Abstract
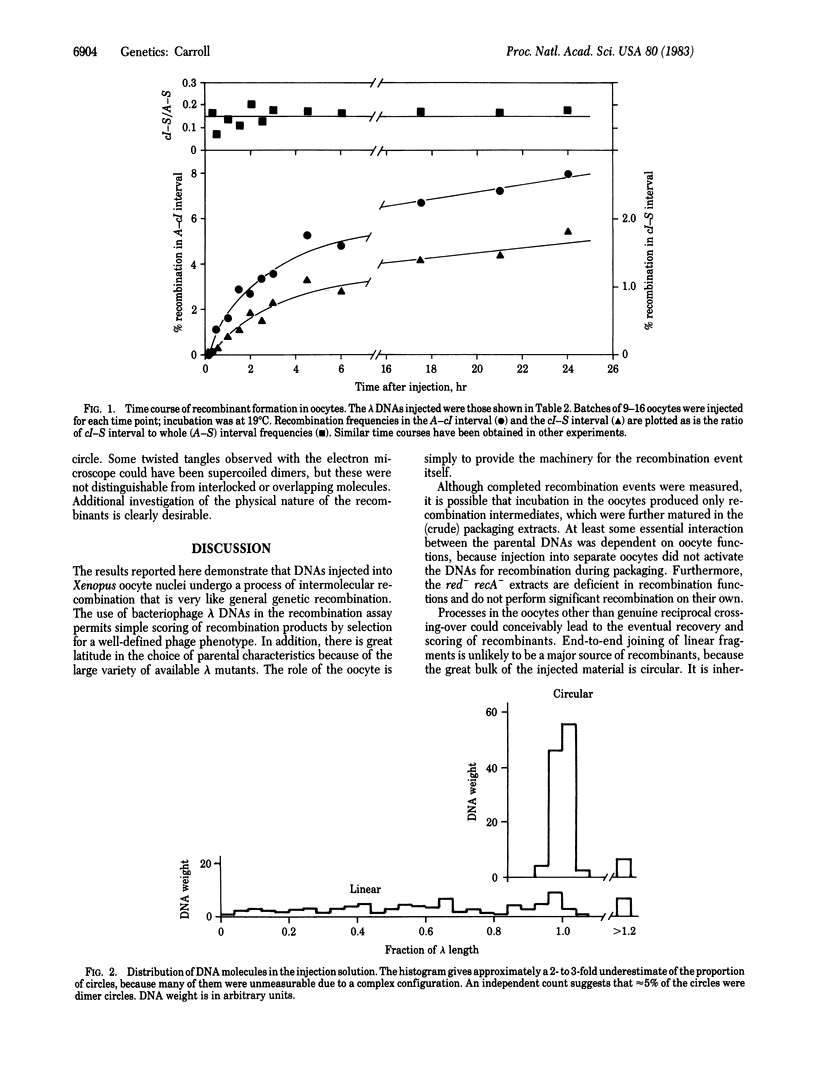
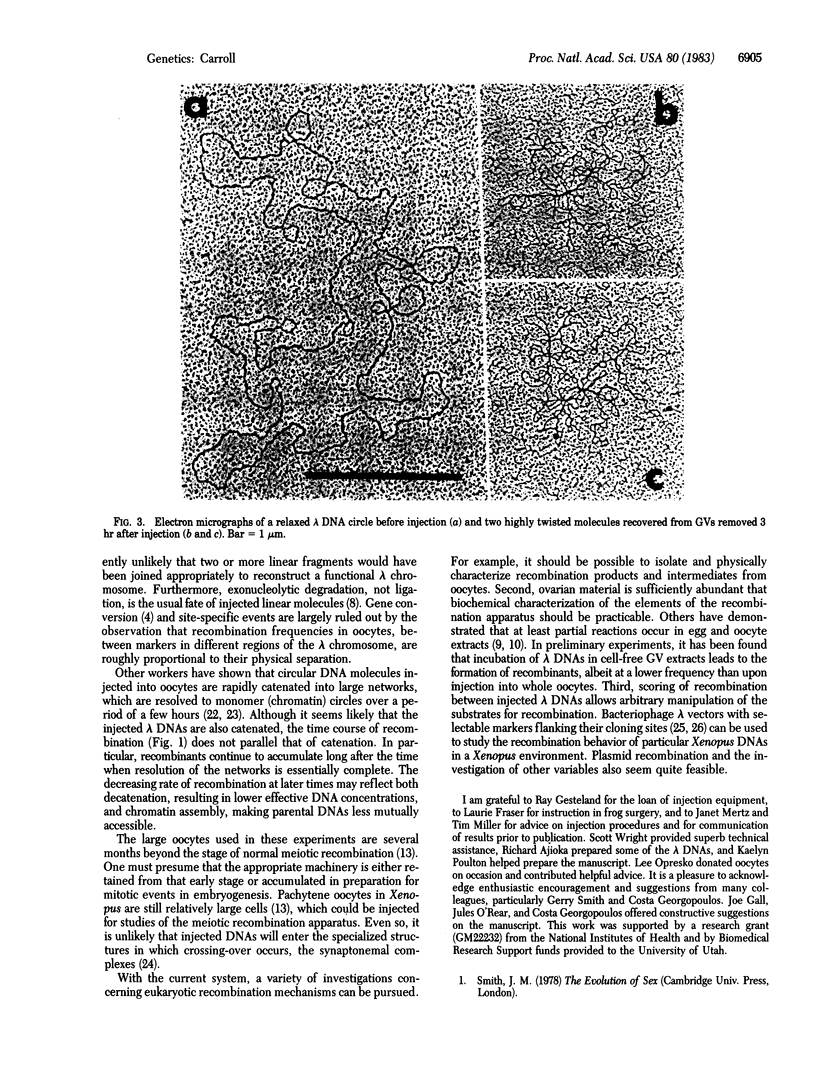
Pairs of genetically marked bacteriophage lambda DNAs have been injected into Xenopus laevis oocyte nuclei. After suitable incubation, DNA was recovered and packaged into phage particles in vitro. When these were plated onto a selective host, phage recombinant for parental markers were observed. Recombination was dependent on both parents being present in the same oocyte nucleus and was roughly proportional to the physical separation of the markers. Thus, the oocytes appear to contain the machinery necessary for performing typical genetic recombination. This system offers a great deal of scope and flexibility for future studies of recombination mechanisms at the molecular level in vertebrates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S., Carpenter A. T., Esposito M. S., Esposito R. E., Sandler L. The genetic control of meiosis. Annu Rev Genet. 1976;10:53–134. doi: 10.1146/annurev.ge.10.120176.000413. [DOI] [PubMed] [Google Scholar]
- Benbow R. M., Krauss M. R. Recombinant DNA formation in a cell-free system from Xenopus laevis eggs. Cell. 1977 Sep;12(1):191–204. doi: 10.1016/0092-8674(77)90197-0. [DOI] [PubMed] [Google Scholar]
- Bird A. P. A study of early events in ribosomal gene amplification. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1179–1183. doi: 10.1101/sqb.1978.042.01.118. [DOI] [PubMed] [Google Scholar]
- Carroll D., Ajioka R. S., Georgopoulos C. Bacteriophage lambda cloning vehicles for studies of genetic recombination. Gene. 1980 Aug;10(3):261–271. doi: 10.1016/0378-1119(80)90055-4. [DOI] [PubMed] [Google Scholar]
- Carroll D., Ajioka R. S. Recombination of a eukaryotic DNA in bacteria. Gene. 1980 Aug;10(3):273–281. doi: 10.1016/0378-1119(80)90056-6. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Echols H., Murialdo H. Genetic map of bacteriophage lambda. Microbiol Rev. 1978 Sep;42(3):577–591. doi: 10.1128/mr.42.3.577-591.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Mortimer R., Lusnak K., Tavares F. Meiotic gene conversion: a signal of the basic recombination event in yeast. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1325–1341. doi: 10.1101/sqb.1979.043.01.152. [DOI] [PubMed] [Google Scholar]
- Gandini Attardi D., Mattoccia E., Tocchini-Valentini G. P. Formation of branched DNA structures by Xenopus laevis oocyte extract. Nature. 1977 Dec 22;270(5639):754–756. doi: 10.1038/270754a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Hohn B. DNA as substrate for packaging into bacteriophage lambda, in vitro. J Mol Biol. 1975 Oct 15;98(1):93–106. doi: 10.1016/s0022-2836(75)80103-3. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Moriya K., Matsumoto T. In vitro study of illegitimate recombination: involvement of DNA gyrase. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):399–408. doi: 10.1101/sqb.1981.045.01.054. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Clarkson S. G., Telford J. L., Birnstiel M. L. Transcription of xenopus tDNAmet1 and sea urchin histone DNA injected into the Xenopus oocyte nucleus. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1077–1082. doi: 10.1101/sqb.1978.042.01.108. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Miller T. J. In vivo catenation and decatenation of DNA. Mol Cell Biol. 1983 Jan;3(1):126–131. doi: 10.1128/mcb.3.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B. Mechanisms of chromosome synapsis at meiotic prophase. Int Rev Cytol. 1973;35:117–134. doi: 10.1016/s0074-7696(08)60353-8. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Smith G. P. Unequal crossover and the evolution of multigene families. Cold Spring Harb Symp Quant Biol. 1974;38:507–513. doi: 10.1101/sqb.1974.038.01.055. [DOI] [PubMed] [Google Scholar]
- Stephens D. L., Miller T. J., Silver L., Zipser D., Mertz J. E. Easy-to-use equipment for the accurate microinjection of nanoliter volumes into the nuclei of amphibian oocytes. Anal Biochem. 1981 Jul 1;114(2):299–309. doi: 10.1016/0003-2697(81)90485-1. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Laskey R. A., Finch J., Gurdon J. B. Selective DNA conservation and chromatin assembly after injection of SV40 DNA into Xenopus oocytes. Dev Biol. 1978 May;64(1):178–188. doi: 10.1016/0012-1606(78)90069-6. [DOI] [PubMed] [Google Scholar]



