Abstract
Kidney function and cardiovascular disease are closely connected and albuminuria is a proven marker of cardiovascular risk. The present study investigated the prevalence and characteristics of albuminuria in patients with hypertension. Outpatients with essential hypertension under medical treatment were enrolled in this study (n = 350, 70.0 ± 11.4 years old). Urine samples were collected for the measurement of albumin concentration, which are expressed as the ratio of urine albumin to creatinine concentration (mg/g Cr). Cross-sectional analyses were also performed of the relationships between urinary albumin and other variables. Urinary albumin was detected in 88.3% of patients, while only 35.4% showed abnormal albuminuria (≥30 mg/g Cr). The presence of abnormal albuminuria was independently correlated with systolic blood pressure, B-type natriuretic peptide, and C-reactive protein by multivariate analysis (P < 0.05). Furthermore, multivariate regression analysis identified systolic blood pressure, serum creatinine, B-type natriuretic peptide, and C-reactive protein as the only factors showing independent correlation with urinary albumin (P < 0.05). Thus, approximately 35% of hypertensive patients had abnormal albuminuria. Urinary albumin was closely associated with blood pressure, C-reactive protein, and B-type natriuretic peptide, indicating that the severity of albuminuria parallels that of systemic inflammation, cardiac load, and blood pressure.
Hypertension is one of the major risk factors for cardiovascular disease, a leading cause of mortality worldwide1,2,3. The kidney is important in the management of hypertension and damage to this organ can start a vicious circle of hypertension and kidney damage2,3. Furthermore, there may be a close connection between kidney dysfunction and cardiovascular disease4,5,6,7.
Recent studies have established microalbminuria, which is association with arterial hypertension, metabolic syndrome, and diabetes mellitus with a prevalence rate of 6.6–36.1%8,9,10,11,12, as an important cardiovascular risk factor4,5,6,7. Left ventricular hypertrophy and increased carotid artery intima-media thickness, both subclinical cardiovascular diseases, are associated with microalbuminuria in individuals at increased risk of cardiovascular disease13,14,15. Moreover, urinary albumin excretion, even at levels below the clinically defined thresholds for microalbminuria, is associated with an increased incidence of cardiovascular and all-cause mortality7,13. Although most of these indicative studies involved high-risk patients, recent studies also demonstrated the importance of urinary excretion of albumin as a cardiovascular risk in the general population4,16.
The data reported thus far clearly underpin the importance of measuring urinary albumin in patients with hypertension, and interventions that reduce urinary albumin may reduce the increased cardiovascular risk in patients with albuminuria, although this latter point remains unproven17,18. More importantly, patients with hypertension may benefit from prevention of the onset or progression of albuminuria and to this end, further characterization of albuminuria in hypertensive patients or of possible factors affecting urinary excretion of albumin could provide useful information. Thus, the present study sought to investigate the prevalence and characteristics of albuminuria in patients with hypertension, and to identify factors closely related to the urinary excretion of albumin.
Results
Figure 1 shows the distribution of the ratio of urinary albumin to urinary creatinine (UACR; mg/g creatinine [mg/g Cr]) in patients with hypertension. Urinary albumin was detected in 88.3% of hypertensive patients and 35.4% of patients showed abnormal albuminuria (≥30 mg/g Cr). Table 1 shows the descriptive data stratified by the presence of abnormal albuminuria. Among subjects studied, 22.0% had diabetes mellitus (15.4% of these were under medication), 44.6% had dyslipidemia (33.4% under medication), and 36.6% had an estimated glomerular filtration rate (eGFR) < 60 ml/min per 1.73 m2. The age distribution of participants was as follows: 20–29 years, 0.3%; 30–39 years, 2.0%; 40–49 years, 2.3%; 50–59 years, 13.4%; 60–69 years, 24.9%; 70–79 years, 37.7%; 80–years, 19.4%. Patients with abnormal albuminuria were older, had higher systolic blood pressure, serum uric acid, fasting plasma glucose, HbA1c, electrocardiogram (ECG) voltage, B-type natriuretic peptide (BNP), and C-reactive protein (CRP), and lower eGFR as compared to those without abnormal albuminuria. As expected from the results in Table 1, the presence of abnormal albuminuria was positively correlated with age, systolic blood pressure, fasting plasma glucose, HbA1c, ECG voltage, BNP, and CRP, and inversely correlated with eGFR by univariate analysis (Table 2). However, only systolic blood pressure, BNP, and CRP independently correlated with abnormal albuminuria in a multivariate logistic regression model where variables with P < 0.25 in the univariate analysis were included as independent variables (Table 2). Similar results were obtained using a model where eGFR was adopted instead of serum creatinine as an index of kidney function, without the inclusion of age and gender (data not shown). In a model where ECG voltage was adopted instead of BNP as an independent variable, ECG voltage independently correlated with abnormal albuminuria (odds ratio, 1.54 [95% CI, 1.13–2.10]; P < 0.01). Furthermore, abnormal albuminuria was independently correlated with mean blood pressure or pulse pressure, but not diastolic blood pressure, after adjustment for possible variables (data not shown). In an analysis of a subgroup of patients without diabetes, systolic blood pressure (1.01 [1.00–1.03], P < 0.05) and BNP (1.12 [1.02–1.23], P = 0.02) independently correlated with abnormal albuminuria.
Figure 1. The distribution of urinary albumin in patients with hypertension.
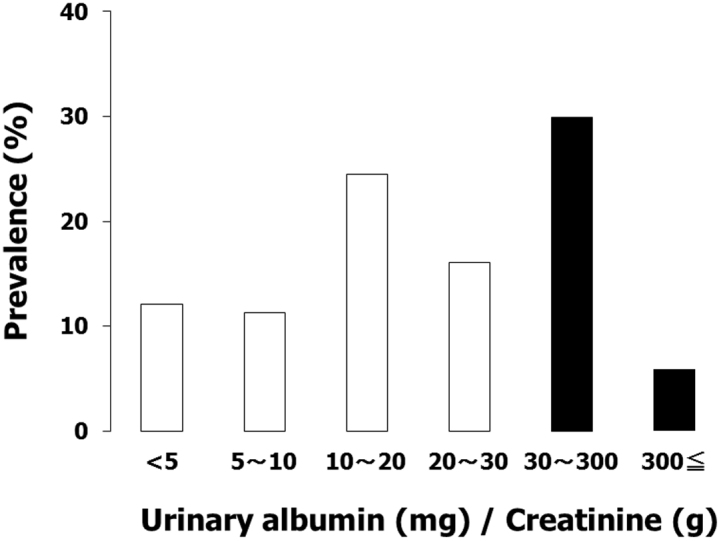
Urinary albumin was detected in 88.3% of hypertensive patients and 35.4% of patients showing abnormal albuminuria.
Table 1. Characteristics of patients with and without abnormal albuminuria.
| All subjects | UACR (mg/g creatinine) | |||
|---|---|---|---|---|
| <30 (n = 226) | ≥30 (n = 124) | P | ||
| Age (years) | 70.0 ± 11.4 | 68.8 ± 11.5 | 72.0 ± 11.1 | 0.01 |
| Gender (male) | 47.3% (164) | 50.7% (114) | 42.6% (52) | 0.16 |
| BMI (kg/m2) | 24.2 ± 3.9 | 24.3 ± 3.6 | 24.0 ± 4.3 | 0.45 |
| SBP (mmHg) | 138.0 ± 20.5 | 136.3 ± 19.0 | 141.0 ± 22.5 | 0.04 |
| DBP (mmHg) | 78.8 ± 12.3 | 78.5 ± 11.9 | 79.4 ± 13.0 | 0.50 |
| Heart rate (bpm) | 74.7 ± 12.4 | 74.2 ± 12.2 | 75.7 ± 12.7 | 0.27 |
| Hemoglobin (g/dl) | 13.3 ± 1.5 | 13.4 ± 1.4 | 13.12 ± 1.6 | 0.18 |
| Serum creatinine (mg/dl) | 0.82 ± 0.24 | 0.80 ± 0.22 | 0.85 ± 0.28 | 0.05 |
| Uric acid (mg/dl) | 5.8 ± 1.5 | 5.7 ± 1.5 | 6.0 ± 1.5 | <0.05 |
| FPG (mg/dl) | 117 ± 29.0 | 115 ± 26.0 | 125 ± 36.9 | <0.01 |
| Triglyceride (mg/dl) | 152 ± 87 | 151 ± 86 | 153 ± 89 | 0.82 |
| HDL cholesterol (mg/dl) | 56.8 ± 15.4 | 56.7 ± 14.6 | 56.8 ± 16.8 | 0.97 |
| LDL cholesterol (mg/dl) | 109 ± 30.4 | 115 ± 34.4 | 118 ± 30.9 | 0.41 |
| HbA1c (%) | 5.6 ± 0.6 | 5.5 ± 0.7 | 5.7 ± 0.9 | 0.04 |
| eGFR (ml/min/1.73 m2) | 65.3 ± 17.2 | 67.2 ± 16.2 | 62.3 ± 18.5 | 0.04 |
| ECG voltage (mV) | 2.86 ± 0.92 | 2.74 ± 0.80 | 3.11 ± 1.09 | <0.01 |
| BNP (ng/l) | 22.4 (11.0, 50.0) | 18.2 (9.6, 38.8) | 26.5 (15.4, 68.3) | <0.001 |
| CRP (mg/dl) | 0.07 (0.02, 0.15) | 0.07 (0.02, 0.14) | 0.09 (0.05, 0.19) | 0.03 |
| Antihypertensive medication | ||||
| RA inhibitors | 68.9% (241) | 66.8% (151) | 72.6% (90) | 0.16 |
| CCBs | 73.7% (258) | 73.9% (167) | 73.4% (91) | 0.51 |
| Diuretics | 39.7% (139) | 35.8% (81) | 46.8% (58) | 0.03 |
| β-blockers | 15.1% (53) | 14.2% (32) | 16.9% (21) | 0.29 |
| α-blockers | 1.7% (6) | 1.8% (4) | 1.6% (2) | 0.64 |
Abbreviations: BMI, body mass index; BNP, B-type natriuretic peptide; CCB, calcium channel blocker; CRP, C-reactive protein; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; RA, renin-angiotensin; SBP, systolic blood pressure.
Values are mean ± s.d., except for BNP and CRP [median (25 and 75 percentiles)].
Table 2. Univariate and multivariate logistic regression analysis demonstrating the relationship of abnormal albuminuria with other variables.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Odds ratio (95%CI) | P | Odds ratio (95%CI) | P | |
| Age (years) | 1.03 (1.01–1.05) | <0.01 | 1.02 (0.99–1.05) | 0.34 |
| Gender (male) | 1.37 (0.88–2.13) | 0.16 | 1.27 (0.65–2.50) | 0.49 |
| BMI (kg/m2) | 0.97 (0.90–1.05) | 0.48 | – | – |
| SBP (mmHg) | 1.01 (1.00–1.02) | <0.05 | 1.01 (1.00–1.03) | 0.04 |
| DBP (mmHg) | 1.01 (0.99–1.02) | 0.50 | – | – |
| Heart rate (bpm) | 1.01 (0.99–1.03) | 0.26 | – | – |
| Hemoglobin (g/dl) | 0.89 (0.75–1.05) | 0.17 | 1.02 (0.82–1.28) | 0.84 |
| Serum creatinine (mg/dl) | 2.39 (0.98–5.82) | 0.05 | 2.10 (0.49–8.97) | 0.31 |
| Uric acid (mg/dl) | 1.16 (1.00–1.35) | 0.05 | 1.09 (0.87–1.36) | 0.47 |
| FPG (mg/dl) | 1.01 (1.00–1.02) | <0.01 | – | – |
| Triglyceride (mg/dl) | 1.00 (0.99–1.00) | 0.82 | – | – |
| HDL cholesterol (mg/dl) | 1.00 (0.99–1.02) | 0.97 | – | – |
| LDL cholesterol (mg/dl) | 1.00 (1.00–1.01) | 0.42 | – | – |
| HbA1c (%) | 1.39 (1.03–1.88) | 0.03 | 1.18 (0.81–1.72) | 0.39 |
| eGFR (ml/min/1.73 m2) | 0.98 (0.97–1.00) | 0.03 | – | – |
| ECG voltage (mV) | 1.54 (1.19–1.98) | <0.01 | ||
| BNP (ng/l) | 1.77 (1.20–2.59) | <0.01 | 1.10 (1.01–1.20) | 0.03 |
| CRP (mg/dl) | 3.64 (1.15–11.5) | 0.03 | 4.31 (1.10–16.8) | 0.03 |
Variables showing a tendency of association with abnormal albuminuria (P < 0.25) in the univariate analysis were included in the multivariate model (eGFR and ECG voltage were excluded from the model due to possible multicollinearity).
Abbreviations: BMI, body mass index; BNP, B-type natriuretic peptide; CRP, C-reactive protein; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
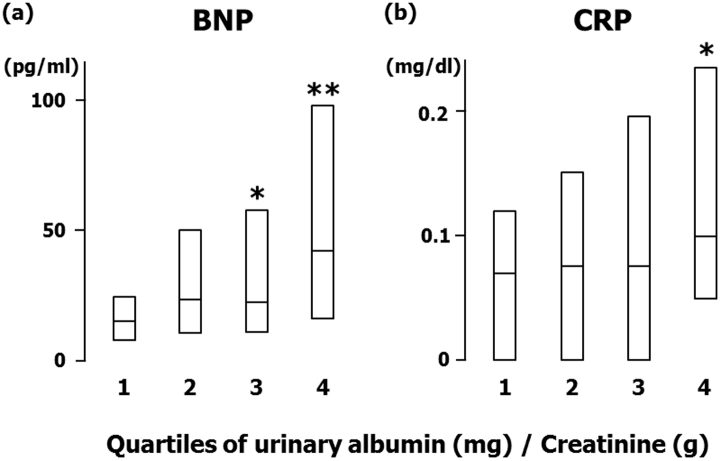
In the next series of analyses, urinary excretion of albumin was taken as a continuous variable and the characteristics of urinary albumin were investigated. Table 3 shows the results of univariate and multivariate regression analysis demonstrating relationships between urinary excretion of albumin and other variables. Univariate analysis revealed that age, systolic blood pressure, serum creatinine, uric acid, fasting plasma glucose, HbA1c, ECG voltage, BNP, and CRP positively correlated with UACR, while eGFR showed an inverse correlation. Then, stepwise method (forward-backward selection) was applied in order to select variables included in a multivariate regression model. Among variables listed in the univariate column in Table 3, systolic blood pressure (standardized coefficient 0.237, P < 0.001), serum creatinine (0.105, P = 0.04), BNP (0.237, P < 0.001), and CRP (0.166, P = 0.001) remained as factors showing significant association with urinary albumin and, thus, were adopted as independent variables in the multivariate regression analysis. Multivariate regression analysis indicated that systolic blood pressure, serum creatinine, BNP, and CRP were the independent predictor of urinary excretion of albumin (Table 3). Similar results were obtained in a regression model where eGFR was adopted instead of creatinine as an index of kidney function and age and gender were not included. ECG voltage was also an independent predictor of urinary albumin (0.202, P < 0.001) in a model where ECG voltage instead of BNP was adopted as an independent variable. BNP and CRP were increased across the quartiles of urinary excretion of albumin (trend, P < 0.0001; Figure 2). In an analysis of a subgroup of patients without diabetes, systolic blood pressure (0.255, P < 0.001), serum creatinine (0.140, P = 0.01), and BNP (0.310, P < 0.001) were independent predictors of urinary albumin.
Table 3. Univariate and multivariate regression analyses demonstrating factors showing correlation with urinary albumin excretion.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| r | P | Standardized coefficient | P | |
| Age (years) | 0.161 | <0.001 | – | – |
| Gender (male) | 0.049 | 0.36 | – | – |
| BMI (kg/m2) | 0.133 | 0.13 | – | – |
| SBP (mmHg) | 0.172 | <0.001 | 0.235 | 0.002 |
| DBP (mmHg) | 0.058 | 0.28 | – | – |
| Heart rate (bpm) | 0.081 | 0.13 | – | – |
| Hemoglobin (g/dl) | −0.081 | 0.16 | – | – |
| Serum creatinine (mg/dl) | 0.157 | <0.01 | 0.103 | 0.04 |
| Uric acid (mg/dl) | 0.177 | <0.001 | – | – |
| FPG (mg/dl) | 0.228 | <0.001 | – | – |
| HDL cholesterol (mg/dl) | −0.023 | 0.67 | – | – |
| LDL cholesterol (mg/dl) | 0.031 | 0.56 | – | – |
| HbA1c (%) | 0.208 | <0.001 | – | – |
| eGFR (ml/min/1.73 m2) | −0.224 | <0.001 | – | – |
| ECG voltage (mV) | 0.204 | <0.001 | – | – |
| BNP (ng/l) | 0.331 | <0.001 | 0.321 | <0.0001 |
| CRP (mg/dl) | 0.182 | 0.001 | 0.156 | 0.04 |
| Use of RA inhibitors | −0.038 | 0.47 | – | – |
Independent variables in the multivariate model were chosen using a stepwise regression analysis where all variables listed in the univariate analysis except eGFR and ECG voltage were included.
Abbreviations: BMI, body mass index; BNP, B-type natriuretic peptide; CRP, C-reactive protein; DBP, diastolic blood pressure; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; RA, renin-angiotensin system; SBP, systolic blood pressure.
Figure 2. Relationship of urinary albumin with (a) B-type natriuretic peptide (BNP) and (b) C-reactive protein (CRP) in hypertensive patients.
The median value [range] of the urinary albumin to creatinine ratio (mg/g creatinine) was 6.0 [0–10.0], 15.0 [11.0–21.0], 28.0 [22.0–45.0], and 112 [45.0–3013] in Quartile 1, 2, 3, and 4, respectively. *P < 0.01, **P < 0.001 vs. quartile 1.
Discussion
Urinary albumin was detected in 88.3% of our hypertensive patients and 35.4% showed abnormal albuminuria (≥30 mg/g Cr). The reported prevalence of microalbuminuria in hypertensive patients based on several studies is 6.6% to 36.1%8,10,11,12. The present results were similar to these published studies, although there seems to be a somewhat higher prevalence of microalbuminuria in Japanese hypertensive patients9. Such a difference may be attributed to different age and/or severity and duration of hypertension between our patient cohort and those studied previously. Indeed, urinary albumin has been correlated with several factors including age and systolic blood pressure9. Furthermore, the higher prevalence of microalbuminuria in Japanese hypertensive patients as compared to Caucasian hypertensive patients suggests ethnic differences in the pathophysiological process involved in microalbuminuria caused by hypertension. Importantly, the prevalence of microalbuminuria is higher in subjects with hypertension than in the general population; around 5% in the general population10,19,20, while higher prevalence is also reported (13.7%)21. Although the difference in the prevalence of microalbuminuria between the present hypertensive patients and general population in the previous reports mostly depends on the difference in blood pressure, patients with hypertension may be at high risk of albuminuria even under appropriate medication.
Only systolic blood pressure, BNP, and CRP were identified as independent predictors of urinary excretion of albumin. Although this was a cross-sectional study and causal relationship between urinary albumin and these factors cannot be elucidated, the results suggest several possibilities. Elevated blood pressure is considered to cause kidney dysfunction, but blood pressure may differentially affect GFR and urinary albumin excretion. Increased systemic blood pressure may cause an increased intraglomerular pressure and, thereby, increase urinary excretion of albumin, whereas deterioration of GFR may reflect some other alterations related to hypertension as well as increased intraglomerular pressure. The concept that the urinary excretion of albumin increases with increasing blood pressure supports the close and independent correlation between urinary albumin and BNP. BNP is secreted from ventricular myocytes in response to increased ventricular filling pressure or volume22,23; thus, an increase in blood pressure could stimulate the secretion of BNP through an increase in cardiac load. Indeed, circulating BNP levels are elevated in patients with hypertension as compared to normotensive subjects24,25. Moreover, since left ventricular load is determined not only by cardiac output and peripheral vascular resistance, but also by the stiffness of conduit arteries and the timing and magnitude of pressure wave reflections, BNP levels, at least in part, reflect the stiffness of conduit arteries. An increase in aortic stiffness, which might be somewhat parallel to the progression of nephrosclerosis, results in pressure-related microvascular damage in the kidney26 and thus could in turn increase the urinary excretion of albumin. Otherwise, an increase in BNP may only reflect left ventricular hypertrophy and a significant correlation between BNP and albuminuria may indicate similar progressive damage to hypertensive organs, in the heart (left ventricular hypertrophy) and kidney (albuminuria). Indeed, there was a significant correlation between ECG voltage and urinary albumin. Significant association between BNP levels and urinary albumin has been previously reported in patients with diabetes27,28. The present study proved this association in non-diabetic hypertensive patients.
Recent studies suggest that albuminuria could be a phenotype of systemic arterial endothelial damage caused by hypertension or diabetes29,30,31, with dysfunction of the endothelium of the glomerular capillary resulting in altered glomerular filtration. Since endothelial dysfunction is triggered by the inflammatory activation of endothelial cells, the close association of CRP, a marker of systemic inflammation, with urinary albumin is convincing. However, CRP was not independently associated with urinary excretion of albumin in a rural Chinese population, although univariate analysis of the same group identified a correlation between CRP and microalbuminuria32. It is possible that the presence of hypertension could clarify the relationship between CRP and urinary albumin. It is well known that inhibitors of the renin-angiotensin system reduce albuminuria and inflammatory biomarkers such as CRP and IL-6, but we did not observe a significant relationship between the use of renin-angiotensin system inhibitors and UACR in this study. In general, inhibitors of the renin-angiotensin system are preferably used in patients with albuminuria, which might have masked urinary albumin-reducing effects of the inhibitors in this cross-sectional analysis. Alternatively, most patients took two or more classes of antihypertensive drugs including renin-angiotensin system inhibitors, which could have masked the beneficial effects of renin-angiotensin system inhibitors.
The present results have some clinical implications. Several clinical trials demonstrated that antihypertensive drugs targeting the renin-angiotensin system reduce urinary excretion of albumin33,34. The present results support the concept that reduction in blood pressure itself has beneficial effects on urinary albumin regardless of antihypertensive drugs used. Furthermore, interventions that prevent hypertensive patients from the progression to atherosclerosis could be useful for reducing urinary albumin. Atherosclerosis is considered to be a chronic inflammation of the arterial wall initiated by inflammatory activation of the endothelium35. Thus, treatment with anti-atherosclerotic or endothelium-protective medications might reduce urinary albumin. Indeed, atorvastatin reduces urinary excretion of albumin in patients with chronic kidney disease36. However, these hypotheses derived from the present study remain unproved until large-scale interventional studies are carried out to clarify the issue. Furthermore, it is not clear whether reduction in urinary albumin brings about beneficial long-term cardiovascular outcomes.
Interpretation of the present results is limited by the cross-sectional nature of our study design. The findings that only support an association between UACR and systolic blood pressure, BNP, and CRP do not yield any conclusions about causation. Indeed, blood pressure was not an independent risk factor of microalbuminuria in the Framingham Offspring Cohort, although age, male sex, diabetes, current smoking, high-normal UACR, and low HDL cholesterol were significant predictors of microalbuminuria. Only well-designed, powerful, and longitudinal studies could attempt to answer the crucial questions of whether an increase in systolic blood pressure, BNP, or CRP promotes urinary excretion of albumin, or vice versa.
In conclusion, the present study demonstrated that the prevalence of abnormal albuminuria was 35.4% and urinary albumin excretion was positively correlated with systolic blood pressure, BNP, and CRP levels in hypertensive patients. These results suggested that not only elevated blood pressure, but also low-grade inflammation and increased stiffness of conduit arterial wall could underlie the urinary excretion of albumin in hypertensive patients.
Methods
Patients and study design
Patients were eligible for inclusion in the study if (1) they had essential hypertension, (2) they were ≥20 years of age, and (3) their antihypertensive medication had not been changed for at least 3 months before enrolment. Blood pressure was measured by a doctor using a validated oscillometric technique (HEM-7070; Omron, Kyoto, Japan) after patients had been seated for 2 min with their back supported and their arms supported at heart level. Proper cuff size was determined based on arm circumference. Three consecutive blood pressure measurements were taken at 2-min intervals and the mean of the second and third measurements was recorded as the blood pressure. All patients were previously diagnosed with hypertension based on clinical blood pressure measured on at least two different occasions. Secondary hypertension was excluded based on medical history and appropriate physical, biochemical, and radiological examinations. Exclusion criteria for the study were secondary hypertension; a history of myocardial infarction, coronary revascularization, heart failure, or stroke; cardiovascular disease; valvular heart disease; atrial fibrillation and severe arrhythmia; overt infection (as assessed by CRP ≥ 1.0 mg/dl) or active inflammatory disease; and malignant disease.
A total of 350 outpatients with essential hypertension under antihypertensive medications in our hypertensive clinic were enrolled in the present study from January, 2007 to December, 2010. Their medical records were retrospectively analyzed and relationships between urinary excretion of albumin and other factors were evaluated. The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Nagoya City University Graduate School of Medical Sciences. All patients provided written informed consent prior to participating in the study.
Biochemical measurement
Plasma BNP levels were measured by radioimmunoassay (Shionoria BNP kit, Shionogi, Osaka, Japan) and CRP levels were determined by latex-enhanced immunonephelometric assay on a BN II analyzer (Dade Behring, Marburg, Germany). The detection limit of the CRP assay was 0.03 mg/dl and CRP values < 0.03 mg/dl were recorded as 0.015 mg/dl. A single-void morning urine sample was collected and urinary albumin was measured by a turbidimetric immunoassay (Autokit Micro Albumin, Wako, Osaka, Japan). The sensitivity limit for albumin was 5 mg/l. The urinary concentration of albumin was taken as 2.5 mg/l in patients with urinary albumin below the sensitivity limit. Urinary excretion of albumin was expressed as the ratio of urinary albumin to urinary creatinine (UACR). Abnormal albuminuria was defined as UACR ≥ 30 mg/g Cr. eGFR was calculated according to the Modification of Diet in Renal Disease equation37 with coefficients modified for Japanese patients38.
Statistical analysis
All analyses were performed using SPSS 17.0 (Chicago, USA). Data in the text and the tables are expressed as mean ± standard deviation except for CRP, BNP, and UACR, which are expressed as the median value with 25 and 75 percentiles. Differences between two groups with a normal distribution were analyzed by unpaired Student's t tests. The UACR, BNP, and CRP were log-transformed before statistical analysis. Yates' corrected chi-square test was used for comparisons between categorical data. Quartiles of the UACR were calculated to investigate the relationship of urinary excretion of albumin with BNP and CRP, and trends across the quartiles were analyzed using ANOVA. Univariate and multivariate logistic regression analyses were performed to examine predictors of abnormal albuminuria. Variables that showed a tendency of association with abnormal albuminuria (P < 0. 25) in univariate analysis were inserted into a multivariate logistic regression model in order to investigate independent predictors of abnormal albuminuria. Univariate and multivariate linear regression analyses were performed in order to investigate the relationships between urinary excretion of albumin and other variables. Stepwise regression analysis was performed in order to select variables adopted in the multivariate model. P < 0.05 was considered statistically significant.
Author Contributions
Y.D. designed the study, S.M. and S.T. collected and analyzed data, and S.M., Y.D. and G.K. interpreted the data. S.M. drafted the manuscript and Y.D., G.K. and N.O. revised the manuscript. All authors reviewed the manuscript.
References
- Kannel W. B. Hypertension as a risk factor for cardiac events: epidemiologic results of long-term studies. J. Cardiovasc. Pharmacol. 21, S27–S37 (1993). [DOI] [PubMed] [Google Scholar]
- Chobanian A. V. et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42, 1206–1252 (2003). [DOI] [PubMed] [Google Scholar]
- Ogihara T. et al. Japanese Society of Hypertension Committee. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens. Res. 32, 3–107 (2009). [PubMed] [Google Scholar]
- Hillege H. L. et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106, 1777–1782 (2002). [DOI] [PubMed] [Google Scholar]
- Sarnak M. J. et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108, 2154–2169 (2003). [DOI] [PubMed] [Google Scholar]
- Manjunath G. et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J. Am. Coll. Cardiol. 41, 47–55 (2003). [DOI] [PubMed] [Google Scholar]
- Matsushita K. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375, 2073–2081 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. et al. MARPLE Study Group. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: final results of a prospective long-term study (MARPLE Study). J. Hypertens. 24, 541–548 (2006). [DOI] [PubMed] [Google Scholar]
- Ohmaru N. et al. Distribution pattern of urine albumin creatinine ratio and the prevalence of high-normal levels in untreated asymptomatic non-diabetic hypertensive patients. Intern. Med. 50, 1621–1629 (2011). [DOI] [PubMed] [Google Scholar]
- Robles N. R. et al. Prevalence of abnormal urinary albumin excretion in a population-based study in Spain: results from the HERMEX Study. Eur. J. Clin. Invest. 42, 1272–1277 (2012). [DOI] [PubMed] [Google Scholar]
- Singh A. K. & Kari J. A. Metabolic syndrome and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 22, 198–203 (2013). [DOI] [PubMed] [Google Scholar]
- Kim Y. S. et al. Prevalence of microalbuminuria and associated risk factors among adult Korean hypertensive patients in a primary care setting. Hypertens. Res. 36, 807–823 (2013). [DOI] [PubMed] [Google Scholar]
- Wachtell K. et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: the LIFE study. Ann. Intern. Med. 139, 901–906 (2003). [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Aoki T., Imahori M. & Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 66, 448–454 (2004). [DOI] [PubMed] [Google Scholar]
- Chico A., Tomas A. & Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine 27, 213–217 (2005). [DOI] [PubMed] [Google Scholar]
- Arnlöv J. et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation 112, 969–975 (2005). [DOI] [PubMed] [Google Scholar]
- Ibsen H. et al. Reduction in albuminuria translates to reduction in cardiovascular events in hypertensive patients: losartan intervention for endpoint reduction in hypertension study. Hypertension 45, 198–202 (2005). [DOI] [PubMed] [Google Scholar]
- Araki S. et al. Reduction in microalbuminuria as an integrated indicator for renal and cardiovascular risk reduction in patients with type 2 diabetes. Diabetes 56, 1727–1730 (2007). [DOI] [PubMed] [Google Scholar]
- Daviglus M. L. et al. Relation of nutrient intake to microalbuminuria in nondiabetic middle-aged men and women: International Population Study on Macronutrients and Blood Pressure (INTERMAP). Am. J. Kidney Dis. 45, 256–266 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konta T. et al. Prevalence and risk factor analysis of microalbuminuria in Japanese general population: the Takahata study. Kidney Int. 70, 751–756 (2006). [DOI] [PubMed] [Google Scholar]
- Tanaka S., Takase H., Dohi Y. & Kimura G. The prevalence and characteristics of microalbuminuria in the general population: a cross-sectional study. BMC Res. Notes. 6, 256 (2013) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukoyama M. et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Invest. 87, 1402–1412 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y. et al. Measurement of plasma brain natriuretic peptide level as a guide for cardiac overload. Cardiovasc. Res. 51, 585–591 (2001). [DOI] [PubMed] [Google Scholar]
- Mukoyama M. et al. Human brain natriuretic peptide, a novel cardiac hormone. Lancet 335, 801–802 (1990). [DOI] [PubMed] [Google Scholar]
- Buckley M. G. et al. Plasma concentrations and comparisons of brain and atrial natriuretic peptide in normal subjects and in patients with essential hypertension. J. Hum. Hypertens. 7, 245–250 (1993). [PubMed] [Google Scholar]
- O'Rourke M. F. & Safar M. E. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46, 200–204 (2005). [DOI] [PubMed] [Google Scholar]
- Yano Y. et al. Plasma brain natriuretic peptide levels in normotensive noninsulin-dependent diabetic patients with microalbuminuria. J. Clin. Endocrinol. Metab. 84, 2353–2356 (1999). [DOI] [PubMed] [Google Scholar]
- Nagai T., Imamura M., Inukai T. & Mori M. Brain natriuretic polypeptide in type 2 NIDDM patients with albuminuria. J. Med. 32, 169–180 (2001). [PubMed] [Google Scholar]
- Pedrinelli R. et al. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet. 344, 14–18 (1994). [DOI] [PubMed] [Google Scholar]
- Malik A. R., Sultan S., Turner S. T. & Kullo I. J. Urinary albumin excretion is associated with impaired flow- and nitroglycerin-mediated brachial artery dilatation in hypertensive adults. J. Hum. Hypertens. 21, 231–238 (2007). [DOI] [PubMed] [Google Scholar]
- Silva A. M. et al. Microalbuminuria is associated with impaired arterial and venous endothelium-dependent vasodilation in patients with Type 2 diabetes. J. Endocrinol. Invest. 33, 696–700 (2010). [DOI] [PubMed] [Google Scholar]
- Jiang L. et al. Metabolic syndrome, C-reactive protein and microalbuminuria in a rural Chinese population: a cross-sectional study. BMC Nephrol. 14, 118 (2013) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselbergs F. W. et al. Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVEND IT) Investigators. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation 110, 2809–2816 (2004). [DOI] [PubMed] [Google Scholar]
- Bakris G. L. et al. Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: results of the IMPROVE trial. Kidney Int. 72, 879–885 (2007). [DOI] [PubMed] [Google Scholar]
- Glass C. K. & Witztum J. L. Atherosclerosis. the road ahead. Cell 104, 503–516 (2001). [DOI] [PubMed] [Google Scholar]
- Bianchi S., Bigazzi R., Caiazza A. & Campese V. M. A controlled, prospective study of the effects of atorvastatin on proteinuria and progression of kidney disease. Am. J. Kidney Dis. 41, 565–570 (2003). [DOI] [PubMed] [Google Scholar]
- Levey A. S. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130, 461–470 (1999). [DOI] [PubMed] [Google Scholar]
- Matsuo S. et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 53, 982–992 (2009). [DOI] [PubMed] [Google Scholar]