Abstract
The mammalian brain depends upon glucose as its main source of energy, and tight regulation of glucose metabolism is critical for brain physiology. Consistent with its critical role for physiological brain function, disruption of normal glucose metabolism as well as its interdependence with cell death pathways forms the pathophysiological basis for many brain disorders. Here, we review recent advances in understanding how glucose metabolism sustains basic brain physiology. We aim at synthesizing these findings to form a comprehensive picture of the cooperation required between different systems and cell types, and the specific breakdowns in this cooperation which lead to disease.
Keywords: glucose metabolism, metabolic coupling, apoptosis, brain-body axis, metabolic brain disease
“Nobody realizes that some people expend tremendous energy merely to be normal.“Albert Camus, Notebooks 1942-1951
Glucose metabolism: fueling the brain
The mammalian brain depends on glucose as its main source of energy. In the adult brain, neurons have the highest energy demand [1], requiring continuous delivery of glucose from blood. In humans, the brain accounts for ~2% of the body weight, but it consumes ~20% of glucose-derived energy making it the main consumer of glucose (~5.6 mg glucose per 100 g human brain tissue per minute [2]). Glucose metabolism provides the fuel for physiological brain function through the generation of ATP, the foundation for neuronal and non-neuronal cellular maintenance, as well as the generation of neurotransmitters. Therefore, tight regulation of glucose metabolism is critical for brain physiology and disturbed glucose metabolism in the brain underlies several diseases affecting both the brain itself as well as the entire organism.
Here, we provide a comprehensive overview of the functional implications and recent advances in understanding the fundamental role of glucose metabolism in physiological and pathological brain function. Although brain energy metabolism has been investigated for decades, certain aspects remain controversial, in particular in the field of energy substrate consumption and utilization. It is beyond the scope of this review to resolve these controversies; rather it is our aim to highlight conflicting concepts and results to stimulate discussion in key areas. To this end, we review the bioenergetics of neurotransmission, the cellular composition of a metabolic network, the regulation of cerebral blood flow (CBF), how peripheral glucose metabolism and energy homeostasis are sensed and controlled by the CNS, and the tight regulation of cellular survival through glucose-metabolizing enzymes.
Glucose is required to provide the precursors for neurotransmitter synthesis and the ATP to fuel their actions as well as the brain’s energy demands not related to signaling. Cellular compartmentation of glucose transport and metabolism is intimately related to local regulation of blood flow, and glucose-sensing neurons govern the brain-body nutrient axis. Glucose metabolism is connected to cell death pathways by glucose-metabolizing enzymes. Thus, disruption of pathways of glucose delivery and metabolism leads to debilitating brain diseases. We highlight the multifaceted role and complex regulation of glucose metabolism in the CNS as well as the physiological and pathophysiological consequences of balanced and disturbed glucose metabolism (Figure 1).
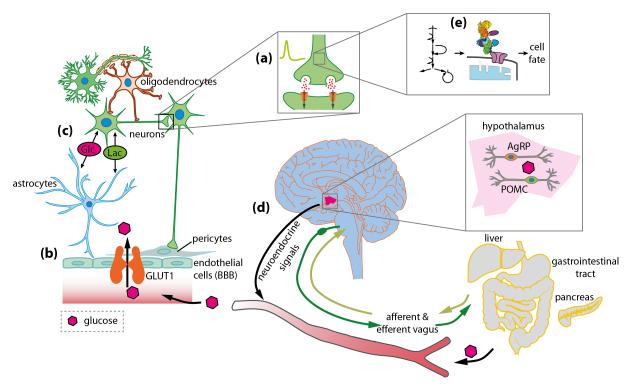
Figure 1. The role of glucose for brain function.
Glucose (Glc) is the main source of energy for the mammalian brain, (a) Specialized centers in the brain, including proopiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons in the hypothalamus, sense central and peripheral glucose levels and regulate glucose metabolism through the vagal nerve as well as neuroendocrine signals.. (b) Glucose supply to the brain is regulated by neurovascular coupling and may be modulated by metabolism-dependent and -independent mechanisms. Glucose enters the brain from the blood by crossing the BBB through glucose transporter 1 (GLUT1), and (c) glucose and other metabolites (e.g. lactate, Lac) are rapidly distributed through a highly coupled metabolic network of brain cells. (d) Glucose provides the energy for neurotransmission, and (e) several glucose-metabolizing enzymes control cellular survival. Disturbed glucose metabolism on any of these levels can be the foundation for the development of a large variety of disorders of the brain (see section on “Disease mechanisms”).
Glucose metabolism: the bioenergetic basis for neurotransmission
The largest proportion of energy in the brain is consumed for neuronal computation and information processing [3], e.g. the generation of action potentials and postsynaptic potentials generated after synaptic events (Figure 1d), and the maintenance of ion gradients and neuronal resting potential [1, 4]. Additionally, glucose metabolism provides the energy and precursors for the biosynthesis of neurotransmitters (for a comprehensive overview see [5]). Importantly, astrocytic glycogen seems to be directly relevant for learning [6]. Furthermore, the glycolytic end product lactate appears to play a role in long-term memory formation [7], but the exact mechanism has not yet been established. Lactate injections [7] alter the intracellular redox state and pH due to co-transport of H+ with lactate, and lactate receptors may also play a role in linking brain energy metabolism and neurotransmission [8, 9]. However, oxidative metabolism both in neurons and astrocytes appears to contribute to sustained learning effects after training, and glycogen can supply carbon for synthesis of glutamate during learning [6].
It has been suggested that action potentials have been rendered highly efficient through evolution [10], and thus most of the energy consumed in the brain is used on synaptic activity [3, 10, 11]. The human cortex alone requires approximately 3×1023 ATP/s/m3 [1], and the energy expenditure to release one synaptic vesicle is roughly calculated to be 1.64×105 molecules ATP [3]. Consequently, a model of energy use in the brain suggests that a considerably larger amount of energy is spent in the grey matter compared with the white matter [12]. In essence, the brain increases its utilization of glucose upon activation [13].
Glucose uptake in the brain – How are neurons and astrocytes fed?
Dependence of the brain on glucose as its obligatory fuel derives mainly from the blood-brain barrier (BBB; Glossary), and its selective permeability for glucose in the adult brain. Glucose cannot be replaced as an energy source but it can be supplemented, as during strenuous physical activity when blood lactate levels are elevated [14] or during prolonged starvation [15] when blood levels of ketone bodies are elevated and BBB monocarboxylic acid transporter (MCT) levels are upregulated. Because entry of neuroactive compounds (e.g., glutamate, aspartate, glycine, D-serine) into brain is highly restricted by the BBB, these compounds must be synthesized from glucose within the brain. The BBB and its transport properties sharply contrast with muscle and liver that do not have tight junctions between their vascular endothelial cells and have different transporter levels for various compounds, enabling these organs to metabolize glucose, monocarboxylic acids, fatty acids, amino acids, and ketone bodies.
Glossary.
Autophagy
an intracellular “recycling” pathway that can be activated under conditions of metabolic stress to inhibit cell death. It involves the lysosomal degradation of cytoplasmic proteins or entire organelles for catabolic regeneration of nutrient pools [61].
Blood-brain barrier
the permeability barrier arising from tight junctions between brain endothelial cells, restricting diffusion from blood to brain. Entry into the brain is limited to molecules that can diffuse across membranes (e.g., oxygen and other gases, lipid-permeable compounds) or have transporter molecules (e.g., glucose transporters). Neuroactive compounds (e.g., glutamate, adrenalin) in blood are highly restricted from entry into brain.
Functional activation
a response by the brain to a specific stimulus (e.g., sensory stimulation) that increases cellular activity and metabolism above the “resting” / baseline value prior to onset of the stimulus. Brain activation has the same meaning but is a more general term that includes increased activity during abnormal or disease states.
Glutamate-glutamine cycle
the release of neurotransmitter glutamate from excitatory neurons, its sodium-dependent uptake by astrocytes, its conversion to glutamine by glutamine synthetase in astrocytes, release of glutamine and uptake into neurons followed by the conversion to glutamate by glutaminase and its repackaging into synaptic vesicles.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
a glycolytic enzyme that reduces NAD+ to NADH and converts D-glyceraldehyde-3-phosphate to 1,3-bisphospho-D-glycerate, an intermediary metabolite in the generation of pyruvate.
Glycolysis
a cytoplasmic pathway for metabolism of one molecule of glucose to produce two molecules of pyruvate, with phosphorylation of 2 ADP to form 2 ATP and reduction of 2 NAD+ to 2 NADH. Cytoplasmic oxidation of NADH can be achieved by conversion of pyruvate to lactate by the lactate dehydrogenase (LDH) reaction or via the malate-aspartate shuttle (MAS) (Figure 2a). The MAS is required to generate pyruvate for oxidation in the TCA cycle, whereas LDH removes this substrate from the cell. Net production of lactate in the presence of adequate levels and delivery of oxygen is sometimes termed “aerobic” glycolysis, contrasting the massive production of lactate under hypoxia or anoxia (“anaerobic” glycolysis).
Hexokinase (HK)
the enzyme catalyzing the first irreversible step in glucose metabolism, the irreversible conversion of glucose to glucose-6-phosphate (Glc-6-P) in an ATP-dependent reaction. The brain has different HK isoforms that have specific functions. HKI is the major isoform in brain for the glycolytic pathway; it has a broad substrate specificity, and it is feedback-inhibited by Glc-6-P. HKII is a minor, hypoxia-regulated isoform in the brain that controls neuronal survival depending on the metabolic state. HKIV (glucokinase, GK) is a minor isoform of hexokinase in the brain that has an important role in glucose-sensing neurons; it is specific for glucose and is not inhibited by Glc-6-P.
Ketogenic diet
a diet that has a high fat and low carbohydrate content so that plasma levels of ketone bodies (acetoacetate and β-hydroxybutyrate) rise and serve as an alternative oxidative fuel.
Metabolic coupling
a synergistic interaction between different cells or cell types in which compounds produced in one cell are used by another cell.
Neurovascular unit
groups of neurons, astrocytes, endothelial cells, vascular smooth muscle cells and pericytes that are involved in local signaling activities, metabolic interactions, and regulation of blood flow.
Tricarboxylic acid (TCA) cycle
a mitochondrial pathway for oxidation of pyruvate to produce 3CO2 and generate FADH2 and NADH that are oxidized via the electron transport chain with conversion of oxygen to water and formation of about 32 ATP per glucose molecule. This ATP yield is less than the theoretical maximum due to proton leakage across the mitochondrial membrane.
The large blood-to-brain concentration gradient drives the facilitative transport of glucose across the endothelial membranes via GLUT1 glucose transporters into extracellular fluid (Figures 1b, 2). The steady-state brain tissue glucose concentration is about 20% of that in arterial plasma. GLUT1 further mediates glucose uptake from extracellular fluid into astrocytes, oligodendroglia, and microglia, whereas GLUT3, which has a much higher transport rate than GLUT1, facilitates neuronal glucose uptake (Figure 1c, 2b) [16]. Glucose transport capacity exceeds demand over a wide range, and the higher transport rate of GLUT3 ensures that neurons have sufficient glucose supplies under varying glucose levels and different activity states [5]. Although astrocytes are generally believed to be involved in the uptake and distribution of brain metabolites [3, 17, 18], modeling predicts that most glucose diffuses from endothelial cells through the gaps between the surrounding astrocytic endfeet, and throughout the extracellular fluid to more distant brain cells, facilitating rapid GLUT3-mediated uptake into neurons [16]. Some glucose may, however, also be taken up into astrocytic endfeet, followed by its diffusion down its concentration gradients to other gap junction-coupled astrocytes, with release to extracellular fluid at sites more distant from the capillary [3, 17, 18].
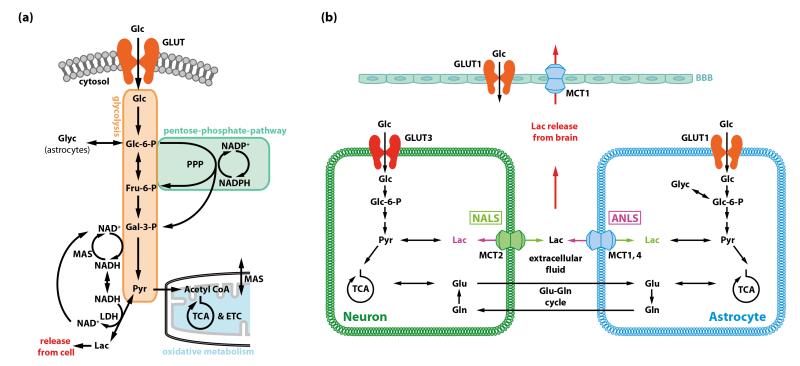
Figure 2. Generation of energy in brain and three models for the fate of lactate derived from glucose metabolism in the brain.
(a) Major pathways of glucose metabolism. Hexokinase uses ATP to phosphorylate glucose to glucose-6-phosphate (Glc-6-P) in the first irreversible step of the glycolytic pathway. Glc-6-P regulates hexokinase activity by feedback inhibition [19], and it is a ‘branch-point’ metabolite that has alternative metabolic fates. Glc-6-P can continue down the glycolytic pathway to generate pyruvate that can then be used in mitochondria by oxidative metabolism via the tricarboxylic acid (TCA) cycle. It can also enter the pentose phosphate shunt pathway (PPP) to generate NADPH for management of oxidative stress and precursors for nucleic acid biosynthesis, and, in astrocytes, it is a precursor for glycogen. Most of the glucose carbon derived from the PPP re-enters the glycolytic pathway downstream of Glc-6-P. The glycolytic pathway produces a net of 2 ATP per molecule of glucose and oxidation of pyruvate via acetyl coenzyme A (acetyl CoA) in the TCA cycle produces about 30 ATP for a total of about 32 ATP. Formation of pyruvate from glucose requires regeneration of NAD+ from NADH produced by the glyceraldehyde-3-phosphate dehydrogenase reaction by the malateaspartate shuttle (MAS). NADH cannot cross the mitochondrial membrane, and the MAS transfers cytoplasmic NADH to the mitochondria where it is oxidized via the electron transport chain (ETC). When glycolytic flux exceeds that of the MAS or the TCA cycle rate, or during hypoxic or anoxic conditions, NAD+ is regenerated by the lactate dehydrogenase (LDH) reaction that converts pyruvate to lactate. Because intracellular accumulation of lactate would cause reversal of the LDH reaction, lactate must be released from the cell by monocarboxylic acid transporters (MCT). Exit of lactate eliminates pyruvate as an oxidizable substrate for that cell and limits the ATP yield per glucose to two. (b) Three models for the fate of lactate generated in brain from blood-borne glucose or astrocytic glycogen. The astrocyte-to-neuron lactate shuttle (ANLS) was proposed on the basis of glutamate-evoked increases in glucose utilization and lactate release by cultured astrocytes (reviewed in [29]). In brief, the model states that Na+-dependent uptake of neurotransmitter glutamate from the synaptic cleft by astrocytes generates a demand for 2 ATP in astrocytes, one to extrude Na+ and one to convert glutamate into glutamine in the glutamate-glutamine cycle (Glossary). The model states that this ATP is generated by the glycolytic pathway and is associated with release of lactate from astrocytes and its uptake by nearby neurons where it is oxidized. Thereby astrocyte-neuron metabolic coupling is linked with the glutamate-glutamine cycle and excitatory neurotransmission. Thus, during brain activation glycolytic upregulation is stated to occur in astrocytes, with astrocyte-derived lactate providing the major fuel for neurons. The neuron-toastrocyte lactate shuttle (NALS) is based on kinetics of glucose uptake into brain cells in response to increased metabolic demand and different model assumptions compared with the ANLS [27]. Here, glucose is predicted to be predominantly taken up into neurons due to their high energy demand and the higher transport rate of the neuronal glucose transporter, GLUT3, compared with the astrocytic glucose transporter, GLUT1 [16]. Lactate is posited to be generated by neurons and taken up by astrocytes. The lactate release model [5] is based on the observed mismatch between total glucose utilization and oxidative metabolism and measured lactate release from brain during brain activation in vivo. If lactate were produced and locally oxidized, total and oxidative metabolism would be similar in magnitude. However, the rise in oxidative metabolism varies with experimental condition and pathways stimulated, it is much less than that of total glucose utilization [5]. Astrocytes have a much faster and greater capacity for lactate uptake from extracellular fluid, and for lactate dispersal among gap junction-coupled astrocytes compared with neuronal lactate uptake and shuttling of lactate to neurons [17]. Astrocytic endfeet surround the vasculature, and can discharge lactate to perivascular fluid for efflux from brain.
Local rates of glucose utilization are driven by functional activities (Figure 1d; Glossary) that consume ATP and generate ADP, which is an obligatory co-substrate for energy-producing reactions. Intracellular glucose is phosphorylated by hexokinase I (HKI, Glossary) to form Glc-6-P, thereby trapping the molecule in the cell, and thus creating a ‘sink’ that draws more glucose into the cell (Figure 2a). The intracellular glucose pool size is maintained as the net balance between rates of its influx, efflux, and metabolism. The Km (half-saturation constant) of HKI for glucose is very low [19], and HKI can therefore operate at maximal velocity as long as the intracellular glucose exceeds about 0.8-1 mmol/L. Glc-6-P governs HKI activity by feedback inhibition such that the in vivo activity of HKI in resting, awake brain is only about 5% of its maximal capacity measured in vitro. Thus, dis-inhibition of HKI by consumption of Glc-6-P can stimulate HKI flux by up to 20-fold, a capacity that greatly exceeds the 4-6-fold rise in the cerebral metabolic rate of glucose (CMRglc) during seizures and ischemia [20, 21]. Glc-6-P is metabolized via the glycolytic pathway to generate ATP, but it is also the substrate for the pentose phosphate shunt pathway (PPP) that generates NADPH to manage oxidative stress and to synthesize nucleic acid precursors (Figure 2a). Phosphofructokinase is considered to be the major regulator of the glycolytic pathway due to its allosteric regulation by many metabolites (e.g., inhibition by ATP, citrate, H+, and activation by ADP, AMP, fructose-6-P, fructose-1,6,-P2, fructose-2,6,-P2, ribose-1,5,-P2) that act in concert to integrate the fluxes of the glycolytic and TCA cycle pathways. Glucose metabolism is also the source for biosynthesis of other compounds required by the brain, including complex carbohydrates that are components of glycoproteins and glycolipids, amino acids, one-carbon donors for methylation reactions, and the supply of neurotransmitter precursors [5, 22]. To summarize, CMRglc is controlled in each cell by the rate of ADP production (i.e., ATP demand) and regulation of rate-controlling enzymes by metabolites.
In astrocytes, Glc-6-P is the precursor for glycogen, a polymer composed of glucose residues. Glycogen is the brain’s only energy reserve (Figure 2a, b). In normal brain, glycogen turnover occurs at normal glucose levels, consistent with its role as an important local energy buffer for astrocytes, and it is mobilized by functional activation or energy deficits [23, 24]. Modeling predicts that glycogenolysis reduces astrocytic glucose utilization by maintaining levels of Glc-6-P sufficient to sustain high feedback inhibition of HKI, thereby sparing glucose for neurons [25]. During severe hypoglycemia or aglycemia, very low rates of glycogenolysis equivalent to only a few percent of normal glucose utilization rates are sufficient to prolong neuronal functions [5, 22].
To maintain glycolytic flux, the NADH produced by the glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Glossary) must be oxidized. Regeneration of NAD+ can occur by two mechanisms, the malate-aspartate shuttle (MAS) or the lactate dehydrogenase (LDH) reaction. The MAS is required for generation of pyruvate as an oxidative fuel because LDH activity is associated with lactate release (Figure 2a, see legend for details).
In resting awake brain, most of the glucose is completely oxidized to CO2 and water, and nearly a stoichiometric amount of O2 (i.e., 6O2 per glucose) is consumed. Due to biosynthetic reactions and slight efflux of lactate from brain, the ratio of oxygen to glucose consumption is generally about 5.5-5.8. Under different conditions ranging from deep anesthesia to the conscious state, the rate of neuronal glucose oxidation is approximately proportional to glutamatergic neurotransmission [26], indicating that the rate of the major energy producing pathway in neurons (TCA cycle, Figure 2) is directly related to the energy demands associated with the flux through the glutamate-glutamine cycle (Glossary, Figure 2b) [5].
During brain activation, glycolysis is usually preferentially upregulated compared with oxygen consumption [5], and the oxygen/glucose utilization ratio falls. Because this phenomenon occurs in normal, normoxic subjects that have excess oxygen delivery to brain, it is sometimes called aerobic glycolysis (Glossary) to distinguish it from the large rise in glycolysis during hypoxia/anoxia. Stimulation of glycolysis generates increased amounts of lactate that can be released from brain (Figure 2) but also have various important functions, including serving as supplementary oxidative fuel for astrocytes and neurons, modulating redox signaling of metabolic state, regulating blood flow [5], or functioning as a mediator of metabolic information [9].
Metabolic interactions among astrocytes and neurons and lactate shuttling
Both neurons [16, 27, 28] and astrocytes [18, 29] have been described as the main consumers of glucose. The cellular contributions to overall glucose utilization has been a very controversial issue for decades because current technology does not have adequate spatiotemporal resolution to quantify metabolic activity in single cells in vivo. Two conflicting concepts describe the predominant cellular fate of glucose during brain activation and propose different directions and magnitudes of shuttling of lactate among neurons and astrocytes. A third model is derived from demonstration of substantial lactate release from brain, irrespective of the originating cell type (Figure 2b) [5, 17].
The astrocyte-to-neuron lactate shuttle (ANLS; Figure 2b) claims that glutamatergic neurotransmission stimulates astrocytic lactate production that serves as an important neuronal fuel during activation [29]. However, this notion remains controversial because glutamate does not stimulate glycolysis in most astrocyte preparations, the cellular origin of lactate in vivo is unknown, substantial lactate oxidation by neurons has not been demonstrated during brain activation, and studies supporting this model [29] have been challenged [5, 22]. Furthermore, the neurotransmitter glutamate itself may directly support energetics of perisynaptic astrocytes that contain mitochondria because the glial glutamate transporter GLAST forms a macromolecular complex linking glutamate uptake with its oxidation [30] that can provide ATP to meet the astrocytic energy demands. Glutamate oxidation at the site of its uptake eliminates the need for glycolysis to generate ATP and the ANLS [31]. The notions that glycogen-derived lactate is necessary as the bioenergetic basis for neuronal memory consolidation [7, 32] and that glucose-derived lactate from oligodendrocytes is required to support axons [33, 34] demand direct experimental proof of the magnitude and contribution of lactate shuttling compared with other energy sources.
The neuron-to-astrocyte lactate shuttle (NALS, Figure 2b) is based on different assumptions than the ANLS and accounts for the kinetics of glucose transporters in neurons and astrocytes. The NALS model predicts predominant neuronal glucose uptake during activation, with transfer of lactate to astrocytes such that the direction of metabolite flux can be context-dependent [16, 27]. Astrocytes have key roles in lactate uptake from extracellular fluid and lactate dispersal to other astrocytes via gap junctional communication; these processes occur at rates 2-4-fold faster than lactate uptake by neurons or astrocytic transfer of lactate to neurons [17]. Thus, astrocytes are poised to take up lactate from interstitial fluid and release lactate from their endfeet to perivascular fluid for discharge to lymphatic drainage systems and venous blood [5, 17]. The total glucose utilization substantially exceeds oxidative metabolism of glucose, with release of sizeable quantities of lactate released from activated brain (Figure 2b) [5, 22].
The use of lactate as a supplemental fuel varies with its availability and physiological state of the subject. In sedentary subjects, the brain lactate level exceeds that in blood, facilitating the efflux of lactate from activated brain regions to blood. In contrast, strenuous physical activity increases glycolysis in muscles and raises blood lactate levels, reversing the direction of the lactate gradient from blood to brain and flooding the entire brain with lactate. Under these conditions, lactate is oxidized in the brain in amounts that rise with blood lactate level. However, lactate oxidation in brain during exercise accompanies increased CMRglc and release of brain-derived lactate to blood [35], suggesting separate routes for lactate efflux and influx. Thus, increased blood lactate level represents a ‘glucose sparing’ physiological state in which use of a supplemental oxidative fuel helps maintain availability of glucose for the glycolytic and pentose phosphate shunt pathways that provide critical functions for the brain.
Glucose metabolism and the regulation of cerebral blood flow
Under resting conditions, local CBF is highest in brain regions with the highest local glucose metabolism. All brain regions are metabolically active at all times, but there is a large heterogeneity among various brain structures. During functional activation, the increase in local CBF usually parallels the increase in CMRglc, whereas the increase in oxygen metabolism is much lower [36]. However, there is at least one example where under peripheral somatosensory stimulation, local CBF in the ipsilateral cortex can decrease despite increased CMRglc [37].
This close correlation of CBF and CMRglc (and, to a lesser extent, the cerebral metabolic rate of oxygen, CMRO2) demands highly dynamic and fine-tuned mechanisms to adapt local glucose and oxygen delivery and carbon dioxide removal via the blood to the actual demand of active brain regions. The traditional ‘metabolic’ hypothesis of neurovascular coupling [38] - mediated by vasoactive metabolic products such as lactate, CO2/H+, or adenosine - has recently been replaced by the currently-favored ‘neuronal’ hypothesis suggesting that neuronal energy demand is communicated to the vasculature (either directly or through astrocytes) within the neurovascular unit (Glossary) in an anticipatory, feed-forward manner by vasoactive neurotransmitters or products of synaptic signaling and that vasodilation occurs independently of glucose metabolism-induced signaling (Figure 1b; for review see [39]). The proposed feed-forward regulation is a reliable basis for a fast adaptation of the regional blood flow to the actual local level of neuronal activity, avoiding risky drops in glucose and oxygen concentrations, which might occur during exclusive metabolic regulation. However, recent studies suggest that changes in the lactate:pyruvate ratio, and therefore the cytosolic NADH:NAD+ ratio [40] or increased lactate production [41, 42] may be at least partially responsible for vasodilation during neuronal activation. Thus, neurovascular coupling regulated by feed-forward signaling may be supplemented or modulated by metabolism-dependent mechanisms [43].
Experimental studies show that direct glucose sensing mechanisms are unlikely to be involved in the activity-induced regulation of CBF. Neither hyperglycemia nor mild-to-moderate hypoglycemia significantly changes the blood flow responses to functional activation [44, 45]. In addition, during acute hypoglycemia, resting CBF only increases significantly when blood and brain glucose are dramatically reduced (for detailed review see [46]).
The consequences of impaired adaptation of CBF to CMRglc are under active investigation. Artificial reduction of the CBF response during functional activation had no impact on evoked neuronal activity in an acute experimental setting [47]. However, it is assumed that chronic global hypoperfusion of the brain may be not only a consequence but also an early cause of neurodegeneration in vascular dementia and Alzheimer’s disease [48] (see below). Thus, fine-tuned CBF-CMRglc- CMRO2 regulation is indispensable for healthy brain.
Brain-body axis – central control over peripheral glucose metabolism
Given that the brain relies on exogenous nutrient supplies, it is not surprising that the brain can increase these supplies, especially glucose, by regulating systemic homeostasis and food intake [49, 50] (Figure 1a). Specialized neuronal networks in the hypothalamic arcuate nucleus and in the hindbrain sense, integrate, and regulate energy homeostasis and glucose levels and signal to the periphery through a dedicated neuronal network [49-51]. Indeed, central glucose sensing and the peripheral regulation of glucose metabolism are tightly linked [52]. In addition to their peripheral action, hormones [49-51] including insulin [53] and glucagon-like peptide-1 (GLP-1) [54, 55] mediate peripheral glucose uptake through neuronal signaling cascades. Furthermore, brain insulin receptors [56] and other metabolic receptors and transporters, such as glucose transporters [57, 58], mediate metabolic signaling in the brain.
In the hypothalamus, pro-opiomelanocortin (POMC) [59], melanin concentrating hormone (MCH) [60], and neuropeptide Y (NPY) / agouti-related peptide (AgRP) neurons sense peripheral glucose levels and regulate energy metabolism in an antagonistic fashion [49]. Defective neuronal maintenance in these cells has severe consequences for peripheral metabolism. Defective autophagy (Glossary and [61]) in POMC neurons can lead to lifelong metabolic defects such as peripheral glucose intolerance and obesity [62, 63]. However, disrupted autophagy in glucose-sensing AgRP neurons promotes leanness and reduced food intake [64]. Interestingly, glucokinase (GK; Glossary) is expressed in select neuronal populations in hypothalamic glucose-sensing formations [65], and a protein complex containing GK and BAD (Bcl-2 antagonist of cell death) might regulate peripheral [66], as well as central glucose sensing.
Besides hormones and nutrients, both afferent and efferent metabolic signals link hindbrain nuclei and the gastrointestinal tract through the vagal nerve [51]. Thus, a complex interplay between the brain, in particular the hypothalamus, and peripheral systems control glucose supply to the brain [49, 51], peripheral nutrient uptake [49, 51] and utilization [67], as well as feeding [67-69]. Notably, the regulation of energy homeostasis through the brain is not limited to glucose metabolism but also includes most other major energy producing systems with close links between these systems [49, 51, 64, 70]. The mechanisms of the brain-body interaction in the regulation of glucose metabolism have recently been reviewed in greater detail [49, 50].
Glucose metabolism and the regulation of cell death
Glucose metabolism is evolutionarily linked to the regulation of cell death [71] (Figures 1e, 3a), and this link is tightly controlled in a similar fashion in many cell types, arguing for a universal role of co-regulated metabolic and apoptotic pathways. Neurons and cancer cells are among the cell types that rely almost exclusively on glucose metabolism for energy generation, and recent evidence suggests that these cells can use similar mechanisms to adapt to substrate deprivation and promote survival [72, 73].
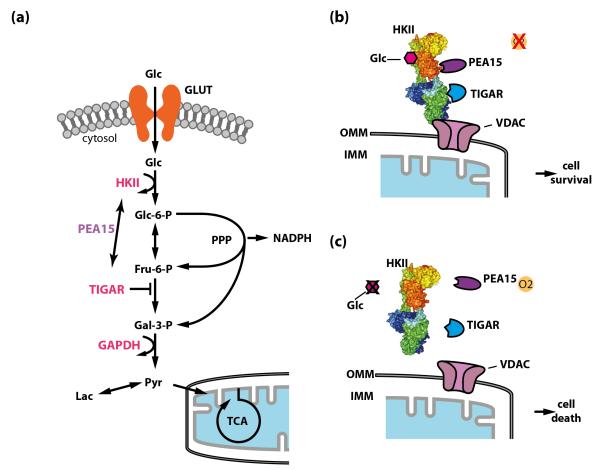
Figure 3. The connection between glucose metabolism and cell death.
(a) Glucose metabolism and cell death regulation intersect at several levels. Glucose metabolizing enzymes, including hexokinase II (HKII), glucokinase (GK), the fructose-2,6-bisphosphatase TIGAR (Tp53-induced Glycolysis and Apoptosis Regulator), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and others, are involved in the regulation of cell death through different mechanisms. Phosphoprotein-enriched in astrocytes (PEA15) might function as a molecular linker between HKII and TIGAR under certain conditions. Flux through the pentose phosphate pathway (PPP) generates NADPH, which is important for neuronal redox environment and inhibits cell death. (b) and (c) The expression of HKII in neurons is upregulated under hypoxic conditions. Together with PEA15 it functions as a molecular switch to regulate neuronal viability depending on the metabolic state [72]. HKII and PEA15 interact and bind to mitochondria through the outer-mitochondrial membrane voltage-dependent anion channel (VDAC). During hypoxia, HKII protects cells from cell death, whereas during glucose deprivation, where HKII detaches from mitochondria and the interaction with PEA15 is destabilized, HKII promotes cell death [72]. HKII also interacts with TIGAR under hypoxic conditions [77]. Similar to PEA15, which increases the capacity of HKII to protect neurons, TIGAR increases the glycolytic activity of HKII. However, the exact mechanistic link is presently unknown. Glc, glucose; GLUT, glucose transporter; Glc-6-P, glucose-6-phosphate; Fru-6-P, fructose-6-phosphate; Gal-3-P, glyceraldehyde-3-phosphate; Lac, lactate; NADPH, reduced nicotinamide adenine dinucleotide phosphate; Pyr, pyruvate; TCA, tricarboxylic acid cycle; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane. HKII was rendered in Pymol using structure 2nzt (RCSB Protein Data Bank).
Hexokinase II (HKII), a hypoxia-regulated HK isoform (Glossary) in the brain, has been demonstrated to control neuronal survival depending on the metabolic state [72] (Figures 1e, 3). HKII restricts or inhibits apoptosis in a variety of different cell types depending on whether or not it is bound to mitochondria [72, 74], and on the availability of glucose [72]. Furthermore, the capacity of HKII to phosphorylate glucose is involved in sensing the metabolic state of the cell (Figure 2a). In addition, HKII elicits its antiapoptotic function through a molecular interaction with PEA15/PED (phosphoprotein enriched in astrocytes / phosphoprotein enriched in diabetes) [72]. HKII activity protects against neuronal cell death after hypoxia [72] and in the presence of oxidative stress [75]. However, HKII increases neuronal cell death under glucose deprivation, thereby functioning as a molecular switch that regulates neuronal survival depending on the metabolic state. Importantly, the capacity of HKII to phosphorylate glucose and its interaction with PEA15 both mediate this effect [72] (Figure 3b, c).
Intriguingly, controlled regulation of glucose metabolism protects both neurons and cancer cells from apoptosis through related mechanisms [72, 73], highlighting the universal importance of sensing the metabolic state of a cell. In both cell types, HKII protects from cell death under hypoxic conditions [72], and increased activity of the PPP provides the reducing environment for inhibiting cytochrome c-mediated apoptosis, thereby preventing cellular damage through oxidative stress [73]. Furthermore, PPP inhibition after selective experimental activation of glycolysis and the concomitant deprivation of NADPH triggers apoptosis in neurons [76]. However, it has not been established whether the fructose-2,6-bisphosphatase TIGAR (Tp53-induced Glycolysis and Apoptosis Regulator), which promotes flux through the PPP and interacts with HKII under hypoxic conditions [77], promotes preferential PPP-flux in neurons and thereby cooperates with HKII to prevent cell death. Nevertheless, increased HKII activity upon interaction with TIGAR [77] is reminiscent of the increased capacity of HKII to inhibit neuronal apoptosis when it is bound to PEA15/PED [72] (Figure 3b, c).
Finally, other enzymes of the glycolytic cascade, including GAPDH, have also been demonstrated to inhibit cell death under certain conditions [78]. However, GAPDH has also been suggested to mediate neuronal apoptosis after DNA damage [79]. Thus, glycolytic enzymes can regulate neuronal cell death in a context-dependent fashion, exerting both pro- and antiapoptotic effects.
Control of apoptosis by glucose-metabolizing enzymes appears not to be a one-way street. As an example, the Bcl-2 family member BAD interacts with GK (also known as hexokinase IV) in liver and pancreatic β-cells to modulate apoptosis in response to changes in glucose levels [66]. However, it is presently unknown whether GK has a role in regulating neuronal viability depending on the metabolic state in select glucose sensing neuronal populations (see below). Furthermore, BclXL, an antiapoptotic Bcl-2 family member has been suggested to increase metabolic efficiency of neurons by decreasing a proton leak within the F1FO ATPase and across the inner mitochondrial membrane [80, 81].
Disturbed metabolism is closely linked to cell death pathways and autophagy [61]. Indeed, GAPDH [78], as well as many players in the apoptotic cascades, regulate autophagy [61]. However, whether these mechanisms involve (dysfunctional) glucose sensing through glycolytic enzymes or if they are controlled by co-regulated apoptotic / autophagic pathways (e.g. by the Bcl-2 family [82]), remains to be established. Thus, disturbed signaling through these pathways is thought to be the pathophysiological basis for a large variety of diseases.
Disease mechanisms
Neurons are largely intolerant of inadequate energy supply, and thus the high energy demand of the brain predisposes it to a variety of diseases if energy supplies are disrupted. A large variety of central nervous system pathologies are the consequence, and sometimes also the cause of disturbed central or peripheral glucose energy metabolism, which can be affected at almost every level of the cellular or biochemical metabolic cascades (Figure 1). Changes in the glucose metabolism of affected patients can efficiently be measured by positron emission tomography (PET) imaging in a large variety of clinical scenarios (Box 1).
Box 1.
Glucose metabolism and functional brain imaging:
Under physiological conditions, cerebral glucose metabolism is tightly correlated to neuronal activity [13]. Imaging of local cerebral glucose metabolism (CMRglc) can therefore be used to visualize areas of increased neuronal activity. The most frequently-used methods of brain metabolic imaging are the detection of radiolabeled glucose by positron emission tomography (PET) in vivo or for diagnostic imaging (Figure I), and by ex vivo autoradiography [106]. The glucose analogues [18F]fluoro-2-deoxyglucose (FDG) or 2-deoxy[14C]glucose are injected intravenously, transported into the brain, and phosphorylated by HK to 2-deoxyglucose-6-phosphate (2-DG-6-P). Fluorescent 2-DG derivatives can be used for fluorescence imaging in animal models [107], but quantitative determination of CMRglc with the fluorescent analogs requires evaluation of the kinetics for competition with glucose for transport and phosphorylation [5]. Labeled 2-DG-6-P is trapped in the tissue and then detected. In the experimental setting, autoradiography of previously-sliced tissue provides a higher spatial resolution than external imagingTo achieve further information on the metabolic pathways of glucose, nuclear magnetic resonance spectroscopy after [13C]glucose infusion can be applied as a powerful tool to measure a large number of metabolic fluxes of glucose noninvasively [108]. Due to low temporal resolution, these methods do not allow to measure fast dynamic changes of glucose metabolism during neuronal activation.
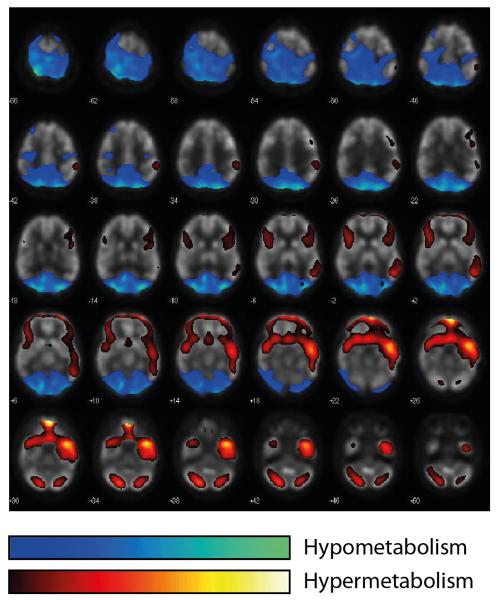
Figure I. Example of a diagnostic [18F]fluoro-2-deoxyglucose PET-CT.
As illustrated in this 23 year-old female patient after a two-month course of severe anti-NMDA-R encephalitis, these patients typically show widespread frontotemporal cortical hypermetabolism as well as bioccipital and cerebellar cortical hypometabolism [103]. For visualtization, hyper- and hypometabolism is colour-coded across the entire brain as depicted in the legend. Images are from superior (top left) to inferior (bottom right). For details on the voxel-based statistical analysis to demonstrate hyper- and hypometabolism and the corresponding cohort study see Leypoldt et al. [103]. Images courtesy of Dr. R. Buchert, Charité.
Neuroglycopenia is a neurodevelopmental syndrome characterized by mental retardation or developmental delay, anomalous coordination and muscle tone, as well as thalamocortical hypometabolism [83]. This syndrome is further characterized by infantile drug-resistant seizures, developmental retardation and microcephaly in many cases [84]. It can be caused by persistent hypoglycemia during development or by deficiency in the major glucose transporter of the blood-brain barrier (BBB), GLUT1 (Figure 1b). Indeed, more than 10% of early-onset absence epilepsy [85] and up to 1% of the common idiopathic generalized epilepsies [86] have been ascribed to GLUT1 deficiency caused by mutations in the SLC2A1 (solute carrier family 2) gene. Early diagnosis of the GLUT1 deficiency syndrome is important because adherence to a ketogenic diet (Glossary) [15] is an effective treatment for most patients [83]; in general, ketogenic diet efficiently suppresses epileptic seizures in childhood drug-resistant epilepsy [87]. The anticonvulsant effect of restricted dietary carbohydrate intake further underscores the relevance of glucose-derived energy for neuronal excitability [15]. Furthermore, inhibition of glycolysis with the glucose analog 2-deoxyglucose in experimental seizures is an effective treatment and illustrates a role for glycolysis-derived NADH for the metabolic regulation of genes involved in epilepsy [88]. Interestingly, BAD appears to be involved in the regulation of neuronal energy substrate utilization independent of its apoptotic function [89]. However, it remains unclear whether GK [66], which has a very restricted expression pattern in the brain, or another HK isoform plays a role in facilitating glucose sensing by BAD in this context.
A thromboembolic occlusion of a brain-supplying artery leads to an acute disruption in blood supply to a specific brain territory, causing cerebral ischemia (Figure 1b). Within minutes, glucose depletion and associated compromised bioenergetic pathways cause extensive neuronal death in the core of the infarction, and over time in the surrounding tissue [90, 91]. Cellular models suggest that increased levels of the mitochondrial-bound glycolytic enzyme HKII can protect neurons from cell death in ischemia [72] (Figure 1e).
Spreading depression is a self-propagating wave of neuronal depolarization in the cortex, which is associated with a variety of neurovascular diseases including stroke, subarachnoid hemorrhage, traumatic brain injury [92], and migraine [93], Spreading depolarizations (SD) disturb cortical glucose metabolism [94], although interestingly it has been demonstrated that hyperglycemia increases the cortical resistance against SD [95].
Although neurodegenerative diseases are not classically thought to be caused by disturbed metabolism, bioenergetic defects are emerging as important pathophysiological mechanisms (Box 2) [3] in several disorders. One of the earliest signs of Alzheimer’s disease (AD) is a reduction in cerebral glucose metabolism, and both human studies and animal models suggest that disturbed glucose metabolism is associated with AD progression [96]. In a mouse model of AD, GLUT1 expression is reduced both at the BBB as well as in astrocytes, which is paralleled by impaired glucose transport and reduced cerebral lactate release during neuronal activation [97] (Figure 1b). Dysregulated glucose metabolism in metabolic disorders such as obesity or type II diabetes mellitus has been linked to AD progression and cognitive impairment [96]. However, a large clinical trial could not demonstrate a beneficial effect of aggressive glucose lowering on cognitive outcome in diabetic patients [98].
Box 2.
Glucose metabolism, cell death and neurodegeneration
Glucose metabolism and the regulation of cell death are tightly coupled [66, 71, 72] (Figure 3).
Autophagy can be activated upon metabolic stress (e.g. starvation) of cells, as well as upon other stressors including hypoxia and inflammation, to promote cellular survival under these conditions [61]. Autophagy, in turn, can be regulated by cell death and metabolic pathways [61], including key regulators of glucose metabolism [78].
Defective autophagy, oxidative stress and bioenergetic stress have been linked to the development of neurodegenerative diseases [61, 96, 109].
Disrupted axonal nutrient supply and a defective metabolic network are associated with neurodegeneration in the central and peripheral nervous system [33, 34].
Future research will elucidate the role of defective glucose metabolism and the extent of the involvement of members of the glycolytic cascade [72, 76-78] in the pathophysiology of neurodegenerative diseases.
In patients suffering from Parkinson’s disease (PD), widespread cortical hypometabolism is accompanied by glucose hypermetabolism in the external pallidum and possibly other subcortical structures [99]. In one model of PD, HKII, which regulates neuronal viability depending on the metabolic state [72] (Figure 1e), has been suggested to inhibit degeneration of dopaminergic neurons [100].
Disturbed metabolism in myelin-producing cells is associated with axonal degeneration. In the brain, defective lactate transporter levels in oligodendrocytes are linked to axonopathy [34] (Figure 1c), and in the peripheral nervous system disrupted oxidative phosphorylation in Schwann cells is related to severe neuropathy [33]. However, demyelination without extensive axonal loss in an animal model of multiple sclerosis [101] hints to a complex underlying mechanism.
Autoantibodies against the NR1 subunit of the N-methyl-D-aspartate receptor (NMDA-R) may inhibit glutamatergic transmission (Figure 1d) by blocking, crosslinking, and initiating the internalization of the receptor. Patients with anti-NMDAR encephalitis have distinct symptoms that occur over weeks and months. The symptoms start with fever, psychosis, and seizures, and progress to abnormal movements, respiratory failure, dysautonomia, and coma [102]. As a consequence of impaired NMDA-R function, and correlating with disease activity, a characteristic pattern of [18F] FDG-PET abnormalities (Box 1, Figure I) includes increased fronto-temporal and decreased occipito-parietal glucose metabolism. Despite these severe and long-lasting symptoms and changes in metabolism, most patients do not have pathological findings in diagnostic MRI-imaging studies. Furthermore, normalization in cerebral glucose metabolism accompanies recovery [103].
Finally, disrupted central glucose sensing, insulin signaling, and defective hypothalamic circuits have been implicated in the pathophysiological mechanism of type 2 diabetes mellitus and obesity [49, 59, 62-64, 67] (Figure 1a). At the same time, dysregulated glucose metabolism in diabetes mellitus can injure the brain through both hypo- and hyperglycemia [104]. Furthermore, cachexia, a severe complication after cerebral ischemia, has been in part ascribed to dysregulation of the hypothalamus-pituitary-adrenal axis and perturbed efferent signaling [105]. Given the role of hypothalamic structures for glucose and nutrient sensing (see above and [49, 51]), disturbed central glucose sensing and impeded central regulation of peripheral metabolism (see above) may contribute to the development of cachexia after CNS damage.
Concluding remarks
Glucose metabolism is closely integrated with brain physiology and function. Although recent studies have shed light on the complex regulation of biochemical, cellular, and systemic pathways, many features of the exact regulation remain controversial or elusive (Box 3). The advent of novel and refined biochemical or genetic tools, screening methods, imaging technologies and systems analyses will allow for the study of cellular, subcellular, and even biochemical mechanisms in the cell or in vivo with unprecedented temporal and spatial resolution. In addition to studying individual biochemical or cellular pathways and their control over intracellular signaling cascades (e.g. programmed cell death), peripheral homeostasis or brain activity, future challenges lie in integrating the parts of the puzzle to form a conclusive picture of the cooperation between different systems and cell types. Ultimately, a thorough understanding of these mechanisms will lead to better insight into the pathophysiology of multiple diverse disorders of the brain and allow the development of novel treatment strategies.
Box 3.
Outstanding questions
Does modeling accurately predict actual energy use of the brain?
Can metabolic substrates be interchanged? Are there functional consequences of using glucose and other metabolites respectively? Future studies need to address the consequences of oxidative vs. glycolytic metabolism for different brain functions.
What metabolic substrates support neurons and other cell types under different functional states of the brain? What is the relevance of shuttling of metabolic substrates between different cell types? Does the direction of metabolic shuttling between cells depend on the (physiological or experimental) context?
How does metabolic coupling among brain cells and shuttling of metabolites sustain brain activity?
Are there additional anatomical or cellular networks in the brain which control peripheral glucose metabolism? Do cell death pathways contribute to central glucose sensing? How does disturbed peripheral metabolism influence central glucose sensing and regulation? How does disrupted central glucose sensing cause systemic metabolic disorders?
What is the functional connection in the regulation of cell death pathways through different members of the glycolytic cascade? What is the functional impact of this connection for different cells of the brain (e.g. neurons, astrocytes, oligodendroglia, etc.)? How does dysbalanced metabolism and subsequent dysregulation of cell death pathways contribute to neurodegenerative diseases or other acute or chronic disorders of the brain?
How can knowledge about cerebral glucose metabolism be exploited for refined therapies of neurodegenerative disorders or other diseases of the brain?
Highlights.
We provide a comprehensive overview of the role of glucose metabolism for normal brain function.
We analyze the contribution of glucose metabolism to brain physiology.
We discuss controversies in energy substrate consumption and utilization.
We highlight the connection between glucose metabolism and cell death.
We review the pathophysiological consequences of balanced and disturbed glucose metabolism.
Acknowledgements
We are grateful to the members of our labs for their contribution to our underlying research. We would like to thank Ralph Buchert, Department of Nuclear Medicine, Charité, for kindly providing and analyzing the PET images. This work was supported by the European Union’s Seventh Framework Programme (FP7/2008-2013) under Grant Agreements 201024 and 202213 (European Stroke Network), the Deutsche Forschungsgemeinschaft (NeuroCure Cluster of Excellence, Exc 257; SyNergy, Munich Cluster for Systems Neurology, Exc 1010), the Bundesministerium für Bildung und Forschung (Center for Stroke Research Berlin, 01 EO 08 01), a Canadian Stroke Network – European Stroke Network Transatlantic Collaboration grant, and the National Institutes of Health (DK081936).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures P.M. has received research funding from Sanofi. A.M. has received research funding and speaker honoraria from Bayer Vital GmbH, Sanofi, and Wyeth Pharma GmbH. U.L. and G.D. have no disclosures.
References
- 1.Howarth C, et al. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erbsloh F, et al. [The glucose consumption of the brain & its dependence on the liver] Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1958;196:611–626. doi: 10.1007/BF00344388. [DOI] [PubMed] [Google Scholar]
- 3.Harris JJ, et al. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Ivannikov MV, et al. Calcium clearance and its energy requirements in cerebellar neurons. Cell Calcium. 2010;47:507–513. doi: 10.1016/j.ceca.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dienel GA. Fueling and imaging brain activation. ASN Neuro. 2012;4:e00093. doi: 10.1042/AN20120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. Journal of neurochemistry. 2009;109(Suppl 1):10–16. doi: 10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki A, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauritzen KH, et al. Lactate Receptor Sites Link Neurotransmission, Neurovascular Coupling, and Brain Energy Metabolism. Cereb Cortex. 2013 doi: 10.1093/cercor/bht136. 10.1093/cercor/bht136 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Bergersen LH, Gjedde A. Is lactate a volume transmitter of metabolic states of the brain? Front Neuroenergetics. 2012;4:5. doi: 10.3389/fnene.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alle H, et al. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- 11.Liotta A, et al. Energy demand of synaptic transmission at the hippocampal Schaffer-collateral synapse. J Cereb Blood Flow Metab. 2012;32:2076–2083. doi: 10.1038/jcbfm.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32:356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokoloff L. Energetics of functional activation in neural tissues. Neurochem Res. 1999;24:321–329. doi: 10.1023/a:1022534709672. [DOI] [PubMed] [Google Scholar]
- 14.van Hall G, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29:1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- 15.Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36:32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson IA, et al. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandhi GK, et al. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. Journal of neurochemistry. 2009;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouach N, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 20.Borgstrom L, et al. Glucose consumption in the cerebral cortex of rat during bicuculline-induced status epilipticus. Journal of neurochemistry. 1976;27:971–973. doi: 10.1111/j.1471-4159.1976.tb05165.x. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, et al. Effect of Ischemia on Known Substrates and Cofactors of the Glycolytic Pathway in Brain. J Biol Chem. 1964;239:18–30. [PubMed] [Google Scholar]
- 22.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dienel GA, et al. A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. Journal of neurochemistry. 2007;102:466–478. doi: 10.1111/j.1471-4159.2007.04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls AB, et al. Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience. 2009;158:284–292. doi: 10.1016/j.neuroscience.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 25.Dinuzzo M, et al. The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem Res. 2012;37:2432–2438. doi: 10.1007/s11064-012-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyder F, Rothman DL. Quantitative fMRI and oxidative neuroenergetics. Neuroimage. 2012;62:985–994. doi: 10.1016/j.neuroimage.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangia S, et al. Response to ‘comment on recent modeling studies of astrocyte-neuron metabolic interactions’: much ado about nothing. J Cereb Blood Flow Metab. 2011;31:1346–1353. doi: 10.1038/jcbfm.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall CN, et al. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer DE, et al. The glutamate transporter, GLAST, participates in a macromolecular complex that supports glutamate metabolism. Neurochem Int. 2012;61:566–574. doi: 10.1016/j.neuint.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dienel GA. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int. 2013 doi: 10.1016/j.neuint.2013.06.015. in press, DOI: 10.1016/j.neuint.2013.1006.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newman LA, et al. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funfschilling U, et al. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature. 2012;485:517–521. doi: 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Overgaard M, et al. Hypoxia and exercise provoke both lactate release and lactate oxidation by the human brain. FASEB J. 2012;26:3012–3020. doi: 10.1096/fj.11-191999. [DOI] [PubMed] [Google Scholar]
- 36.Fox PT, et al. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 37.Devor A, et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J Neurosci. 2008;28:14347–14357. doi: 10.1523/JNEUROSCI.4307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. J Physiol. 1890;11:85–158. 117. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attwell D, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vlassenko AG, et al. Regulation of blood flow in activated human brain by cytosolic NADH/NAD+ ratio. Proc Natl Acad Sci U S A. 2006;103:1964–1969. doi: 10.1073/pnas.0510632103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vafaee MS, et al. Oxygen consumption and blood flow coupling in human motor cortex during intense finger tapping: implication for a role of lactate. J Cereb Blood Flow Metab. 2012;32:1859–1868. doi: 10.1038/jcbfm.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gordon GR, et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon GR, et al. Bidirectional control of arteriole diameter by astrocytes. Exp Physiol. 2011;96:393–399. doi: 10.1113/expphysiol.2010.053132. [DOI] [PubMed] [Google Scholar]
- 44.Wolf T, et al. Excessive oxygen or glucose supply does not alter the blood flow response to somatosensory stimulation or spreading depression in rats. Brain Res. 1997;761:290–299. doi: 10.1016/s0006-8993(97)00354-5. [DOI] [PubMed] [Google Scholar]
- 45.Powers WJ, et al. Effect of stepped hypoglycemia on regional cerebral blood flow response to physiological brain activation. Am J Physiol. 1996;270:H554–559. doi: 10.1152/ajpheart.1996.270.2.H554. [DOI] [PubMed] [Google Scholar]
- 46.Nehlig A. Cerebral energy metabolism, glucose transport and blood flow: changes with maturation and adaptation to hypoglycaemia. Diabetes Metab. 1997;23:18–29. [PubMed] [Google Scholar]
- 47.Leithner C, et al. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J Cereb Blood Flow Metab. 2010;30:311–322. doi: 10.1038/jcbfm.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicolakakis N, Hamel E. Neurovascular function in Alzheimer’s disease patients and experimental models. J Cereb Blood Flow Metab. 2011;31:1354–1370. doi: 10.1038/jcbfm.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lam CK, et al. CNS regulation of glucose homeostasis. Physiology (Bethesda) 2009;24:159–170. doi: 10.1152/physiol.00003.2009. [DOI] [PubMed] [Google Scholar]
- 50.Grayson BE, et al. Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci. 2013;14:24–37. doi: 10.1038/nrn3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012;16:296–309. doi: 10.1016/j.cmet.2012.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lam TK, et al. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- 53.Filippi BM, et al. Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab. 2012;16:500–510. doi: 10.1016/j.cmet.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Hayes MR, et al. Intracellular signals mediating the food intakesuppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sandoval DA, et al. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 57.Chari M, et al. Glucose transporter-1 in the hypothalamic glial cells mediates glucose sensing to regulate glucose production in vivo. Diabetes. 2011;60:1901–1906. doi: 10.2337/db11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mounien L, et al. Glut2-dependent glucose-sensing controls thermoregulation by enhancing the leptin sensitivity of NPY and POMC neurons. FASEB J. 2010;24:1747–1758. doi: 10.1096/fj.09-144923. [DOI] [PubMed] [Google Scholar]
- 59.Parton LE, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 60.Kong D, et al. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kroemer G, et al. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coupe B, et al. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab. 2012;15:247–255. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushik S, et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 2012;13:258–265. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaushik S, et al. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynch RM, et al. Localization of glucokinase gene expression in the rat brain. Diabetes. 2000;49:693–700. doi: 10.2337/diabetes.49.5.693. [DOI] [PubMed] [Google Scholar]
- 66.Danial NN, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 67.Joly-Amado A, et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 2012;31:4276–4288. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aponte Y, et al. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Q, et al. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi CX, Tschop MH. Brain-gut-adipose-tissue communication pathways at a glance. Dis Model Mech. 2012;5:583–587. doi: 10.1242/dmm.009902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King A, Gottlieb E. Glucose metabolism and programmed cell death: an evolutionary and mechanistic perspective. Curr Opin Cell Biol. 2009;21:885–893. doi: 10.1016/j.ceb.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Mergenthaler P, et al. Mitochondrial hexokinase II (HKII) and phosphoprotein enriched in astrocytes (PEA15) form a molecular switch governing cellular fate depending on the metabolic state. Proc Natl Acad Sci U S A. 2012;109:1518–1523. doi: 10.1073/pnas.1108225109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaughn AE, Deshmukh M. Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c. Nat Cell Biol. 2008;10:1477–1483. doi: 10.1038/ncb1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Majewski N, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 75.Gimenez-Cassina A, et al. Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J Biol Chem. 2009;284:3001–3011. doi: 10.1074/jbc.M808698200. [DOI] [PubMed] [Google Scholar]
- 76.Herrero-Mendez A, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 77.Cheung EC, et al. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colell A, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 79.Ishitani R, Chuang DM. Glyceraldehyde-3-phosphate dehydrogenase antisense oligodeoxynucleotides protect against cytosine arabinonucleoside-induced apoptosis in cultured cerebellar neurons. Proc Natl Acad Sci U S A. 1996;93:9937–9941. doi: 10.1073/pnas.93.18.9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alavian KN, et al. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen YB, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galluzzi L, et al. Non-apoptotic functions of apoptosis-regulatory proteins. EMBO Rep. 2012;13:322–330. doi: 10.1038/embor.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pascual JM, et al. Brain glucose supply and the syndrome of infantile neuroglycopenia. Arch Neurol. 2007;64:507–513. doi: 10.1001/archneur.64.4.noc60165. [DOI] [PubMed] [Google Scholar]
- 84.Leen WG, et al. Glucose transporter-1 deficiency syndrome: the expanding clinical and genetic spectrum of a treatable disorder. Brain. 2010;133:655–670. doi: 10.1093/brain/awp336. [DOI] [PubMed] [Google Scholar]
- 85.Arsov T, et al. Early onset absence epilepsy: 1 in 10 cases is caused by GLUT1 deficiency. Epilepsia. 2012;53:e204–e207. doi: 10.1111/epi.12007. [DOI] [PubMed] [Google Scholar]
- 86.Arsov T, et al. Glucose transporter 1 deficiency in the idiopathic generalized epilepsies. Ann Neurol. 2012;72:807–815. doi: 10.1002/ana.23702. [DOI] [PubMed] [Google Scholar]
- 87.Neal EG, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 88.Garriga-Canut M, et al. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 89.Gimenez-Cassina A, et al. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron. 2012;74:719–730. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dirnagl U, et al. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 91.Mergenthaler P, et al. Pathophysiology of stroke: lessons from animal models. Metab Brain Dis. 2004;19:151–167. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- 92.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 93.Vecchia D, Pietrobon D. Migraine: a disorder of brain excitatory-inhibitory balance? Trends Neurosci. 2012;35:507–520. doi: 10.1016/j.tins.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 94.Sakowitz OW, et al. Clusters of spreading depolarizations are associated with disturbed cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2013;44:220–223. doi: 10.1161/STROKEAHA.112.672352. [DOI] [PubMed] [Google Scholar]
- 95.Hoffmann U, et al. Glucose modulation of spreading depression susceptibility. J Cereb Blood Flow Metab. 2013;33:191–195. doi: 10.1038/jcbfm.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Merlini M, et al. Vascular beta-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAbeta mice. Acta Neuropathol. 2011;122:293–311. doi: 10.1007/s00401-011-0834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Launer LJ, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Borghammer P, et al. Glucose metabolism in small subcortical structures in Parkinson’s disease. Acta Neurol Scand. 2012;125:303–310. doi: 10.1111/j.1600-0404.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 100.Corona JC, et al. Hexokinase II gene transfer protects against neurodegeneration in the rotenone and MPTP mouse models of Parkinson’s disease. J Neurosci Res. 2010;88:1943–1950. doi: 10.1002/jnr.22357. [DOI] [PubMed] [Google Scholar]
- 101.Skripuletz T, et al. De- and remyelination in the CNS white and grey matter induced by cuprizone: the old, the new, and the unexpected. Histol Histopathol. 2011;26:1585–1597. doi: 10.14670/HH-26.1585. [DOI] [PubMed] [Google Scholar]
- 102.Dalmau J, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leypoldt F, et al. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83:681–686. doi: 10.1136/jnnp-2011-301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scheen AJ. Central nervous system: a conductor orchestrating metabolic regulations harmed by both hyperglycaemia and hypoglycaemia. Diabetes Metab. 2010;36(Suppl 3):S31–38. doi: 10.1016/S1262-3636(10)70464-X. [DOI] [PubMed] [Google Scholar]
- 105.Scherbakov N, et al. Body weight after stroke: lessons from the obesity paradox. Stroke. 2011;42:3646–3650. doi: 10.1161/STROKEAHA.111.619163. [DOI] [PubMed] [Google Scholar]
- 106.Sokoloff L, et al. The [14C]Deoxyglucose Method for Measurement of Local Cerebral Glucose Utilization. In: Boulton A, et al., editors. Carbohydrates and Energy Metabolism. Humana Press; 1989. pp. 155–193. [Google Scholar]
- 107.Yao J, et al. Noninvasive photoacoustic computed tomography of mouse brain metabolism in vivo. Neuroimage. 2012;64C:257–266. doi: 10.1016/j.neuroimage.2012.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Graaf RA, et al. State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo. A practical guide. NMR Biomed. 2011;24:958–972. doi: 10.1002/nbm.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol. 2012;8:108–117. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]