Abstract
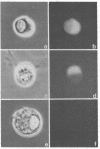
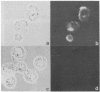
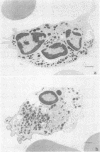
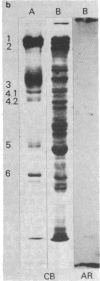
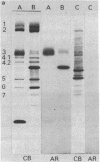
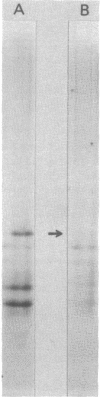
Band 3, the major transmembrane polypeptide of erythrocytes, mediates the exchange of anions (chloride and bicarbonate) across the membrane. We suspected that band 3 was present on nucleated somatic cells as well as erythrocytes because the senescent cell antigen that is immunologically related to band 3 is present on lymphocytes, platelets, adult liver cells, and embryonic kidney cells; and antibodies prepared against the senescent cell antigen isolated from leukocytes react with erythrocyte band 3. For this reason, we examined human fibroblasts, lung cells, neutrophils, mononuclear leukocytes, squamous epithelial (mouth) cells, lung squamous epithelial carcinoma, mouse neuroblastoma cells, and rat hepatocytes for immunoreactive forms of band 3 by using monospecific antibodies to erythrocyte band 3. The results demonstrated that polypeptides sharing common antigenic determinants with erythrocyte band 3 are present in nucleated somatic cells as determined by immunofluorescence, immunoelectron microscopy, and immunoautoradiography. Peptide mapping revealed substantial sequence homology between erythrocyte band 3 and the band 3-like protein of leukocytes. Immunofluorescence studies indicate that the band 3-like proteins in nucleated cells participate in antibody-induced cell surface capping.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. D., Kay M. M. Homeostatic removal of senescent murine erythrocytes by splenic macrophages. Exp Hematol. 1981 Mar;9(3):297–307. [PubMed] [Google Scholar]
- Bennett V., Davis J., Fowler W. E. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982 Sep 9;299(5879):126–131. doi: 10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. Immunoreactive forms of human erythrocyte ankyrin are present in diverse cells and tissues. Nature. 1979 Oct 18;281(5732):597–599. doi: 10.1038/281597a0. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Rodkey L. S. Autoregulation of an antibody response via network-induced auto-anti-idiotype. J Exp Med. 1979 Jul 1;150(1):67–85. doi: 10.1084/jem.150.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cheng S., Levy D. Characterization of the anion transport system in hepatocyte plasma membranes. J Biol Chem. 1980 Apr 10;255(7):2637–2640. [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F., Korsgren C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibres on non-erythroid cells. Nature. 1982 Oct 14;299(5884):648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. Erythroid spectrin, brain fodrin, and intestinal brush border proteins (TW-260/240) are related molecules containing a common calmodulin-binding subunit bound to a variant cell type-specific subunit. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4002–4005. doi: 10.1073/pnas.79.13.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. F-actin-binding and cross-linking properties of porcine brain fodrin, a spectrin-related molecule. J Biol Chem. 1982 Aug 25;257(16):9781–9787. [PubMed] [Google Scholar]
- Goodman S. R., Zagon I. S., Kulikowski R. R. Identification of a spectrin-like protein in nonerythroid cells. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7570–7574. doi: 10.1073/pnas.78.12.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Karadsheh N. S., Uyeda K. Changes in allosteric properties of phosphofructokinase bound to erythrocyte membranes. J Biol Chem. 1977 Nov 10;252(21):7418–7420. [PubMed] [Google Scholar]
- Kay M. M., Goodman S. R., Sorensen K., Whitfield C. F., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981 Feb 5;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Mendoza J., Diven J., Denton T., Union N., Lajiness M. Age-related changes in the immune system of mice of eight medium and long-lived strains and hybrids. I. Organ, cellular, and activity changes. Mech Ageing Dev. 1979 Dec;11(5-6):295–346. doi: 10.1016/0047-6374(79)90009-5. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Multiple labelling technique used for kinetic studies of activated human B lymphocytes. Nature. 1975 Apr 3;254(5499):424–426. doi: 10.1038/254424a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Sorensen K., Wong P., Bolton P. Antigenicity, storage, and aging: physiologic autoantibodies to cell membrane and serum proteins and the senescent cell antigen. Mol Cell Biochem. 1982 Nov 26;49(2):65–85. doi: 10.1007/BF00242486. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Steck T. L. Association of glyceraldehyde-3-phosphate dehydrogenase with the human red cell membrane. A kinetic analysis. J Biol Chem. 1980 Jul 10;255(13):6314–6321. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Lin S., Spudich J. A. Binding of cytochalasin B to a red cell membrane protein. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1471–1476. doi: 10.1016/s0006-291x(74)80449-3. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Beutler E. Comparison of structure and function of human erythrocyte and human muscle actin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):935–938. doi: 10.1073/pnas.76.2.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Colaço C. A., Lazarides E. Involvement of spectrin in cell-surface receptor capping in lymphocytes. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1626–1630. doi: 10.1073/pnas.80.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Expression of the beta subunit of spectrin in nonerythroid cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):363–367. doi: 10.1073/pnas.80.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhany J. M., Shaklai N. Functional properties of human hemoglobin bound to the erythrocyte membrane. Biochemistry. 1979 Mar 6;18(5):893–899. doi: 10.1021/bi00572a025. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Koziarz J. J., Singh M. K., Reddy G., Köhler H. Preparation and analysis of seven major, topographically defined fragments of band 3, the predominant transmembrane polypeptide of human erythrocyte membranes. Biochemistry. 1978 Apr 4;17(7):1216–1222. doi: 10.1021/bi00600a013. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Strapazon E., Steck T. L. Interaction of the aldolase and the membrane of human erythrocytes. Biochemistry. 1977 Jun 28;16(13):2966–2971. doi: 10.1021/bi00632a025. [DOI] [PubMed] [Google Scholar]
- Taverna R. D., Langdon R. G. D-glucosyl isothiocyanate, an affinity label for the glucose transport proteins of the human erythrocyte membrane. Biochem Biophys Res Commun. 1973 Sep 18;54(2):593–599. doi: 10.1016/0006-291x(73)91464-2. [DOI] [PubMed] [Google Scholar]
- Tolson N. D., Boothroyd B., Hopkins C. R. Cell surface labelling with gold colloid particulates: the use of avidin and staphylococcal protein A-coated gold in conjunction with biotin and fc-bearing ligands. J Microsc. 1981 Aug;123(Pt 2):215–226. doi: 10.1111/j.1365-2818.1981.tb01296.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]