons from PD25-40 to PD45-55 with an increasing rostro-caudal gradient, whereas negligible changes were found in the dorsal and middle regions. In contrast, the number of CR-positive interneurons in the ventral hippocampus remained unchanged across the three age groups studied. Together, these results describe for the first time that GABAergic circuits in the ventral hippocampus undergo protracted development during adolescence, in particular the PV-positive cell population circumscribed to the ventral region of the ventral hippocampus.
Keywords: adolescence, interneurons, ventral hippocampus, parvalbumin, calretinin
Introduction
The neurobiological substrates underlying the periadolescent vulnerability to the onset of psychiatric disorders such as schizophrenia and drug abuse are incompletely understood (Paus et al., 2008). This is due in part to our limited knowledge of the normative development of brain structures and connections during the adolescent stage. At the behavioral level, it is well known that many cognitive abilities are refined during the transition from adolescence to adulthood (Best and Miller, 2010). These include working memory, decision-making, and inhibitory control, all of which have been attributed to the protracted maturation and functional remodeling of the prefrontal cortex during adolescence (Casey et al., 2000). However, recent studies have also highlighted the involvement of the ventral hippocampus since afferents originating from this limbic region have long been recognized to play a role in amplifying prefrontal-dependent cognitive processes (Ishikawa and Nakamura, 2003). In particular, lack of ventral, but not dorsal, hippocampal modulation increases premature responses in prefrontal-dependent goal-directed tasks indicative of reduced inhibitory control (Abela et al., 2012; Chudasama et al., 2012), as is often seen in teenagers (Geier and Luna, 2009) and in psychiatric cases of schizophrenia and drug abuse (Chambers et al., 2001; Potenza and Chambers, 2001). Thus, it is plausible that the ventral hippocampus also undergoes major structural and functional remodeling during adolescence as is seen in the prefrontal cortex.
At the cellular level, GABAergic interneurons are of great interest as a potential basis for the functional maturation of cognitive processes and inhibitory control during adolescence (O’Donnell, 2011; Uhlhaas and Singer, 2011). More importantly, disruption of GABAergic circuits in both prefrontal cortex and hippocampus has been repeatedly observed in schizophrenia (see review by (Benes and Berretta, 2001; Konradi et al., 2011; Lewis et al., 1999). These findings have been validated in animal models in which disruptions of the ventral hippocampus early in development lead to GABAergic-dependent behavioral and physiological impairments in adulthood consistent with those observed in schizophrenia (reviewed in (Grace, 2010; Lodge and Grace, 2011; Tseng et al., 2009). We recently found that markers of GABAergic interneurons change distinctively in the prefrontal cortex from juveniles to young adults (Caballero et al., 2013), providing a framework for understanding the susceptibility of prefrontal GABAergic circuits during adolescence. Here, we sought to determine whether parvalbumin (PV)- and calretinin (CR)-positive interneurons in the ventral hippocampus display similar periadolescent trajectories to those seen in the prefrontal cortex. To this end, we performed immunohistochemical staining and compared the distribution of ventral hippocampal PV- and CR-positive interneurons along the ventro-dorsal and rostro-caudal axis in juvenile (postnatal day -PD- 25-35), adolescent (PD45-55), and young adult (PD65-75) rats.
Materials and Methods
All experimental procedures were conducted according to the USPHS Guide for Care and Use of Laboratory Animals, and were approved by the Rosalind Franklin University Institutional Animal Care and Use Committee.
Experimental groups
Three postnatal age groups of male Sprague-Dawley rats (Harlan, Indianapolis, IN) were compared in the present study: PD25-35, PD45-55 and PD65-75. All animals were allowed to acclimate to the animal facility for at least 5 days before being used. They were group-housed (2–3 rats/cage) with food and water available ad libitum, and maintained at a constant temperature (21–23°C) and humidity in a 12 hour light-dark cycle.
Immunohistochemical measures of PV and CR-positive cells in the ventral hippocampus
All immunohistochemical procedures were conducted following a previously reported protocol (Caballero et al., 2013). Briefly, rats were deeply anesthetized with 8% chloral hydrate (400 mg/kg i.p.), transcardially perfused with saline followed by 4% PFA in phosphate buffered saline (0.1M PBS), and the brains removed and fixed in 4% PFA for 24 hours, and stored in 30% sucrose. Serial 50μm-thick coronal sections of the ventral hippocampus were collected using a freezing stage (PFS-30MP controller, Physitemp Instruments, Clifton, NJ) on a sliding microtome (HM430, Thermo Scientific, FL). After staining, slides were observed under a Nikon E400 microscope with a 10X objective, and digitally acquired with an ORCA-AG deep cooled digital camera (Hamamatsu, Bridgewater, NJ). For every experiment, juvenile samples were included in the processed material, and the digital camera exposure was first set such that PV-positive cells in the juvenile group were visible and within the dynamic range at the final magnification of the image. All subsequent images of the remaining age groups were taken at an identical exposure. For each animal, six sections 300 μm apart spanning the rostro-caudal axis of the ventral hippocampus (within −4.8 mm to −6.3 mm from bregma; Figure 1a) were imaged and analyzed. Each of the rostro-caudal sections was sampled along the dorso-ventral axis with three 866x660μm-images (shaded boxes in Figure 1a), which were defined as dorsal, middle, and ventral regions. To facilitate the identification of cells, images were magnified to 150%, and all PV or CR-positive cells “in focus” contained within the hippocampal boundaries in each of the shaded boxes were counted regardless of their morphology or fluorescent intensity. Cell counts were performed by an individual blind to the experimental groups using ImageJ (NIH, USA, http://rsb.info.nih.gov/ij/). Data from two adjacent rostro-caudal sections were pooled, and defined as L1 (two most rostral levels), L2 (two central levels), and L3 (two most caudal levels).
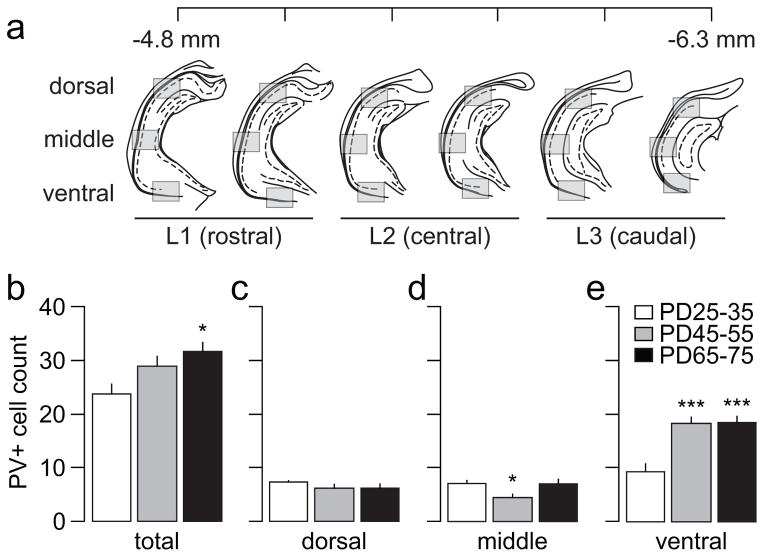
Figure 1.
(a) Diagram depicting the approximate rostro-caudal levels (at 300 μm intervals) and the dorso-ventral regions of the ventral hippocampus sampled for PV-positive cell quantification (see Materials and Methods for details). (b) Summary of the mean PV-positive cell count obtained across the 6 rostro-caudal sections sampled in juvenile (PD25-35, n=8), adolescent (PD45-55, n=8), and adult rats (PD65-75, n=8). Overall, the results indicate a global increase in the mean number of PV-positive cells in adult animals compared to juveniles (main effect of age F(2,21)=5.01, p=0.017 one-way ANOVA; *p<0.05 vs. juvenile, Tukey post-hoc). (c–e) Distribution of the mean PV-positive cell count obtained across the dorsal-ventral regions. One-way ANOVA analysis revealed a significant main effect of age in the middle (F(2,21)=4.62, p=0.022) and ventral (F(2,21)=17.43, p<0.0005) regions (*p<0.05, ***p<0.0005 vs. juvenile, Tukey post-hoc).
Statistics
All data are expressed as mean ± SEM, and the statistical significance (p<0.05) among age groups was determined by one-way or two-way ANOVA, followed by Tukey post-hoc test when applicable (StatSoft, Tulsa, OK).
Results
Periadolescent upregulation of PV-positive interneurons in the ventral hippocampus
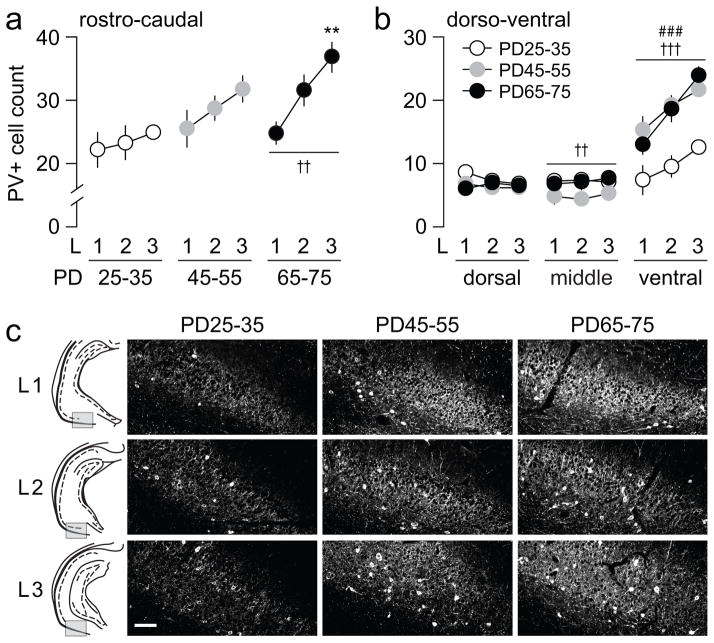
Visual inspection of the ventral hippocampus sections showed that PV-positive cell bodies were found associated almost exclusively with the stratum pyramidale and oriens, as previously described (Freund and Buzsaki, 1996). In the present study, six rostro-caudal serial sections of the ventral hippocampus (Fig 1a) were used to estimate whether the overall density of PV-positive interneurons (i.e. number of cells per area sampled at 10X; see Materials and Methods for details) in this brain region changes during the adolescent transition to adulthood. For this purpose, three age groups of rats corresponding to PD25-35, PD45-55, and PD65-75 were compared. At each rostro-caudal level, PV-positive cell density was sampled in three regions within the dorso-ventral axis: dorsal, middle, and ventral (Fig 1a). Overall, we found a significant age-dependent progressive increase in the number of PV-positive cells per section sampled (Fig 1b). Further analyses revealed that the density of PV-positive neurons within the dorsal and middle regions remained relatively unchanged across the three age groups examined (Fig 1c,d). However, the ventral region displayed a highly significant upregulation of PV-positive cells after PD45 (Fig 1e). A similar age-dependent effect was observed throughout the three rostro-caudal levels studied (Fig 2). The data revealed an increase in the rostro-caudal gradient for PV-positive interneurons that begins to emerge in the PD45-55 group, and became markedly significant after PD65 (Fig 2a). We next asked whether such an age-dependent rostro-caudal gradient occurs in all three dorso-ventral regions sampled. We found that the distribution of PV-positive cells within the dorsal and middle regions failed to follow the rostro-caudal gradient observed. In contrast, PV-positive interneurons in the ventral region increased steadily from rostral to caudal levels in all three age groups examined (Fig 2b,c). It is notable that by PD45, the density of PV-positive cells in the ventral region became undistinguishable from that of PD65-75 in all rostro-caudal levels (Fig 2b,c).
Figure 2.
(a) Analysis of PV-positive cells across the 3 rostro-caudal levels (L1, L2, and L3) within each age group. A significant main effect of level (F(2,21)=7.52, ††p<0.005) was observed only in the adult (PD65-75) group. Compared to the rostral level (L1), a significant elevation of mean PV-positive cells per section was observed in the most caudal (L3) level (**p<0.005 vs. L1, Tukey post-hoc test). (b) Further analyses of the distribution of PV-positive cells within the 3 dorso-ventral regions revealed an effect of age in both middle (F(2,63)=6.43, ††p<0.005) and ventral regions (F(2,63)=27.85, †††p<0.0005) without a significant age x level interaction (two-way ANOVA). A main effect of level was also observed in the ventral region (F(2,63)=18.16, ###p<0.0005). (c) Examples of PV immunohistochemical staining from the ventral region across the 3 rostro-caudal levels of the ventral hippocampus illustrating the distribution of PV-positive cells observed in the 3 age groups studied. Scale bar = 100 μm
Distribution of ventral hippocampal CR-positive interneurons during periadolescence
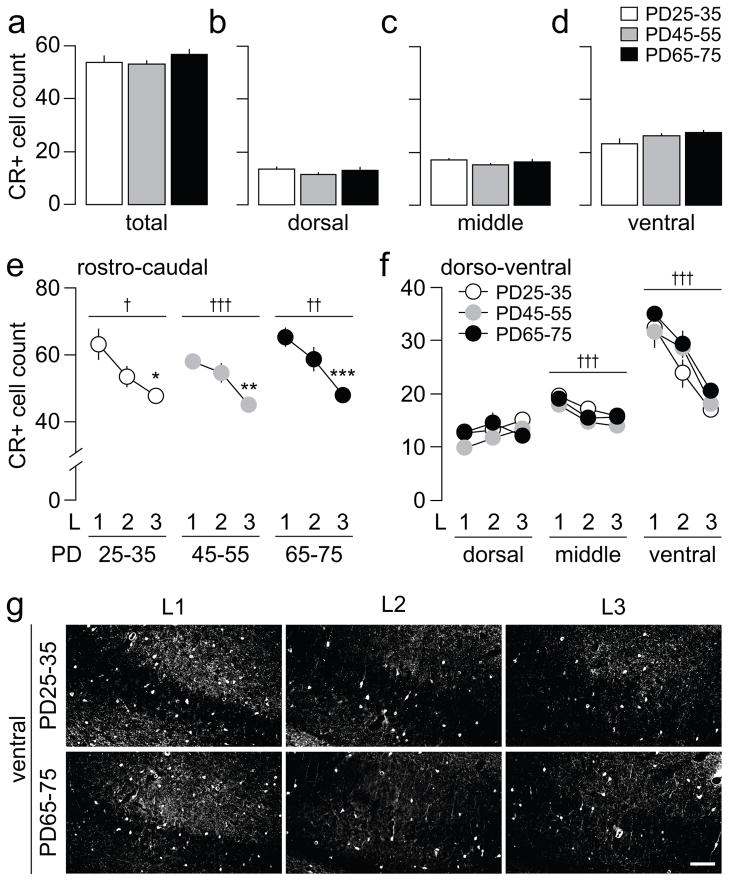
We next examined the distribution of CR-positive cells across the ventral hippocampal slices, as described above. Unlike PV-positive interneurons, the overall density of CR-positive cells remained unchanged across the three age groups studied (Fig 3a). Further analyses did not reveal any age difference in the distribution of CR-positive interneurons within the dorso-ventral regions (Fig 3b–d). Likewise, an age-independent pattern of CR-positive cell distribution was observed across the three rostro-caudal levels, such that the density of CR-positive interneurons became progressively smaller towards the caudal level (Fig 3e). Similar to PV-positive interneurons, the rostro-caudal gradient observed in CR-positive cells is mainly attributable to changes in CR expression in the ventral region. While the dorsal and middle regions showed non-significant or small rostro-caudal changes, the ventral region experienced the greatest decline in CR-positive interneurons in the rostro-caudal direction (Fig 3f). However, none of these changes in CR-positive cell distribution were age-dependent.
Figure 3.
(a) Summary of the distribution of CR-positive cells across the 6 rostro-caudal sections of the ventral hippocampus (Fig 1a) from juvenile (PD25-35, n=8), adolescent (PD45-55, n=8), and adult rats (PD65-75, n=8). The results indicate no significant differences in any of the three age groups examined. (b–d) Statistical analyses revealed no significant age differences in the distribution of CR-positive cells within the 3 dorsal-ventral regions studied. (e) One-way ANOVA analysis of the distribution of CR-positive cells across the 3 rostro-caudal levels (L1, L2, and L3) showed a significant effect of levels in all 3 age groups (juveniles: F(2,21)=6.02, †p<0.01; adolescents: F(2,21)=11.26, †††p<0.0005; adults: F(2,21)=10.89, ††p<0.005). Note the progressive reduction in the mean number of CR-positive neurons from the rostral level L1 towards the caudal level L3 (*p<0.01, **p<0.005, ***p<0.0005 vs. L1, Tukey post-hoc test). (f) Further analysis of the distribution of CR-positive cells within the 3 dorso-ventral regions indicates a rostro-caudal level effect only in the middle (F(2,63)=12.11, †††p<0.0005) and ventral regions (F(2,63)=40.44, †††p<0.0005). No significant main effect of age or age x level interaction was observed at any of the regions examined. (g) Examples of CR immunohistochemical staining from the ventral region across the 3 rostro-caudal levels (L1, L2, L3) of the ventral hippocampus illustrating the distribution of CR-positive cells observed in juveniles and adults. Scale bar = 100 μm
Discussion
In the present study, we determined the developmental trajectory of PV- and CR-positive interneurons along the dorso-ventral and rostro-caudal axes of the ventral hippocampus in juvenile, adolescent, and adult rats. Similar to what we have observed in the medial prefrontal cortex (Caballero et al., 2013), our results indicate that PV-positive interneurons in the ventral hippocampus undergo upregulation during the periadolescent transition to adulthood in a region-specific manner. An age-dependent increase in PV-positive interneurons was observed only in the ventral region after PD45, and across all the rostro-caudal levels of the ventral hippocampus. Interestingly, the magnitude of such periadolescent PV upregulation is more pronounced towards caudal levels of the ventral region. The increase in caudal levels accounts for the characteristic rostro-caudal gradient increase in PV-positive cells observed in the PD65-75 ventral hippocampus. More importantly, such a pattern begins to emerge during adolescence (PD45-55), but is absent in juveniles. In contrast, no apparent age-dependent changes were observed in CR-positive cells. Together, these results provide for the first time evidence for the protracted development of the ventral hippocampal GABAergic system during adolescence, and imply a window of vulnerability for PV-positive interneurons that appears to be circumscribed to the ventral region of the ventral hippocampus.
In adulthood, PV- and CR-positive interneurons in the ventral hippocampus follow an opposite pattern of distribution within the rostro-caudal axis. While PV expression increases, CR expression decreases towards the caudal levels of the ventral hippocampus, a gradient attributable to changes in cell density mainly within the ventral regions across the rostro-caudal axis. A similar opposing PV-to-CR relationship has been observed in the medial prefrontal cortex (Caballero et al., 2013). Notably, the decreasing rostro-caudal gradient in CR-positive cells is already present in the ventral hippocampus of juvenile animals. In contrast, any age-dependent changes in the distribution of PV-positive interneurons begins to emerge after PD45 and becomes significant in the PD65-75 age group, a time during which the PV-positive population increases particularly in the ventral region. The fact that CR remained unchanged during the time frame studied here, suggests that development of CR-positive cells in the ventral hippocampus occurs relatively earlier than that of PV-positive interneurons.
Similar to the prefrontal cortex, a dysregulation of hippocampal function by reduced local GABAergic interneurons has been implicated in the pathophysiology of schizophrenia (Benes and Berretta, 2001; Heckers and Konradi, 2002; Small et al., 2011). This fact is in accordance with a number of postmortem studies describing a reduction in the number of PV-positive interneurons in the hippocampus (Konradi et al., 2011; Torrey et al., 2005; Zhang and Reynolds, 2002), which would be expected to result in increased hippocampal activity and metabolism. Accordingly, several studies have shown increased metabolic activity in the hippocampus in first episode patients with schizophrenia (Molina et al., 2005), which in some cases has been correlated with the degree of positive and cognitive symptoms of the disease (Heckers et al., 1998; Liddle et al., 1992; Molina et al., 2005). Therefore, it is possible that the hippocampal dysfunction observed in schizophrenia results from a neurodevelopmental defect that disrupts the normative periadolescent increase of local PV-positive interneurons reported here.
In summary, the protracted periadolescent increase of PV-positive interneurons in the ventral hippocampus might explain why this structure is more susceptible to genetic and environmental insults whose onset may only be manifested until late adolescence/early adulthood. Such insults could converge in the inability to control oxidative metabolism during the adolescent transition to adulthood (Do et al., 2009; Steullet et al., 2010) when the energetic demand increases, especially for hippocampal PV-positive cells (Gulyas et al., 2006).
Acknowledgments
Supported by Rosalind Franklin University (KYT), the Brain Research Foundation (KYT), and the National Institutes of Health Grant R01-MH086507 (KYT). The authors would like to thank Daryn Cass and Daniel Thomases for their useful editorial comments.
Footnotes
Conflict of Interest
The authors have no biomedical financial interests or potential conflicts of interest to disclose.
References
- Abela AR, Dougherty SD, Fagen ED, Hill CJ, Chudasama Y. Inhibitory Control Deficits in Rats with Ventral Hippocampal Lesions. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs121. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81(6):1641–60. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Flores-Barrera E, Cass DK, Tseng KY. Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence. Brain Struct Funct. 2013 Feb 12; doi: 10.1007/s00429-013-0508-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54(1–3):241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Doobay VM, Liu Y. Hippocampal-prefrontal cortical circuit mediates inhibitory response control in the rat. J Neurosci. 2012;32(32):10915–24. doi: 10.1523/JNEUROSCI.1463-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19(2):220–30. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacol Biochem Behav. 2009;93(3):212–21. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res. 2010;18(3–4):367–76. doi: 10.1007/s12640-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Buzsaki G, Freund TF, Hirase H. Populations of hippocampal inhibitory neurons express different levels of cytochrome c. Eur J Neurosci. 2006;23(10):2581–94. doi: 10.1111/j.1460-9568.2006.04814.x. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal neurons in schizophrenia. J Neural Transm. 2002;109(5–6):891–905. doi: 10.1007/s007020200073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat Neurosci. 1998;1(4):318–23. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci. 2003;23(31):9987–95. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1–3):165–73. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46(5):616–26. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–86. doi: 10.1192/bjp.160.2.179. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32(9):507–13. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina V, Sanz J, Sarramea F, Benito C, Palomo T. Prefrontal atrophy in first episodes of schizophrenia associated with limbic metabolic hyperactivity. J Psychiatr Res. 2005;39(2):117–27. doi: 10.1016/j.jpsychires.2004.06.008. [DOI] [PubMed] [Google Scholar]
- O’Donnell P. Adolescent onset of cortical disinhibition in schizophrenia: insights from animal models. Schizophr Bull. 2011;37(3):484–92. doi: 10.1093/schbul/sbr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9(12):947–57. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Chambers RA. Schizophrenia and pathological gambling. Am J Psychiatry. 2001;158(3):497–8. doi: 10.1176/appi.ajp.158.3.497-a. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30(7):2547–58. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57(3):252–60. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204(2):295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37(3):514–23. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55(1–2):1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]