Background: β1-AR trafficking is regulated by its PDZ that binds to a complex composed of SAP97, AKAP79, and PKA.
Results: Type I PDZ-mediated recycling of the β1-AR was dependent on PKA-mediated phosphorylation of Ser312, whereas trafficking by non-PDZs was PKA-independent.
Conclusion: Divergent trafficking pathways were involved in β1-AR recycling by type I PDZs versus non-PDZs.
Significance: These results provide a roadmap for GPCR trafficking pathways.
Keywords: Adrenergic Receptor, Confocal Microscopy, G Protein-coupled Receptor (GPCR), Protein Kinase A (PKA), Trafficking
Abstract
The β1-adrenergic receptor (β1-AR) is a target for treatment of major cardiovascular diseases, such as heart failure and hypertension. Recycling of agonist-internalized β1-AR is dependent on type I PSD-95/DLG/ZO1 (PDZ) in the C-tail of the β1-AR and on protein kinase A (PKA) activity (Gardner, L. A., Naren, A. P., and Bahouth, S. W. (2007) J. Biol. Chem. 282, 5085–5099). We explored the effects of point mutations in the PDZ and in the activity of PKA on recycling of the β1-AR and its binding to the PDZ-binding protein SAP97. These studies indicated that β1-AR recycling was inhibited by PKA inhibitors and by mutations in the PDZ that interfered with SAP97 binding. The trafficking effects of short sequences differing in PDZ and SAP97 binding were examined using chimeric mutant β1-AR. β1-AR chimera containing the type I PDZ of the β2-adrenergic receptor that does not bind to SAP97 failed to recycle except when serine 312 was mutated to aspartic acid. β1-AR chimera with type I PDZ sequences from the C-tails of aquaporin-2 or GluR1 recycled in a SAP97- and PKA-dependent manner. Non-PDZ β1-AR chimera derived from μ-opioid, dopamine 1, or GluR2 receptors promoted rapid recycling of chimeric β1-AR in a SAP97- and PKA-independent manner. Moreover, the nature of the residue at position −3 in the PDZ regulated whether the β1-AR was internalized alone or in complex with SAP97. These results indicate that divergent pathways were involved in trafficking the β1-AR and provide a roadmap for its trafficking via type I PDZs versus non-PDZs.
Introduction
Trafficking of G protein-coupled receptors (GPCRs)2 is a process initiated by sustained exposure of GPCR to high concentrations of their respective agonists (1, 2). This process is initiated by coordinated activities of G protein receptor kinase (GRK) and β-arrestin to promote the internalization of the agonist-activated GPCR (3, 4). Subsequently, internalized GPCRs are sorted between divergent recycling and lysosomal pathways that determine the functional outcome of trafficking on the GPCR (1). For example, some sequestered GPCRs, such as the β1-AR, β2-adrenergic receptor (β2-AR), μ-opioid receptor, and others, recycle to the plasma membrane rapidly and efficiently (1, 2). In contrast, other GPCRs, such as δ-opioid receptor, vasopressin 2 receptors, and others, do not recycle but are diverted instead to late endosomes/lysosomes, where they are eventually degraded (1, 2). The intricacies of molecular sorting of GPCR are still obscure; thus, a better characterization of GPCR sorting domains and their role in regulating the trafficking itinerary of the GPCR is warranted.
A primary region involved in sorting of internalized GPCR between recycling and lysosomal retention was the type I PDZ sequence found in the extreme C terminus of many recycling GPCRs (5). Type I PDZ domains are sequences that correspond to X(S/T)XØ motifs, where X at positions (P) −1 and −3 is any amino acid, S/T at P = −2 corresponds to either serine or threonine, and Ø at P = 0 is a hydrophobic amino acid (6). In the case of the β2-AR, the tripeptide SLL and multiple variations of this sequence that conformed to the type I PDZ architecture were sufficient for recycling of the β2-AR and in promoting the recycling of non-recycling GPCR, such as the δ-opioid receptor (7, 8). These results indicated that type I PDZ sequences were interchangeable between recycling GPCR and that they provided a primary recycling signal to non-recycling GPCR (7, 8).
Type I PDZ ligands mediate their effects on recycling by binding to PDZ-binding domains found in many scaffolding proteins (9). The type I PDZ in the C terminus of the β2-AR interacted with the PDZ-binding domain of Na+/H+-exchange regulatory factor (NHERF)-1, and NHERF-2 (10). Similarly, the type I PDZ in the β1-AR interacted with PDZ domain-containing proteins related to the membrane-associated guanylate kinase family, such as PSD-95, SAP97, MAGI (I–III), GIPC, and CAL (9–12). Among these PDZ-binding proteins, SAP97 played a prominent role in mediating the recycling of the agonist-internalized β1-AR (11). The effect of SAP97 on recycling was dependent on its bipartite binding to the β1-AR PDZ and to the protein kinase A anchoring protein 79 (AKAP79)-PKA complex (13). Thus, the interaction between the β1-AR and SAP97 created a SAP97-AKAP79-PKA complex that was tethered by SAP97 to the type I PDZ of the β1-AR (11, 13–14).
In addition to the type I PDZ, the recycling of the β1-AR was dependent on a PKA substrate serine at position 312 in the third intracellular loop of the β1-AR (15). Cross-talk between PKA anchored at the PDZ and Ser312 was essential for recycling of the β1-AR (11), whereas inactivation of the PDZ or delocalization of PKA from AKAP79 interfered with recycling of the β1-AR (11, 14–16). Moreover, mutagenesis of Ser312 to alanine prevented the recycling of the β1-AR even in the presence of the active type I PDZ complex, whereas mutagenesis of Ser312 to the phosphoserine mimic aspartic acid (S312D) promoted the recycling of the β1-AR even when its type I PDZ was inactivated (11, 14–16). Therefore, coordinated bipartite regulation between the PDZ and Ser312 was involved in imparting the trafficking signal (i.e. phospho-Ser312) onto the β1-AR (11, 14–16).
The requirement for cross-talk between the PDZ and other domains in the GPCR would suggest that although degenerate type I PDZ sequences were sufficient to promote the recycling of some GPCR, there might be instances where recycling required cross-talk between the constituents of the PDZ complex and other trafficking domains. We tested this hypothesis by point mutations within the β1-AR PDZ and by replacement of 10 amino acids in the C-tail of the β1-AR with corresponding sequences in recycling GPCR. The results indicated that most but not all type I PDZ sequences promoted the recycling of chimeric β1-AR in a PKA- and SAP97-dependent manner. In addition, we determined that chimeric β1-ARs that do not contain type I PDZs but rather contain other recycling sequences would recycle in a PKA- and SAP97-independent manner. Therefore, we propose that trafficking of the β1-AR in the context of its natural or other type I PDZs proceeded by different mechanisms than did its trafficking by non-PDZ containing sequences.
EXPERIMENTAL PROCEDURES
cDNA Constructs, siRNA, and Reagents
Modifications in the β1-AR were generated by PCR to introduce a FLAG sequence (DYKDDDDK) upstream of the N-terminal region and the desired mutation in the C terminus of the β1-AR. siRNA sequences to human SAP97, 5′-GATATCCAGGAACATAAAT-3′, or its control 5′-ccataatacaaGgtataa-3′ were cloned in the pcDNA 6.2-GW/EmGFP-miR vector (Invitrogen). Human embryonic kidney-293 (HEK-293) cells stably expressing moderate β1- or β2-AR densities of between 0.9 ± 0.04 and 1.3 ± 0.05 pmol of receptor/mg protein were used in these studies. Cy3-conjugated anti-FLAG (M2) antibody was from Sigma, anti-SAP97 antibody was from Enzo Life Sciences (Farmingdale, NY), and anti-β1-AR (sc-567) and anti-β-actin (sc-47778) antibodies were from Santa Cruz Biotechnology, Inc. H-89 and myristoylated PKA inhibitor 14-22 amide (mPKI) were from EMD-Millipore (Billerica, MA).
Acid Strip Confocal Recycling Microscopy and Dual Microscopy Protocols
Cells were labeled with Cy3-labeled anti-FLAG IgG (4 μg/ml) for 1 h at 37 °C in the absence or presence of 3 μm H-89 or mPKI to ensure selective inhibition of PKA (17). Cy3-labeled cells were washed in HBSS and incubated in culture medium containing 0.1 mm ascorbic acid. Cells were exposed to 10 μm isoproterenol for 30 min to promote the internalization of the β-AR and then washed in 0.5 m NaCl, 0.2 m acetic acid (pH 3.5) for 4 min on ice to remove antibody bound to extracellular GPCR (13, 18–19). Recycling of internal GPCR was initiated by culturing the cells with a 100 μm concentration of the β-antagonist alprenolol at 37 °C for 15, 30, or 60 min, followed by fixing the slides in 4% paraformaldehyde and 4% sucrose (pH 7.4). At the completion of recycling (i.e. the 60-min slide), the slide was exposed to a second acid wash to strip the Cy3-labeled antibody from the externalized receptor population and then fixed.
Confocal fluorescence microscopy was performed on coded slides, and images were acquired using an Olympus FV1000 confocal microscope (11, 13–14). To calculate the recycling kinetics, a boundary was drawn around the inner circumference of cells using ImageJ software (National Institutes of Health). Pixel intensities inside the boundary of isoproterenol-treated cells were set arbitrarily as 100%. The percentage ratios of pixel intensities inside the boundary of alprenelol-treated cells to that of isoproterenol-treated cells were calculated. The time constant (τ) for receptor recycling was determined by fitting the percentage internal receptor pixel data to a single exponential decay function of time from the equation, y = yo + Ae−1/τ, where yo and A are constants.
Effect of Isoproterenol on the Distribution of the β1-AR and SAP97 in HEK-293 Cells
Cells stably expressing equivalent levels of FLAG WT β1-AR or FLAG β1-GluR1(10) were grown in culture plates with a poly-l-lysine-treated glass window. The next day, the cells were transfected with SAP97-GFP. After 24–36 h, the cells were labeled with Cy3-conjugated anti-FLAG IgG for 60 min at 10 °C. The cells were maintained at this temperature and visualized by confocal microscopy (GFP, λex = 488 nm, λem = 505–530 nm; Cy3, λex = 543 nm, λem = 560 nm) using the multitracking configuration of the microscope. Then the culture plates were warmed on the microscope stage to 37 °C and treated with 10 μm isoproterenol and visualized every 10 min for 30 min. Finally, the medium was aspirated and replaced with warm culture medium containing 100 μm alprenolol and imaged after 60 min for Cy3 (pseudo-red) and GFP (pseudo-green) simultaneously.
Co-immunoprecipitation and β-AR Degradation Assays
Co-immunoprecipitations between SAP97 and the various FLAG-β1-AR or β2-AR constructs were performed as described previously (11, 13–14). Briefly, equal amounts of cellular protein lysates were incubated with preimmune IgG-agarose beads (control) or with anti-FLAG IgG-agarose beads for 4 h at 4 °C. Eluted immune complexes were separated by SDS-polyacrylamide gel electrophoresis and subjected to Western blotting to detect the FLAG epitope or SAP97.
In degradation assays, HEK-293 cells stably expressing equivalent amounts of the indicated FLAG-tagged β-AR (between 0.9 and 1.3 pmol of β1-AR/mg protein) were cultured in complete culture medium containing 0.1 mm ascorbic acid (7). The cells were exposed to buffer or to 10 μm isoproterenol for 0, 3, and 6 h. At each time point, cell membranes were extracted as described (7). Equal amounts of protein lysates (∼30 μg of protein) were subjected to Western blotting, and band intensities of FLAG-epitopes were quantified by direct imaging. Each nitrocellulose filter was then stripped and reprobed with anti-β-actin IgG to correct for minor differences in protein loading or transfer.
Statistics
Data are presented as means ± S.E., except where indicated. For comparison between two groups of data, Student's unpaired t test was used to determine significance. Multiple groups were compared by one-way analysis of variance with Newman-Keuls post-tests using prism software (Graphpad, Inc., San Diego, CA). Results were considered significant at p < 0.05.
RESULTS
Characterization of the Amino Acids in the β1-AR PDZ That Were Involved in Recycling of the Human β1-AR
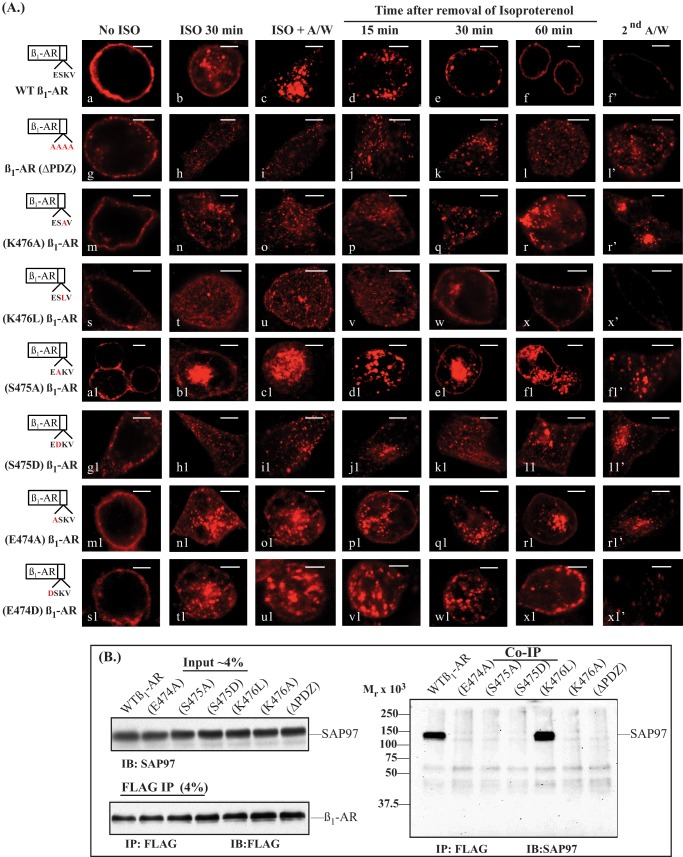
HEK-293 cells expressing WT β1-AR, β1-AR with point mutations in the type I PDZ between amino acids 474 and 477 or β1-AR with an inactivated PDZ (β1-ARΔPDZ) were used to determine the effect of these mutations on trafficking of the β1-AR (Fig. 1). Generally, mutagenesis of one or all of the amino acids in the PDZ domain of the β1-AR did not interfere with its membranous distribution (Fig. 1A, a, g, m, s, a1, g1, m1, and s1). Isoproterenol resulted in rapid endocytosis of membranous β1-AR into intracellular vesicles (Fig. 1A, b, h, n, t, b1, h1, n1, and t1). Exposing the cells to an acetic acid/NaCl solution that stripped surface-exposed antibody (ISO + A/W), revealed the internal distribution of β1-AR (Fig. 1A, images c, i, o, u, c1, i1, o1, and u1). The effect of each point mutation on recycling was initiated by adding the β-adrenergic receptor antagonist alprenolol to promote the recycling of internal receptors. In cells expressing WT β1-AR, agonist-internalized β1-AR recycled back to the cell membrane with an estimated half-life (t0.5) of recycling of 21 ± 6 min (Fig. 1A, d–f, and Table 1). These receptors were externalized and inserted properly in the membrane because a second acid wash stripped >90% of the recycled Cy3 pixels (Fig. 1A, f′). As expected, mutagenesis of the type I PDZ amino acids in the β1-AR to alanine (β1-ARΔPDZ) prevented its recycling (Fig. 1A, j–l′), and its intracellular pixels were not stripped by the second acid wash (11, 13, 14).
FIGURE 1.
Characterization of the residues in the PDZ domain of the β1-AR that are involved in recycling and in binding to SAP97. A, HEK-293 cells expressing the FLAG-tagged WT β1-AR (a–f′) and β1-AR constructs in which the entire PDZ was inactivated (β1-ARΔPDZ; g–l′) or single point mutations in the PDZ (m–x1′) were plated on poly-l-lysine-covered glass slides. Cells were prelabeled for 1 h with Cy3-anti-FLAG antibody and fixed (a, g, m, s, a1, g1, m1, and s1). The rest of the slides were exposed to 10 μm isoproterenol for 30 min (b, h, n, t, b1, h1, n1, and t1) and then acid-washed and fixed (c, i, o, u, c1, i1, o1, and u1). The rest of the slides were subjected to recycling conditions for the indicated time period and then fixed. Slides incubated with 100 μm alprenolol for 1 h were exposed to acid wash and then fixed (f′, l′, r′, x′, f1′, l1′, r1′, and x1′). The distribution of fluorescent pixels was obtained using confocal microscopy, and the colors shown are pseudo-colors. Scale bars, 5 μm. B, HEK-293 cells were transiently transfected with FLAG-tagged β1-AR and rat SAP97 cDNAs for 2 days. The cells were lysed, and about 4% of the crude cell lysate (input) was subjected to Western blotting (IB) with anti-SAP97 IgG to verify that equal amounts of SAP97 were loaded onto the resin. Then equal amounts of the remaining cell lysate proteins were incubated with anti-FLAG IgG resin, followed by washing and eluting the resin with Laemmli sample buffer. The eluates were divided into two portions. One portion representing 4% of the eluate by volume was probed with anti-FLAG antibody to verify that equal amounts of the β1-AR were bound and eluted from the affinity resin. The rest of the FLAG IPs, representing about 90% of resin eluates, were probed with the anti-SAP97 antibody (n = 4). A/W, acid wash.
TABLE 1.
Recycling kinetics of ß1-AR and ß2-AR chimera in HEK-293 cells
| Recycling β-AR construct | Additional conditions | t0.5 for recycling | Statistical significance |
|---|---|---|---|
| min | |||
| WT β1-AR | 21 ± 6 | ||
| WT β1-AR | +3 μm H-89/mPKI | No recycling | NDa |
| WT β1-AR | +Scrambled shRNA | 22 ± 8 | p > 0.05b |
| WT β1-AR | +SAP97 shRNA | No recycling | |
| (K476L) β1-AR | 24 ± 7 | p > 0.05b | |
| (E474D) β1-AR | 38 ± 9 | p < 0.01b | |
| (V477I) β1-AR | 19 ± 7 | p > 0.05b | |
| (V477L) β1-AR | 41 ± 10 | p < 0.01b | |
| WT ß2-AR | 25 ± 6 | ||
| WT β2-AR | +Scrambled shRNA | 27 ± 7 | p > 0.05c |
| WT β2-AR | +SAP97 shRNA | 28 ± 8 | p > 0.05c |
| WT β2-AR | +3 μm H-89 | 23 ± 7 | p > 0.05c |
| WT β2-AR | +3 μm mPKI | 25 ± 8 | p > 0.005 |
| (S312D) β1/β2-AR(10) | 20 ± 7 | p > 0.05b | |
| (S312D) β1/β2-AR(10) | +Scrambled shRNA | 21 ± 7 | p > 0.05b |
| (S312D) β1/β2-AR(10) | +SAP97 shRNA | 23 ± 8 | p > 0.05b |
| (S312D) β1/β2-AR(10) | +3 μm H-89 | 25 ± 8 | p > 0.05b |
| (S312D) β1/β2-AR(10) | +3 μm mPKI | 24 ± 7 | p > 0.05b |
| β1-AR aquaporin(10) | 25 ± 8 | p > 0.05b | |
| β1-AR aquaporin(10) | +Scrambled shRNA | 29 ± 9 | p > 0.05b |
| β1-AR aquaporin(10) | +SAP97 shRNA | No recycling | |
| β1-AR GluR1(10) | 22 ± 7 | p > 0.05b | |
| β1-AR GluR1(10) | +Scrambled shRNA | 27 ± 9 | p > 0.05b |
| β1-AR GluR1(10) | +SAP97 shRNA | No recycling | |
| β1-AR μ-opioid(12) | 21 ± 6 | p > 0.05b | |
| β1-AR μ-opioid(12) | +3 μm H-89 | 20 ± 6 | p > 0.05b |
| β1-AR GluR2(10) | 20 ± 6 | p > 0.05b | |
| β1-AR GluR2(10) | +3 μm H-89 | 22 ± 7 | p > 0.05b |
| β1-AR dopamine R1 | 27 ± 8 | p > 0.05b | |
| β1-AR dopamine R1 | +3 μm H-89 | 30 ± 11 | p > 0.05b |
a Not determined.
b Relative to t0.5 of WT β1-AR recycling.
c Relative to t0.5 of WT β2-AR recycling.
To examine whether the character of the residue at P = −1 would affect the recycling of the β1-AR, Lys476 was mutated to Ala (K476A) and to Leu (K476L). (K476A) β1-AR did not recycle, whereas (K476L) β1-AR recycled normally with an estimated t0.5 of 24 ± 7 min and was reinserted into the cell membrane (Fig. 1A, p–r′ and v–x′). Thus, the character of the mutated residues at P = −1 in the β1-AR seemed to affect its trafficking.
As expected, the Ser475 at P = −2 to Ala mutant ((S475A) β1-AR) did not recycle (Fig. 1A, d1–f1′). To find out if the phosphorylation of this serine would affect the trafficking of the β1-AR, Ser475 was mutated to its phosphoserine mimic aspartic acid ((S475D) β1-AR). The (S475D) β1-AR mutant did not recycle (Fig. 1A, j1-l1′). These results indicated that a serine and perhaps a threonine residue at P = −2 were essential for the activity of this PDZ.
Mutagenesis of Glu474 at P = −3 to Ala ((E474A) β1-AR) inhibited the recycling of this β1-AR mutant (Fig. 1A, p1–r1′). This was surprising because crystal structures of PDZ ligands bound to PDZ-binding domains have not implicated this amino acid in binding to the PDZ binding pocket (20–22). To determine the preferred character of the residue at P = −3, Glu474 was mutated to arginine ((E474R) β1-AR) and leucine ((E474L) β1-AR), but both of these β1-AR mutants did not recycle (data not shown). However, the β1-AR with a Glu474 to aspartic acid mutation ((E474D) β1-AR) recycled slowly yet efficiently, with an estimated t0.5 of recycling of 38 ± 9 min (Fig. 1A, v1–x1′, and Table 1).
Binding of β1-AR PDZ Point Mutants to SAP97
The recycling of the β1-AR is dependent on SAP97 binding to the type I PDZ of the β1-AR (11). Co-immunoprecipitations between the full-length β1-AR mutants described in Fig. 1A and SAP97 indicated that SAP97 was co-immunoprecipitated by the WT β1-AR and by (K476L) β1-AR only (Fig. 1 B). The (K476A) β1-AR construct and the constructs in which the residues at P = −2 and −3 were mutated failed to bind SAP97. Thus, the results of recycling in Fig. 1A and co-immunoprecipitations in Fig. 1B were in agreement, indicating that those β1-AR PDZ point mutants that bound SAP97 were the ones that recycled.
Effect of the Residue at P = 0 on Trafficking and on Binding of the β1-AR to SAP97
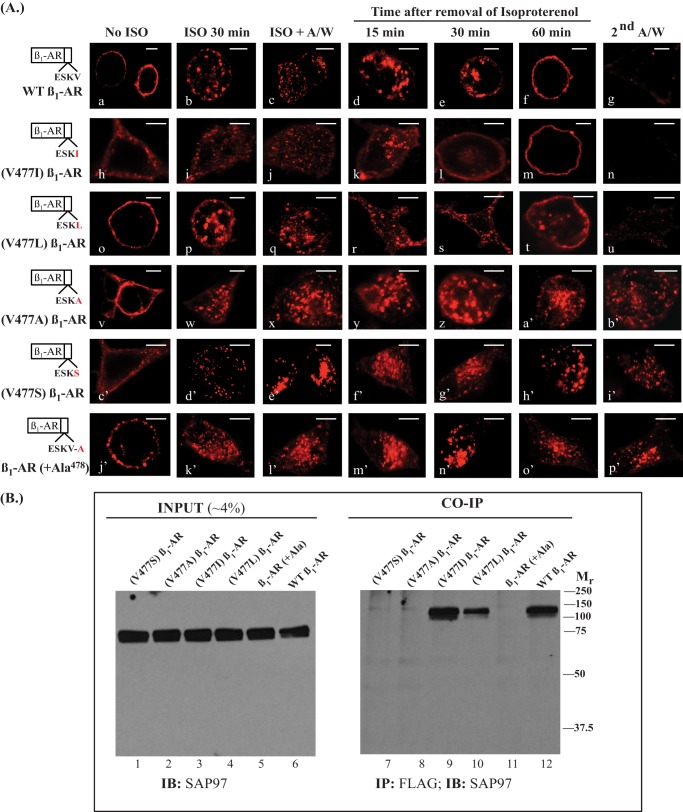
As noted earlier, the preferred amino acids at P = 0 were hydrophobic amino acids, such as Ile, Leu, and Val (5). However, there are reports that mutagenesis of the terminal Val of the β1-AR to Leu (ESKL) produced robust association with NHERF-1, which does not bind to the β1-AR PDZ (12, 23). β1-AR chimera in which Val477 was mutated to Leu ((V477L) β1-AR) or to Ile ((V477I) β1-AR) recycled with different recycling kinetics (Fig. 2A, h–n and o–u). The t0.5 for recycling of the internalized (V477I) β1-AR construct was 19 ± 7 min, which was not significantly different from t0.5 for recycling of the WT β1-AR (15). The recycling of the internalized (V477L) β1-AR construct was incomplete within 1 h. We estimated that ∼63 ± 16% of internal (V477L) β1-AR pixels recycled in the first 1 h. However, (V477L) β1-AR recycled completely after 2 h, with an estimated t0.5 of 41 ± 10 min, which was significantly slower than the t0.5 of WT β1-AR (p < 0.05, n = 4).
FIGURE 2.
Identification of the residues at position 477 in the β1-AR that are involved in recycling and binding to SAP97. A, HEK-293 cells expressing the point mutations of the valine residue at position 477 in the β1-AR were plated on poly-l-lysine-covered glass slides. Cells were subjected to internalization and recycling as described in the legend to Fig. 1A. Scale bars, 5 μm. B, HEK-293 cells were transiently transfected with FLAG-tagged β1-AR and SAP97 cDNAs for 2 days. Cell lysates were prepared, and 4% of input lysate proteins were probed by Western blotting (IB) for the expression of SAP97 (lanes 1–6). Then equal amounts of the rest of the lysates were incubated with anti-FLAG IgG resin, eluted, and subjected to Western blotting for the co-IP of SAP97 (lanes 7–12). A/W, acid wash.
Mutagenesis of Val477 to either Ala ((V477A) β1-AR) or Ser ((V477S) β1-AR) inhibited the recycling of these β1-AR mutants (Fig. 2A, v–b′ and c′–i′). Moreover, the addition of a single Ala residue after Val477 ((+Ala478) β1-AR) inhibited the recycling of this β1-AR mutant (Fig. 2A, j′–p′). Co-immunoprecipitations between the various P = 0 mutants of the β1-AR and SAP97 showed that (V477L) β1-AR and (V477I) β1-AR co-immunoprecipitated SAP97, whereas the other constructs did not (Fig. 2B). The results in Fig. 2B indicated that the amount of SAP97 that was immunoprecipitated by the (V477I) β1-AR construct (lane 9) was equivalent to the amount that was immunoprecipitated by the WT β1-AR (lane 12). However, the amount of SAP97 immunoprecipitated by the (V477L) β1-AR construct (Fig. 2B, lane 10) was ∼30% of the amount of SAP97 that was co-immunoprecipitated with either the (V477I) β1-AR or the WT β1-AR (Fig. 2B). These differences partially explain the slower recycling kinetics of (V477L) β1-AR. Thus, the recycling data and SAP97 binding experiments were once more in agreement, indicating that SAP97 binding to the type I PDZ of the β1-AR was apparently required for the recycling of the β1-AR.
Role of the Type I PDZ of the β2-AR in Regulating the Trafficking of the β1-AR
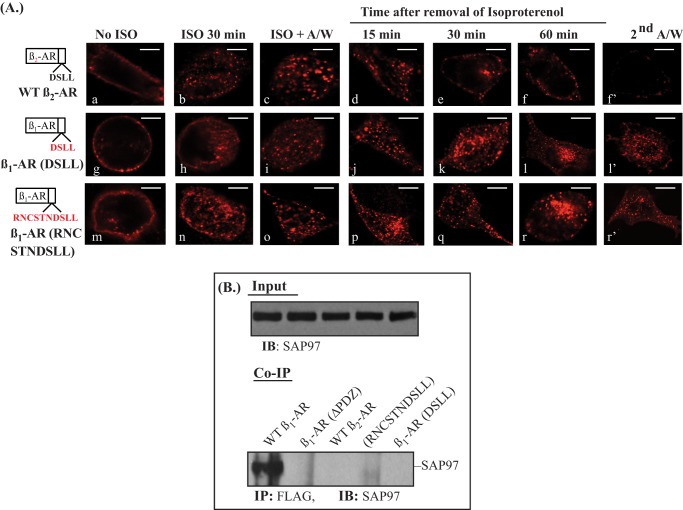
To determine if type I PDZ sequences derived from other recycling type A GPCR would support the recycling of the β1-AR, we swapped the last 4 or 10 amino acids in the β1-AR with their counterparts in the β2-AR (Fig. 3A). As expected, the natural type I PDZ sequence of the β2-AR, DSLL, supported the internalization of WT β2-AR in response to isoproterenol, and internal WT β2-AR recycled efficiently with a t0.5 = 25 ± 6 min (Fig. 3A, a–f′). The β1-AR with a substituted β2-AR PDZ (β1-AR (DSLL)) was internalized efficiently in response to isoproterenol, but internal β1-AR (DSLL) did not recycle even after 3 h from the removal of isoproterenol (Fig. 3A, g–l′). Similarly, the β1/β2-AR(10) mutant, in whom the last 10 amino acids in the β1-AR were replaced by the last 10 amino acids of the β2-AR did not recycle (Fig. 3, images m–r′). These results could be interpreted three ways. One interpretation would be that type I PDZ domains were not interchangeable among the various GPCRs. Another would be that the recycling of the β1-AR was dependent on SAP97, which does not bind to the β2-AR PDZ (23). A third interpretation would be that additional structural motifs in the β1-AR were required for its trafficking.
FIGURE 3.
Effect substituting the β2-AR-derived C-tail sequences on recycling of the β1-AR. A, HEK-293 cells stably expressing the WT β2-AR or β1/β2-AR chimera in whom the last 4 or 10 amino acids of the β1-AR were mutagenized into the corresponding sequences of the human β2-AR were used. The recycling of these β1/β2-adrenergic receptor chimeras was determined as described in the legend to Fig. 1A. B, HEK-293 cells were transiently transfected with FLAG-tagged β1-AR, β2-AR, or β1-ARΔPDZ or with β1/β2-AR chimera and SAP97 cDNAs for 2 days. Equal amounts of cell lysate proteins were incubated with anti-FLAG IgG resin, eluted, and subjected to Western blotting for the co-IP of SAP97. To normalize for input protein levels, ∼4% of each cell lysate were subjected to Western blotting (IB) and probed with the anti-SAP97 antibody. A/W, acid wash.
Role of SAP97 in Regulating the Trafficking of β2-AR and β1/β2-AR-Chimera
To sort between these alternatives, we determined first if SAP97 was involved in trafficking and binding to these β1/β2-AR chimeras. Co-immunoprecipitation experiments between SAP97 and each of the constructs described in Fig. 3A indicated that the WT β2-AR, β1-AR (DSLL), and the β1/β2-AR(10) did not co-immunoprecipitate SAP97 (Fig. 3B).
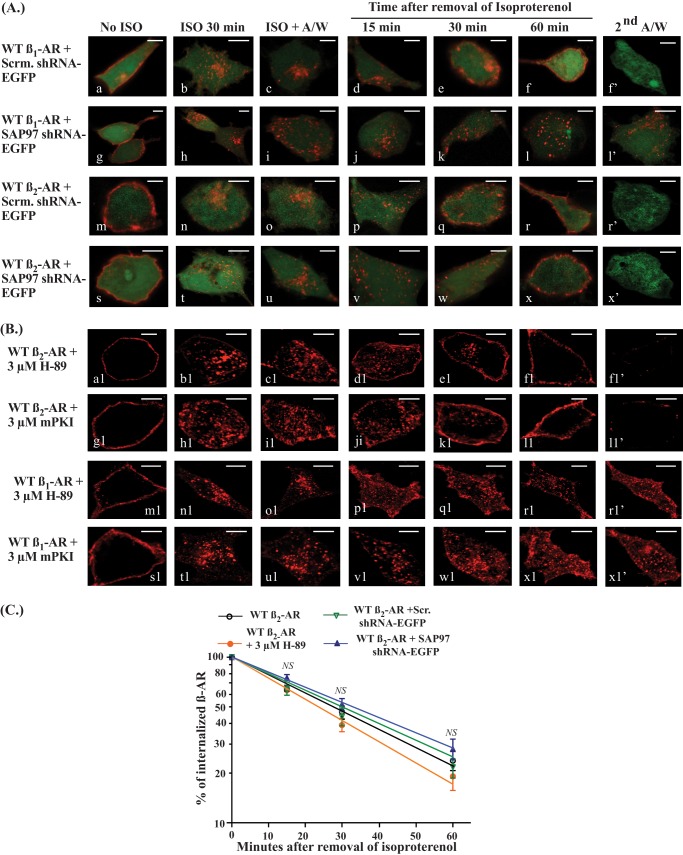
In a second series of experiments, the role of SAP97 was explored by determining the effect of knockdown of SAP97 on trafficking of the WT β1-AR versus the WT β2-AR (Fig. 4). HEK-293 cells expressing the FLAG-tagged β1-AR or β2-AR with either the scrambled EGFP-shRNA or SAP97-EGFP shRNA (pseudo-green color) were used in these trafficking assays (Fig. 4A). In cells that expressed scrambled shRNA, Cy-3-labeled β1-AR or β2-AR (pseudo-red color) was internalized normally and internal β1-AR or β2-AR recycled efficiently and were externalized (Fig. 4, images d–f′ and p–r′, respectively). From these data, we determined that WT β1-AR recycled with a t0.5 of 22 ± 8 min, and WT β2-AR recycled with a t0.5 of 27 ± 7 min (Fig. 4C). In cells in which SAP97 was knocked down by its shRNA, the β1-AR was internalized normally, but internal WT β1-AR did not recycle (Fig. 4, g–l′). In cells expressing the SAP97 shRNA, the β2-AR was internalized and internal WT β2-AR recycled efficiently and completely with a t0.5 of 28 ± 8 (Fig. 4, A (s–x′) and D). These results indicated that the β2-AR PDZ in the context of the β2-AR facilitated its recycling in a SAP97-independent manner but could not support the recycling of chimeric β1/β2-AR.
FIGURE 4.
Effect of SAP97 and H-89/mPKI on recycling of WT β1-AR or WT β2-AR. A, HEK-293 cells stably expressing the FLAG-tagged WT β1-AR and either the scrambled shRNA-EGFP (a–f′) or hSAP97 shRNA-EGFP (g–l′) were used. Similarly, cells stably expressing the FLAG-tagged WT β2-AR with either the scrambled shRNA-EGFP (m–r′) or hSAP97 shRNA-EGFP (s–x′) were used. These four types of cells were subjected to internalization and recycling as described in the legend to Fig. 1A. The distribution of fluorescent pixels was obtained using confocal microscopy, and the colors shown are pseudo-colors. Scale bars, 5 μm. B, HEK-293 cells stably expressing the WT β2-AR or WT β1-AR were preincubated for 30 min with 3 μm H-89 or the myristoylated peptide inhibitor of PKA (mPKI) and then subjected to internalization and recycling as described in the legend to Fig. 1A. Scale bars, 5 μm. C, pixels inside a 300-nm boundary in the isoproterenol/acid-washed images of the indicated β1-AR construct were set arbitrarily to 100% to indicate 100% internalization. The ratios in alprenolol-treated cells (at 15, 30, and 60 min) were calculated and expressed as the percentage for each time period. The ratios from 15–20 independent images for each condition were calculated and expressed as the mean ± S.E. for each time period. The data were plotted as a logarithmic y axis to calculate the t0.5 for recycling, as described under “Experimental Procedures.” The mean and S.E. for each time period were compared among the four different groups by one-way analysis of variance with Newman-Keuls post-tests. Statistical results are expressed as NS to indicate no significant difference or *, **, and *** to indicate p < 0.05, p < 0.01, and p < 0.001, respectively. A/W, acid wash.
Role of PKA in Trafficking of the β1-AR Versus the β2-AR
The relationship between the type I PDZ of the β1-AR and SAP97 is complex because SAP97 binds simultaneously to the type I PDZ of the β1-AR via its PDZ-2 and to AKAP79 via the i3 domain of SAP97 (13). AKAP79-mediated targeting of PKA to the β1-AR played a crucial role in recycling of the β1-AR because this pool of PKA was involved in phosphorylating Ser312 in the third intracellular loop of the β1-AR (11, 14–16). Because SAP97 was not involved in the recycling of the β2-AR, we wondered whether PKA was involved in the trafficking of the WT β2-AR. To address this question, HEK-293 cells stably expressing WT β1-AR or WT β2-AR were pretreated with a 3 μm concentration of the PKA inhibitors H-89 and mPKI (Fig. 4B). We used two different PKA inhibitors because micromolar concentrations of H-89 might compete with isoproterenol binding to β-AR (24). In cells pretreated with H-89 or mPKI, the WT β2-AR was sequestered and internal β2-AR recycled with a t0.5 of 23 ± 7 and 25 ± 8 min, respectively (Fig. 4, B (a1–l1) and C, and Table 1). Under these conditions, the WT β1-AR was internalized, but internal β1-AR did not recycle (Fig. 4B, m1–x1′). These results indicate that recycling of the β1-AR was dependent on PKA and SAP97, whereas recycling of the β2-AR was dependent on the type I PDZ but independent of SAP97 or PKA (Fig. 4, A and B).
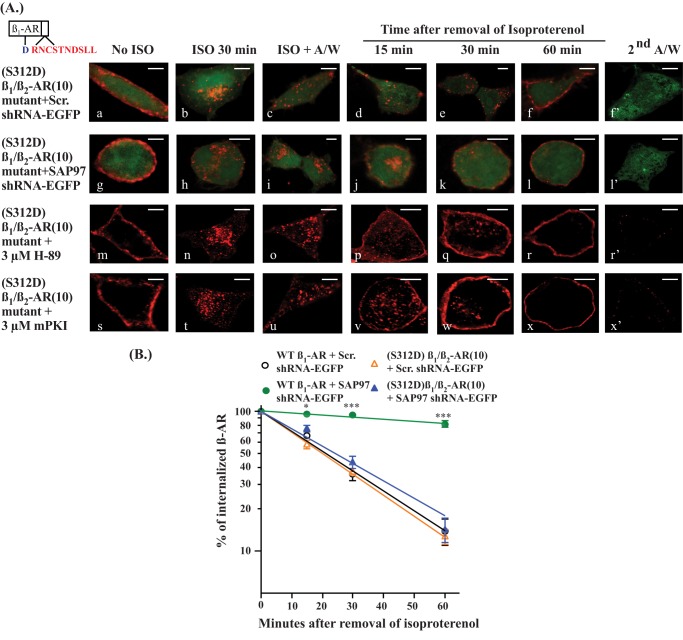
Role of Ser312 in Regulating the Trafficking of β1/β2-AR Chimeras
Thus far, we have shown that inhibition of PKA, inactivation of the PDZ, or knockdown of SAP97 somehow inhibited the recycling of the β1-AR. A hypothesis presented by Gardner et al. (11) suggested that phospho-Ser312 in the third intracellular loop was the ultimate recycling signal because the functional roles of the PDZ, PKA, and SAP97 converged on phosphorylating this serine. Because the β1/β2-AR(10) mutant did not recycle, we wondered if its recycling could be restored when Ser312 was mutated to its phosphoserine mimic aspartic acid ((S312D) β1/β2-AR(10)). Trafficking studies indicated that (S312D) β1/β2-AR(10) recycled with a t0.5 of 20 ± 7 min (Table 1). The recycling kinetics of (S312D) β1/β2-AR(10) were not altered by control or SAP97 shRNAs (Fig. 5A, a–f′ and g–l′, and Table 1). Therefore, trafficking of the (S312D) β1/β2-AR(10) mutant in HEK-293 cells was independent of SAP97, which does not bind to the β2-AR PDZ. Pretreatment of cells expressing (S312D) β1/β2-AR(10) with H-89 or mPKI did not affect the trafficking kinetics of this β1-AR mutant (Fig. 5A, m–r′ and s–x′, and Table 1). These results indicated that substituting Ser312 with Asp312 circumvented the roles of the PDZ, SAP97, and PKA in regulating the trafficking of the β1-AR.
FIGURE 5.
Effect of the serine 312 to aspartic acid mutation on recycling of β1/β2-AR(10) chimera. A, HEK-293 cells were transiently transfected with the FLAG-tagged (S312D) β1-AR mutant in which the last 10 amino acids were mutagenized to the corresponding sequence in the β2-AR ((S312D) β1/β2-AR(10)) with either the scrambled shRNA-EGFP (a–f′) or hSAP97 shRNA-EGFP (g–l′). In addition, cells that expressed ((S312D) β1/β2-AR(10)) were preincubated for 30 min with 3 μm H-89 (m–r′) or mPKI (s–x′) prior to subjecting these cells to internalization and recycling as described in the legend to Fig. 1A. The distribution of fluorescent pixels was obtained using confocal microscopy, and the colors shown are pseudo-colors. Scale bars, 5 μm. B, the recycling data were plotted and analyzed as described in the legend to Fig. 4C, and the means ± S.E. were expressed as NS to indicate no significant difference or *, **, and *** to indicate p < 0.05, p < 0.01, and p < 0.001, respectively.
Characterization of the Role of Other Type I PDZs in Trafficking of the β1-AR
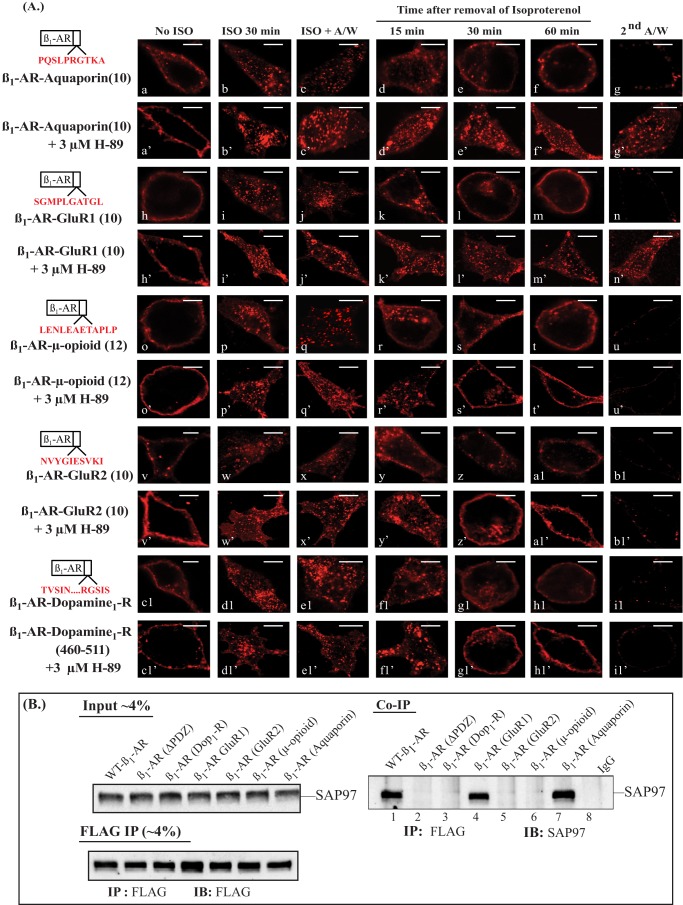
A PDZ substitution strategy was used to determine if other type I PDZs could support the trafficking of the β1-AR. This strategy involved substituting the last 10 amino acids in the β1-AR with the corresponding sequence in the C termini of other recycling proteins (Table 2). Initially, we substituted the type I PDZ sequences of GluR1 and aquaporin-2 into the β1-AR because the translocation of GluR1 and aquaporin-2 from internal endosomes to the membrane was PDZ-dependent (18–19, 25–27). In HEK-293 cells, β1-AR GluR1(10) and β1-AR aquaporin(10) chimera were expressed on the cell membrane, and both were internalized into punctate structures in response to isoproterenol (Fig. 6A, a–c and h–j). Replacement of isoproterenol with alprenolol promoted rapid and efficient recycling of internal β1-AR GluR1(10) and β1-AR aquaporin(10) chimeras back into the cell membrane with a t0.5 of 25 ± 8 and 22 ± 7 min, respectively (Fig. 6A, d–f and k–m, and Table 1). The recycling of internal β1-AR aquaporin(10) and β1-AR GluR1(10) was inhibited by H-89 (Fig. 6A, a′–g′ and h′–n′). Moreover, the recycling of these constructs was also inhibited in cells that expressed the SAP97 shRNA (Table 1).
TABLE 2.
Sequence replacements in the C-tail of the β1-adrenergic receptor
With the exception of the dopamine receptor-recycling signal, all other signals are located at the distal cytoplasmic tail of the indicated protein. The underlined amino acids denote the crucial elements of the sequence.
| Ligand | Accession no. | Sequence | PDZ type | Recycling | PKA- dependent | Reference |
|---|---|---|---|---|---|---|
| β2-AR | NM_000024 | RNCSTNDSLL | I | Yes | ND | 6 |
| GluR1 | NM_000827 | SGMPLGATGL | I | Yes | Yes | 19, 25 |
| Aquaporin-2 | NM_000486 | PQSLPRGTKA | I | Yes | Yes | 26, 27 |
| μ-Opioid receptor | L22455 | LENLEAETAPLP | C-terminal non-PDZ | Rescued δ-opioid receptor recycling | ND | 31, 42 |
| Dopamine 1 receptor | NM_000794 | 360TVSINNNGAAMFSSHHEPRGSIS382 | Internal non-PDZ | Rescued δ-opioid-R recycling | ND | 32 |
| Ionotropic (GluR2) | NM_000826 | NVYGIESVKI | II | NDa | ND | 8, 33 |
a ND, not determined.
FIGURE 6.
Effect of additional substitutions in the C-tail of the β1-AR on recycling and binding to SAP97. A, the last 10 amino acids of the β1-AR were mutagenized into the sequences described in Table 1. HEK-293 cells stably expressing equivalent levels of these FLAG-tagged β1-adrenergic receptor chimeras were preincubated for 30 min in buffer or with 3 μm H-89, and their internalization and recycling were determined as described in the legend to Fig. 1A. B, HEK-293 cells stably expressing the β1-AR chimeras described in A were transiently transfected with SAP97 cDNA for 2 days. The cells were lysed, and about 4% of the crude cell lysate (input) was subjected to Western blotting (IB) and probed with anti-SAP97 IgG to verify that equal amounts of SAP97 were loaded onto the resin. Equal amounts of the remaining cell lysate proteins were incubated with anti-FLAG IgG resin, followed by washing and eluting the resin with Laemmli sample buffer. The eluates were divided into portions. One portion representing 4% of the eluate by volume was probed with anti-FLAG antibody to verify that equal amounts of the β1-AR were bound and eluted from the affinity resin. The rest, representing about 95% of resin eluates, were probed for the co-IP of SAP97 (n = 4). A/W, acid wash.
Co-immunoprecipitations between SAP97 and these β1-AR chimeras were conducted to find out if SAP97 binding correlated with the recycling phenotypes of β1-AR GluR1(10) and β1-AR aquaporin(10). In the control portion of this experiment, we determined that WT β1-AR co-immunoprecipitated SAP97, whereas β1-ARΔPDZ did not interact with SAP97 (Fig. 6B, right, lanes 1 and 2). As expected, β1-AR GluR1(10) co-immunoprecipitated SAP97 (Fig. 6, lane 4), because the type I PDZ of GluR1 binds avidly to SAP97 (28–30). In addition, we discovered that the type I PDZ in the C terminus of aquaporin-2 interacted with SAP97 because β1-AR aquaporin(10) co-immunoprecipitated SAP97 in 6 of 6 co-IP experiments (Fig. 6B, lane 7).
Characterization of the Role of Sequences Unrelated to type I PDZs in Regulating the Trafficking of the β1-AR
Trafficking studies have shown that sequences that did dot correspond to type I PDZ architecture could also support the recycling of many receptors. For example, the type II (XØXØ) PDZ in the C-tail of GluR2 or the non-PDZ sequence in the C-tail of the μ-opioid receptor or an internal non-PDZ sequence between amino acids 360 and 382 of the dopamine 1 receptor were capable of supporting the recycling of non-recycling GPCR, such as δ-opioid receptors (31–33) (Table 2). To determine if these domains could substitute for the type I PDZ and support the recycling of the β1-AR, the C-terminal 10–12 amino acids of the β1-AR were mutated into these sequences (Table 2). Expression of these β1-AR chimeric constructs indicated that they were expressed at the membrane and were internalized into distinct punctate structures in response to isoproterenol (Fig. 6A, o–q, v–x, and c1–e1). Internal non-PDZ β1-AR-chimeras recycled efficiently and were inserted properly into the plasma membrane (Fig. 6A, r–u, y–b1, and f1–i1). To find out if the recycling of β1-AR GluR2(10) was dependent on the type II PDZ, each of the two hydrophobic amino acids in P = 0 or in P = −2 was mutated to alanine (Table 2). These constructs were internalized normally in response to isoproterenol but did not recycle (data not shown). Therefore, the type II-PDZ that supported the recycling of the GluR2 also supported the recycling of the internalized the β1-AR.
Pretreatment of cells expressing β1-AR μ-opioid(12), β1-AR GluR2(10), or β1-AR dopamine 1 receptor with H-89 did not inhibit isoproterenol-mediated internalization; nor did it affect the t0.5 for recycling of these receptors (Fig. 6A, o′–u′, v′–b1′, and c1′–i1′, and Table 1). In addition, SAP97 was transiently transfected into HEK-293 cells that expressed moderate densities of these receptors in order to determine if SAP97 binding correlated with their recycling phenotypes (Fig. 6B). FLAG-IPs did not co-immunoprecipitate SAP97, indicating that β1-AR μ-opioid (12), β1-AR GluR2(10), and β1-AR dopamine 1 receptor recycled in a PKA-, type I PDZ-, and SAP97-independent manner.
Identification of a Role for the P = −3 Residue in the Type I PDZ in Regulating Intracellular Trafficking of GPCR and SAP97
GPCRs are internalized in response to agonists either alone or with their respective PDZ-binding protein (1, 9). For example, the PDZ-binding protein SAP97 dissociated from the β1-AR PDZ (ESKV) after its internalization (11). Similarly, the PDZ-binding protein NHERF-1 dissociated from the PDZ (DSLL) of the internalized β2-AR (34). Conversely, the PDZ-binding protein associated with the type I PDZ (STAV) of the corticotropin-releasing hormone receptor was internalized with the GPCR (35). Because the GPCRs that were internalized with their PDZ-binding protein contained a neutral amino acid at P = −3 and those that were internalized alone contained an acidic amino acid at P = −3, we wondered whether an acidic versus neutral amino acid at P = −3 was somehow involved in regulating this outcome (see “Discussion”).
To properly address the role of acidic versus neutral amino acids at P = −3 in this phenomenon, we needed to identify another functional type I PDZ, which differed from the β1-AR PDZ in the charge of its residue at P = −3 yet had similar functional and biochemical characteristics. We believe that the type I PDZ of the GluR1 (ATGL) fulfilled all of these criteria, because it lacked an acidic residue at P = −3, yet like the β1-AR, it bound to SAP97 via its second PDZ and supported the recycling of the β1-AR GluR1(10) mutant (13, 30).
HEK-293 cells stably expressing equivalent densities of the WT β1-AR or the β1-AR GluR1(10) were transiently transfected with SAP97-GFP. These cells were stained live with Cy3 anti-FLAG IgG and imaged by dual fluorescence confocal microscopy. At time t = 0, Cy3-labeled FLAG WT β1-AR or β1-AR GluR1(10) (pseudo-red pixels) were colocalized with SAP97-GFP (pseudo-green pixels) at the cell surface (Fig. 7, A and B, 0 min, respectively, n = 20). After the addition of isoproterenol, the WT β1-AR and β1-AR GluR1(10) were internalized into endocytic vesicles (Fig. 7, A and B, ISO 30 min, respectively, n = 20). The distribution of SAP97-GFP indicated that the majority of the pixels of internalized β1-AR GluR1(10) were co-localized with SAP97-GFP (overlapping internal yellow pixels), whereas the pixels of internalized WT β1-AR were not localized with SAP97-GFP (Fig. 7, A and B, ISO 30 min). Confocal images that were taken l h after the addition of alprenolol revealed that both the WT β1-AR and the β1-AR GluR1(10) recycled back and were colocalized with SAP97-GFP in the membranous region of the cell (Fig. 7, A and B, ALP 60 min, n = 20). Thus, it appears that the nature of the P = −3 amino acid could affect the association of the internalized GPCR with their PDZ-binding protein.
FIGURE 7.
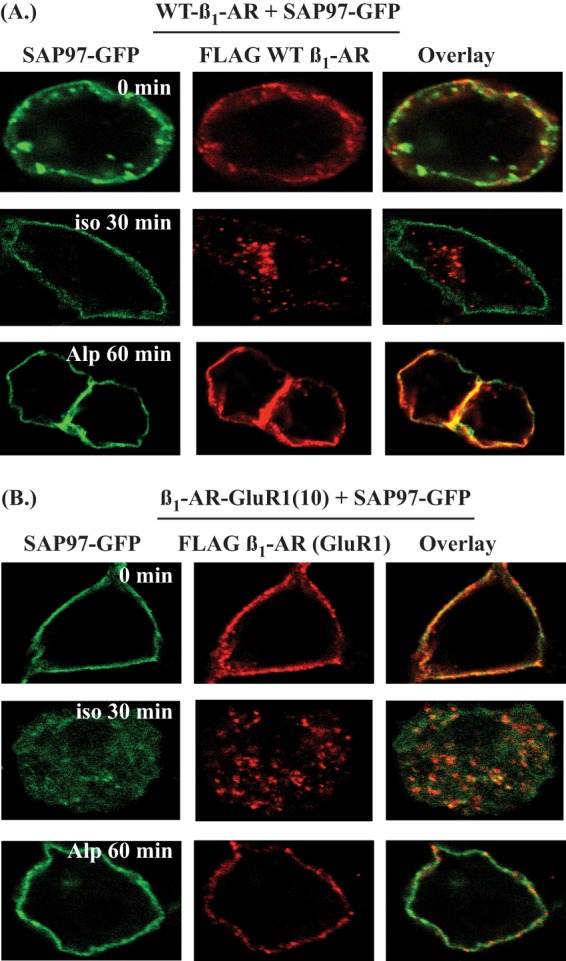
Effect of the polarity of the amino acid at position −3 in the type 1 PDZ on the association of SAP97 with internal β1-AR constructs. Shown is confocal microscopy imaging of SAP97-GFP (green) and FLAG β1-AR labeled with Cy3-conjugated anti-FLAG M2 IgG (red) in cells expressing either the WT β1-AR (A) or cells expressing the β1-AR GluR1(10) (B) prior to (0 min) and 30 min after (ISO 30 min) adding 10 μm isoproterenol. Thereafter, the isoproterenol-exposed cells were washed and incubated for 60 min at 37 °C (ALP 60 min) with 100 μm alprenolol and imaged. The image is representative of 20 cells.
Role of GPCR Recycling in Protecting the GPCR from Degradation after Chronic Agonist Exposure
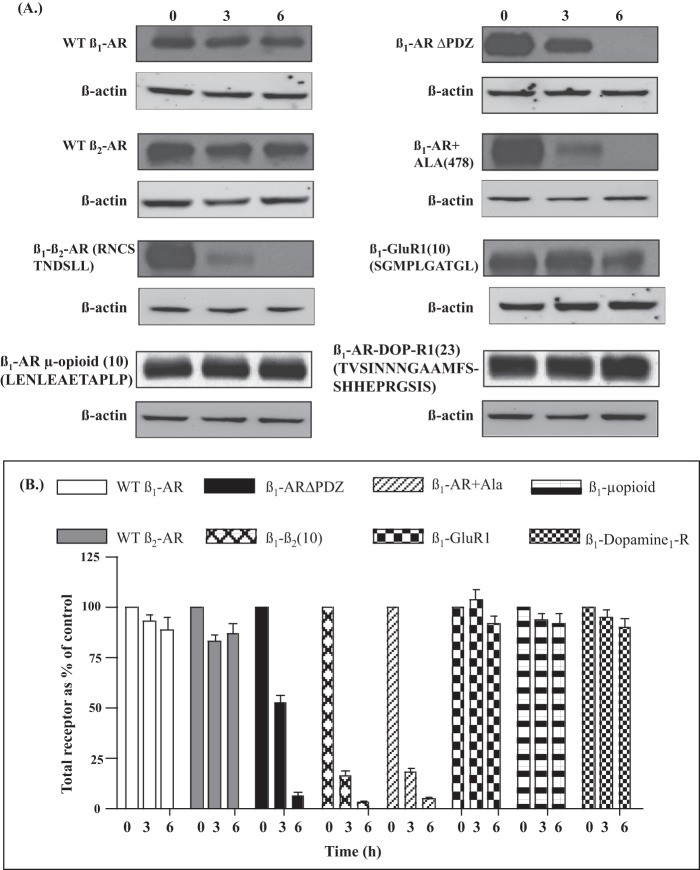
A major role of recycling is to restore the functional efficiency of the GPCR signaling pathway through a process termed resensitization (1, 15). We have determined the effect of prolonged agonist exposures on the fate of biotinylated WT β1-AR and (S312A) β1-AR in HEK-293 cells (36). These studies revealed that the recycling WT β1-AR was not significantly degraded even after 6 h of continuous exposure to agonist, whereas the non-recycling (S312A) β1-AR was markedly (>80%) degraded (36). To investigate whether the recycling phenotype of the β1-AR chimeras would also inhibit their lysosomal proteolysis, HEK-293 cells stably expressing equivalent densities of β1-AR C-tail chimeras were tested by immunoblotting for agonist-induced proteolysis (Fig. 8). The anti-FLAG antibody detected undegraded FLAG-WT β1-AR and WT β2-AR in cell membranes that were prepared after 6 h of continuous exposure to saturating concentrations of isoproterenol (Fig. 8A). Similarly, β1-AR GluR1(10) was not significantly degraded, consistent with its ability to recycle. In contrast, β1-ARΔPDZ and (+Ala478) β1-AR were completely degraded by 6 h, which was consistent with their inability to recycle. The β1/β2-AR(10) mutant in which the β2-AR “recycling signal” replaced the β1-AR PDZ was extensively degraded within 3 h, indicating that although this construct contained an active type I PDZ, its failure to recycle was also associated with rapid proteolysis.
FIGURE 8.
Effect of selected substitutions in the C-tail of the β1-AR on agonist-induced degradation of mutant receptor chimeras. A, HEK-293 cells expressing the indicated FLAG-tagged receptors were exposed to 10 μm isoproterenol for 0, 3, or 6 h. Total cell extracts were probed by Western blotting with anti-FLAG IgG to compare the levels of β1-AR chimeras and with anti-β-actin to equalize the amounts of loaded protein lysates. B, band and intensities of anti-FLAG and anti-β-actin were quantified by chemiluminescent imaging, and anti-FLAG intensities were normalized for each construct. Bars, mean receptor levels (relative to 0 h of agonist exposure) were determined from n = 4 independent experiments per construct. Error bars, S.E.
To determine whether SAP97 binding to the β1-AR was required for its stability, we tested the effect of chronic agonist exposure on the stability of β1-AR μ-opioid(12) or β1-AR dopamine 1 receptor. Replacing the last 10 amino acids of the β1-AR with the “recycling domains” of the μ-opioid or the dopamine 1 receptor significantly protected these receptors from proteolysis, although they did not bind SAP97 (Fig. 8, A and B). Taken together, these data indicated that a variety of type I PDZ and non-PDZ recycling sorting signals that supported recycling also afforded significant protection for the GPCR. A roadmap illustrating putative trafficking pathways of the various β1-AR constructs that were used in this study is presented in Fig. 9 and described under “Discussion.”
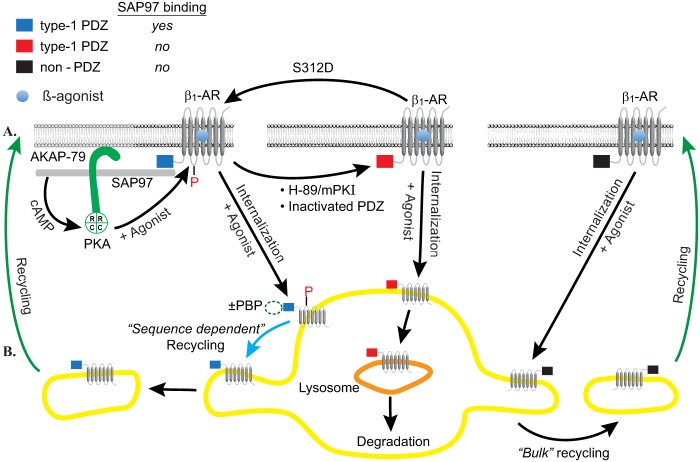
FIGURE 9.
Roadmap for divergent trafficking pathways of chimeric β1-AR. A, organization of membranous β1-AR mutants and chimeras. Left, WT or β1-AR-chimeras with a type I PDZ that bind to SAP97 (blue PDZ) assemble as a complex composed of SAP97-AKAP79-PKA. Activation of these GPCRs increases cyclic AMP, which promotes the dissociation of the catalytic subunit of PKA from PDZ-bound PKA. The catalytic subunit of PKA phosphorylates Ser312 in the third intracellular loop to imprint the recycling signal onto the β1-AR (red P). Middle, β1-AR-chimeras with an inactive PDZ or with a type I PDZ that did bind SAP97 (red PDZ), did not assemble a SAP97-anchored complex. Right, β1-AR-chimeras with a type II PDZ or non-PDZ recycling sequences do not assemble a complex (black PDZ). Activation of all of these receptors, however, increased cyclic AMP and promoted the binding of β-arrestin to the GRK-phosphorylated GPCR. In the left panel, we hypothesize that acidic amino acids at P = −3 in WT or β1-AR mutants would interact with β-arrestin to promote the dissociation of the complex anchored at the PDZ prior to the internalization of the various GPCRs into early endosomes, whereas PDZs with neutral residues at P = −3 are internalized with their PDZ-binding protein (±PBP). B, trafficking roadmap of internalized β1-AR mutants and chimeras. Left, trafficking roadmap of β1-AR mutants with a phospho-Ser312. These GPCRs traffic by a process termed “sequence-dependent trafficking,” in which they are sorted from early endosomes into membrane extensions (blue arrow) that coalesce into “recycling endosomes”. Middle, β1-AR with type I PDZs, but without a phospho-Ser312, were retained within the “body” of early endosomes. These early endosomes eventually matured into late endosomes/lysosomes, in which the retained “cargo” is eventually degraded. Right, internalized β1-AR with recycling non-PDZs traffic out of early endosomes into “recycling endosomes” by a process termed “bulk or sequence-independent recycling.” β1-AR-chimeras that traffic to recycling endosomes recycle back and fuse with the cell membrane (green arrows).
DISCUSSION
Type I PDZs in the C termini of many GPCR participate in a variety of receptor signaling and trafficking events (5, 8, 13). For example, a type I PDZ-mediated interaction between the corticotropin-releasing factor type I receptor and serotonin 2 receptor was required for corticotropin releasing factor-mediated increase in the density of serotonin 2 receptors in primary prefrontal cortical neurons (37). The type I PDZ of the β1-AR was also required for proper localization of the β1-AR into the cell membrane of cardiomyocyte-like cells (38). The bulk of the data on PDZ domains, however, relates to their role in trafficking of internalized GPCR (1, 2). The contribution of individual residues of type I PDZ in trafficking and in binding to PDZ-binding proteins of certain receptors, such as the β2-AR and the ionotropic GluR1, has been extensive (5, 8, 15, 29). Based upon these studies, the functional residues in type I PDZs were the hydrophobic residue at P = 0 and Ser/Thr at P = −2 (5, 31). These results were in agreement with crystal structural data between the type I PDZ of GluR1 (ATGL) or the E6 protein of human papillomavirus-18 (ETQV) with PDZ2 of SAP97 (21, 22). These complexes showed strong interactions between the hydrophobic amino acid at P = 0 and the βB domain of PDZ2 and between the Ser/Thr amino acids at P = −2 and His383 in the binding pocket of PDZ2 of SAP97 (21, 22).
Functional and Biochemical Characterization of Residues in the Type I PDZ of the β1-AR
The paucity of data concerning the role of individual residues in PDZ-mediated regulation of GPCR trafficking prompted a detailed analysis of the contribution of each residue in the type I PDZ of the β1-AR toward its trafficking. Our analysis indicated that a hydrophobic amino acid at P = 0 and a serine at P = −2 were paramount for recycling of the β1-AR and for binding to SAP97 (Figs. 1 and 2). However, the role of the Ser/Thr residue at P = −2 in trafficking and signaling was more complex than previously envisioned because the substitution of a phosphoserine mimic at this position prevented the binding of SAP97 and inhibited its recycling (Fig. 1A, g1–l1′). In addition, mutagenesis of the supposedly degenerate residues at P = −1 and P = −3 to alanine inhibited recycling and SAP97 binding to these β1-AR mutants (Fig. 1). Mutagenesis of the residues in P = −1 or P = −3 to Leu produced divergent effects on β1-AR recycling. Whereas Leu at P = −3 inhibited recycling, Leu at P = −1 supported the recycling of the β1-AR and SAP97 binding. These results indicate that the nature of the residues at P = −1 and P = −3 was not innocuous and suggest that a reevaluation of the nature of these residues in trafficking of other GPCR is warranted.
Are Type I PDZs Interchangeable between Recycling GPCRs?
A PDZ substitution strategy between β1-AR and β2-AR was used to determine if PDZ substitutions between these GPCRs would promote the recycling of internalized β1-AR chimera. This strategy when used by others indicated that transplanting the β1-AR PDZ (ESKV) or the β2-AR PDZ (DSLL) into the C-tail of μ-opioid- and δ-opioid receptors promoted rapid recycling of these opioid receptors (7, 8). Thus, we wanted to find out if the β2-AR PDZ would substitute for the β1-AR PDZ in promoting the trafficking of the β1-AR, although it did not bind to SAP97 (23). Surprisingly, the β2-AR PDZ did not support the recycling of the β1-AR (Fig. 3). An interpretation of the results of Fig. 3 would suggest that type I PDZs were not universally interchangeable in promoting GPCR recycling, which we attribute to major differences in the contribution of these PDZs toward the recycling of their respective GPCR.
Role SAP97 in Regulating the Trafficking of the β1-AR
The results of Figs. 1–3 suggested that β1-AR recycling correlated with SAP97 binding to the transplanted type I PDZ. Because the β2-AR PDZ did not bind to SAP97, this might explain why the transplanted β2-AR PDZ could not support the recycling of β1/β2-AR(10) chimera. To more properly evaluate this notion, the repertoire of exchanged type I PDZs was expanded to include the type I PDZ of GluRI, which binds SAP97 (28–30), and the type I PDZ of aquaporin-2, in which the status of SAP97 binding was unknown. Once more, an excellent correlation was obtained between the binding of these transplanted type I PDZs to SAP97 and their ability to support the recycling of the β1-AR (Fig. 6). Therefore, loss of SAP97 binding upon transplanting the β2-AR PDZ was apparently the primary cause for failure of β1-AR (DSLL) or β1/β2-AR(10) to recycle.
The discovery that SAP97 was a binding partner to the type I PDZ of aquaporin-2 is novel and potentially significant because trafficking of aquaporin-2 in renal epithelial cells in response to vasopressin was regulated by its PDZ (26–27). Thus, renal SAP97 might play an important role in aquaporin-2-mediated conservation of water in epithelial collecting ducts (39).
Cross-talk between PKA Bound to the PDZ and Ser312 Is Required for Recycling of the β1-AR
An interpretation of the results presented thus far would imply that unless type I PDZs bind SAP97, they would not be universally interchangeable in promoting the recycling of the β1-AR. Our speculation, however, is that this is a narrow interpretation of the results because it overlooks a much broader array of mechanisms for GPCR recycling that were highlighted by the point mutants and by the PDZ-switching strategies that were used here.
Functional reliance of the β1-AR PDZ on SAP97 binding was related to the bipartite nature of SAP97 binding. In Nooh et al. (13), we determined that SAP97 binds simultaneously to the type I PDZ of the β1-AR via its PDZ2 binding domain and to an AKAP5-PKA complex via the i3 domain of SAP97. Analysis of the contribution of each member of the β1-AR PDZ complex to recycling of the β1-AR indicated that cross-talk between this complex and Ser312 was involved in setting the trafficking itinerary of the β1-AR (11, 14, 16). This notion is supported by many observations. First, delocalization of PKA by the st-Ht-31 peptide, inhibited the phosphorylation of Ser312 and recycling of the β1-AR (15). Second, manipulations that inactivated the β1-AR PDZ or knocked down either SAP97 or AKAP5 inhibited the phosphorylation of Ser312 and the recycling of the β1-AR (11, 13, 16). Finally, deletion of the second PDZ or the i3 domain of SAP97 or the PKA-binding domain of AKAP5 prevented the recycling of the β1-AR (13, 14). These data provide strong evidence for the involvement of cross-talk between the complex bound to type I PDZ and Ser312 in promoting the recycling and in protecting the β1-AR from degradation.
The targeting of PKA to the type I PDZ of the β1-AR was a primary requirement for cross-talk. This would explain why inhibition of PKA with H-89 or mPKI prevented the recycling of the β1-AR and why β1-AR chimeras, such as β1-AR GluR1(10) and β1-AR aquaporin(10), that bound SAP97 and were capable of targeting PKA to the transplanted type I PDZ supported the recycling of their respective β1-AR chimera. Therefore, a better interpretation of the β2-AR PDZ substitution data would be that β1/β2-AR chimeras did not recycle because the transplanted PDZ failed to target PKA to the β1-AR. Consequently, mutagenesis of Ser312 in β1/β2-AR(10) into its phosphoserine mimic Asp312 converted the non-recycling phenotype of these receptors into a recycling phenotype that recycled independently of PKA, SAP97, and the type I PDZ (Fig. 5). Therefore, recycling of β1-AR constructs with an Asp312 mutation did not require cross-talk because it was mediated by the ultimate recycling signal of the β1-AR (i.e. phospho-Ser312).
Description of “Sequence-dependent” β1-AR Trafficking Pathways
A mechanism for cross-talk between the type I PDZ in the C-tail and Ser312 in the third intracellular loop in regulating the trafficking of the β1-AR is described in Fig. 9. In this figure, we show bipartite binding of SAP97 to organize a SAP97-AKAP5-PKA complex at the type I PDZ of the β1-AR (blue PDZ in Fig. 9). Agonist-mediated activation of the β1-AR acutely increases the levels of cyclic AMP. Cyclic AMP binds to the regulatory subunit of PKA that was bound to the β1-AR PDZ to release the catalytic subunit of PKA. The catalytic subunit of PKA phosphorylates Ser312 and imprints a recycling signal on the β1-AR, which is later internalized by the combined actions of GRK, β-arrestin, clathrin, and other coat proteins (4). In this regard, we speculate that trafficking of the β1-AR with an active PDZ through early endosomes was distinct from that of the transferrin receptor (Tfr) because Tfr recycled by “bulk” sorting that was largely independent of specific cytoplasmic sequences (40). This concept was derived from co-localization studies that have shown early colocalization between the Tfr and β1- or β2-AR in Rab5a and EEA1-positive early endosomes, followed by divergent trafficking itineraries between the Tfr and these β-ARs in later time frames (34, 36). In this view, β1- and β2-AR recycled in a sequence-regulated manner through specific cis-acting sequences present on the receptor's cytoplasmic surface. For example, specialized endosomal tubular domains were involved in PDZ-dependent trafficking of the β2-AR in early endosomes (34). These tubular domains were kinetically and biochemically distinct from the domains that mediated bulk or sequence-independent recycling of the Tfr (34). In the case of the β1-AR, sequence-dependent recycling of this receptor was mediated by phospho-Ser312, which was phosphorylated by the PDZ-anchored pool of PKA. To distinguish the sequence-dependent recycling roadmap from the bulk recycling roadmap, we proposed in Fig. 9 that sequence-dependent recycling in early endosomes would involve an additional sorting step that we depicted with a blue arrow in the left-hand panel of Fig. 9.
The second intracellular pathway is the “lysosomal sorting pathway,” which is trafficked by β1-AR chimera with PDZ inactivating mutations or with transplanted type I PDZs that did not bind SAP97 (red PDZ in the middle panel of Fig. 9). These receptors were not sorted out of early endosomes but instead were retained within the “body” of early endosomes, which later matured into late endosomes/lysosomes (41). This roadmap was derived from trafficking of non-recycling β1-AR mutants, such as β1-AR ΔPDZ or (S312A) β1-AR, and other nonrecycling GPCRs, such as δ-opioid receptors, which were retained in early endosomes (orange PDZ in the middle panel of Fig. 9). Subsequently, these endosomes matured into lysosomes in which cargo, including non-recycling β1-AR mutants, would be eventually degraded (2, 31, 40–42) (Fig. 7).
Fig. 9 also indicates that interconversion between these pathways was possible. On the one hand, inactivation of the PDZ, inhibition of PKA, or knockdown of SAP97 would divert the otherwise recycling β1-AR toward the “lysosomal sorting pathway” (Figs. 1–4 and 8). On the other hand, β1-AR-chimeras with a S312D mutation would recycle via the sequence-dependent trafficking pathway independently of SAP97, PDZ, or PKA (Fig. 5).
Recycling of β1-AR without a Type I PDZ Was PKA- and SAP97-independent
In the other set of the substitutions, we discovered that recycling of the β1-AR was imparted by sequences belonging to type II PDZ and to non-PDZ sequences that were involved in the recycling of other GPCRs (black non-PDZ in the right-hand panel of Fig. 9). In this report, we add three salient features to this novel phenomenon, namely that recycling of β1-AR μ-opioid(12), β1-AR GluR2(10), and β1-AR dopamine 1 receptor chimeras was not dependent on a type I PDZ, PKA, or SAP97.
Therefore, in the classification described in the legend to Fig. 9, recycling of non-PDZ β1-AR chimera was largely independent of specific cytoplasmic sequences (black PDZ in the right-hand panel of Fig. 9). This mode of trafficking is commonly associated with constitutively recycling proteins, such as the Tfr and LDL receptors (40, 41). These proteins recycle through endosomal recycling tubules that mediate direct recycling to the plasma membrane (34, 41). These recycling tubules are biochemically distinct from those that mediate sequence-dependent recycling (34). Nevertheless, a common feature between sequence-dependent and sequence-independent pathways was recycling of the β1-AR, which preserved the integrity of this receptor after long term agonist exposure (Fig. 8). This phenomenon tends to preserve the densities of these GPCRs over several rounds of GPCR internalization and recycling and to restore the intensity of GPCR signaling even after prolonged exposures to desensitizing concentrations of the agonist (1, 15).
Role of the P = −3 Residue in Type I PDZs in Regulating the Internalization of PDZ-binding Proteins
There are conflicting reports concerning the GPCR complex that is internalized in response to agonist exposure. Our investigation suggested that the nature of the residue at P = −3 played a major role in regulating this outcome. The hypothesis behind this line of investigation was derived from studies that examined the phosphorylation of β1- and β2-AR by GRKs.
In studies involving the β1-AR, Hu et al. (43) determined that transiently overexpressed GRK5 decreased the association of the β1-AR with the type I PDZ-binding protein PSD-95 in HEK-293 cells. This effect required the kinase activity of GRK5, suggesting that GRK5 regulated the association between PSD-95 and the β1-AR by phosphorylating the β1-AR (43). Although no specific Ser/Thr were identified, it was presumed that the phosphorylation of Ser475 at P = −2 was involved in reversing the association between the β1-AR and PSD-95 (43). Other studies showed that endogenous GRK-2 in HEK-293 cells would phosphorylate Ser411 at P = −2 in the β2-AR, but no link between phospho-Ser411 and desensitization or internalization of the β2-AR was established (44, 45). However, there is ample evidence that GRK-mediated phosphorylation of three serines within a 10-amino acid segment between residues 355 and 364 in the β2-AR was involved in internalization and desensitization of the β2-AR and in recruitment of β-arrestin to the cell membrane (46–49).
Because the results of Fig. 1 indicated that mutagenesis of Ser475 at P = −2 to Ala or to its phosphoserine mimic Asp inhibited the recycling of the β1-AR, if this serine were to be phosphorylated by GRKs under normal physiological conditions, neither the β1-AR nor the β2-AR would recycle. Accordingly, we reasoned that the Hu et al. (43) hypothesis did not need the phosphorylation of the P = −2 serine to be invoked. Instead, GRK-mediated phosphorylation of the GPCR was sufficient to provide the substrate(s) for the binding β-arrestin. Thus, we hypothesized that the nature of the amino acid at P = −3 could regulate an interaction between this residue and β-arrestin, which somehow affected the association between the GPCR and its PDZ-binding protein. This notion was gleaned from the elegant study by Irannejad et al. (50), which revealed that the binding of β-arrestin to GRK-phosphorylated Ser/Thr residues of the β2-AR preceded the internalization of the β2-AR. Thus, it is conceivable that neutral versus negatively charged “acidic” amino acids at P = −3 would interact differently with β-arrestin in regulating the interaction of the GPCR with its PDZ-binding protein. This scenario would also preserve the integrity of the type I PDZ to promote the trafficking of the internalized GPCR in a sequence-dependent manner back to the cell membrane.
We tested this assumption by using two β1-AR constructs, one with an acidic amino acid at P = −3 (ESKV) and the other with a neutral amino acid at P = −3 (ATGL). These β1-AR constructs were selected because their PDZs bind selectively to the second PDZ of SAP97 (13, 29–30). We do not believe that mutagenesis of the acidic residue in P = −3 of the WT β1-AR or other GPCR to a neutral or basic amino acid would provide a valid test for this hypothesis for two reasons. First, mutagenesis of the P = −3 amino acid in the β1-AR to other amino acids affected its binding to SAP97 (Fig. 1B). Second, it should be ascertained that the chosen PDZs would bind to the same PDZ-binding protein (i.e. SAP97) and interact with the same residues in the PDZ-binding pocket (i.e. PDZ2). Using this strategy, we determined that expression of WT β1-AR or β1-AR GluR1 chimera in HEK-293 did not affect the distribution of SAP97, but their association with SAP97 after sequestration was markedly different (Fig. 7). The results of these experiments were incorporated in the roadmap illustration to indicate that PDZ-binding proteins would be internalized with the GPCR, depending on the nature of the amino acid at P = −3 (Fig. 9, ±PBP).
These results provide the first mechanistic evidence for this type of dynamic regulation and suggest that the nature of the P = −3 amino acid in the type I PDZ of a given GPCR regulated the association between the internalized GPCR and its PDZ-binding partner. This hypothesis was further supported by studies that examined the association between internalized GPCR and their PDZ-binding proteins. On the one hand, the PDZ-binding protein NHERF-1 dissociated from the PDZ (ETPM) of the platelet G protein-coupled P2Y12 receptor and from the PDZ of the β2-AR (DSLL) after the internalization of these GPCRs (34, 51). On the other hand, the PDZ-binding proteins associated with the type I PDZ (STAV) of the corticotropin-releasing hormone receptor and with the type I PDZ of chemokine X5 receptor (LTTF) were internalized with the GPCR (35, 52).
Therefore, internalization of GPCR with their respective PDZ-binding protein is another major difference between sequence-dependent and sequence-independent trafficking mechanisms. The functional significance of reversible or irreversible in trans interactions between internal GPCR and PDZ-binding proteins is currently unknown. However, recent results indicated that these interactions were of profound functional significance because they regulated spatial signaling of the GPCR (50).
Acknowledgments
We thank the confocal microscopy unit at the NEI Vision Core (NEI, National Institutes of Health, Grant 5P30EY13080-10, D. Johnson, principal investigator), the Molecular Resources Center for DNA sequencing, and Daniel Morse for figure preparation.
This work was supported, in whole or in part, by National Institutes of Health Grant HL-085848 (to S. W. B.).
- GPCR
- G protein-coupled receptor
- PDZ
- PSD-95/DLG/ZO1
- β1-AR and β2-AR
- β1- and β2-adrenergic receptor, respectively
- GRK
- G protein-coupled receptor kinase
- HEK-293
- human embryonic kidney 293
- NHERF
- Na+/H+ exchange regulatory factor
- mPKI
- myristoylated PKA inhibitor 14-22 amide
- IP
- immunoprecipitation.
REFERENCES
- 1. Hanyaloglu A. C., von Zastrow M. (2008) Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 2. Marchese A., Paing M. M., Temple B. R., Trejo J. (2008) G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 48, 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Violin J. D., Ren X. R., Lefkowitz R. J. (2006) G-protein-coupled receptor kinase specificity for β-arrestin recruitment to the β2-adrenergic receptor revealed by fluorescence resonance energy transfer. J. Biol. Chem. 281, 20577–20588 [DOI] [PubMed] [Google Scholar]
- 4. Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Ménard L., Caron M. G. (1996) Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271, 363–366 [DOI] [PubMed] [Google Scholar]
- 5. Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. (1999) A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature 401, 286–290 [DOI] [PubMed] [Google Scholar]
- 6. Sheng M., Sala C. (2001) PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 24, 1–29 [DOI] [PubMed] [Google Scholar]
- 7. Gage R. M., Kim K. A., Cao T. T., von Zastrow M. (2001) A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J. Biol. Chem. 276, 44712–44720 [DOI] [PubMed] [Google Scholar]
- 8. Gage R. M., Matveeva E. A., Whiteheart S. W., von Zastrow M. (2005) Type I PDZ ligands are sufficient to promote rapid recycling of G protein-coupled receptors independent of binding to N-ethylmaleimide-sensitive factor. J. Biol. Chem. 280, 3305–3313 [DOI] [PubMed] [Google Scholar]
- 9. Magalhaes A. C., Dunn H., Ferguson S. S. (2012) Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br. J. Pharmacol. 165, 1717–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall R. A., Premont R. T., Chow C. W., Blitzer J. T., Pitcher J. A., Claing A., Stoffel R. H., Barak L. S., Shenolikar S., Weinman E. J., Grinstein S., Lefkowitz R. J. (1998) The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature 392, 626–630 [DOI] [PubMed] [Google Scholar]
- 11. Gardner L. A., Naren A. P., Bahouth S. W. (2007) Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human β1-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J. Biol. Chem. 282, 5085–5099 [DOI] [PubMed] [Google Scholar]
- 12. He J., Bellini M., Inuzuka H., Xu J., Xiong Y., Yang X., Castleberry A. M., Hall R. A. (2006) Proteomic analysis of β1-adrenergic receptor interactions with PDZ scaffold proteins. J. Biol. Chem. 281, 2820–2827 [DOI] [PubMed] [Google Scholar]
- 13. Nooh M. M., Naren A. P., Kim S. J., Xiang Y. K., Bahouth S. W. (2013) SAP97 controls the trafficking and resensitization of the β1-adrenergic receptor through its PDZ2 and I3 domains. PLoS One 8, e63379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X., Nooh M. M., Bahouth S. W. (2013) Role of the AKAP79/150 protein in β1-adrenergic receptor trafficking and signaling in mammalian cells. J. Biol. Chem. 288, 33797–33812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gardner L. A., Delos Santos N. M., Matta S. G., Whitt M. A., Bahouth S. W. (2004) Role of the cyclic AMP-dependent protein kinase in homologous resensitization of the β1-adrenergic receptor. J. Biol. Chem. 279, 21135–21143 [DOI] [PubMed] [Google Scholar]
- 16. Gardner L. A., Tavalin S. J., Goehring A. S., Scott J. D., Bahouth S. W. (2006) AKAP79-mediated targeting of the cyclic AMP-dependent protein kinase to the β1-adrenergic receptor promotes recycling and functional resensitization of the receptor. J. Biol. Chem. 281, 33537–33553 [DOI] [PubMed] [Google Scholar]
- 17. Davies S. P., Reddy H., Caivano M., Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ehlers M. D. (2000) Reinsertion and degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 [DOI] [PubMed] [Google Scholar]
- 19. Snyder E. M., Philpot B. D., Huber K. M., Dong X., Fallon J. R., Bear M. F. (2001) Internalization of ionotropic glutamate receptors in response to mGluR1 activation. Nat. Neurosci. 4, 1079–1085 [DOI] [PubMed] [Google Scholar]
- 20. Morais Cabral J. H., Petosa C., Sutcliffe M. J., Raza S., Byron O., Poy F., Marfatia S. M., Chishti A. H., Liddington R. C. (1996) Crystal structure of a PDZ domain. Nature 382, 649–652 [DOI] [PubMed] [Google Scholar]
- 21. Liu Y., Henry G. D., Hegde R. S., Baleja J. D. (2007) Solution structure of the hDlg/SAP97 PDZ2 domain and its mechanism of interaction with HPV-18 papillomavirus E6 protein. Biochemistry 46, 10864–10874 [DOI] [PubMed] [Google Scholar]
- 22. Von Ossowski I., Oksanen E., von Ossowski L., Cai C., Sundberg M., Goldman A., Kienänen K. (2006) Crystal structure of the second PDZ domain of SAP97 in complex with a GluR-A C-terminal peptide. FEBS J. 273, 5219–5229 [DOI] [PubMed] [Google Scholar]
- 23. Heydorn A., Søndergaard B. P., Ersbøll B., Holst B., Nielsen F. C., Haft C. R., Whistler J., Schwartz T. W. (2004) A library of 7TM receptor C-terminal tails. Interactions with the proposed postendocytic sorting proteins ERM-binding phosphoprotein 50 (EBP50), N-ethylmaleimide-sensitive factor (NSF), sorting nexin 1 (SNX1), and G protein-coupled receptor-associated sorting protein (GASP). J. Biol. Chem. 279, 54291–54303 [DOI] [PubMed] [Google Scholar]
- 24. Penn R. B., Parent J. L., Pronin A. N., Panettieri R. A., Jr., Benovic J. L. (1999) Pharmacological inhibition of protein kinases in intact cells. Antagonism of β-adrenergic receptor ligand binding by H-89 reveals limitations of usefulness. J. Pharmacol. Exp. Ther. 288, 428–437 [PubMed] [Google Scholar]
- 25. Oh M. C., Derkach V. A., Guire E. S., Soderling T. R. (2006) Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 281, 752–758 [DOI] [PubMed] [Google Scholar]
- 26. Klussmann E., Maric K., Wiesner B., Beyermann M., Rosenthal W. (1999) Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J. Biol. Chem. 274, 4934–4938 [DOI] [PubMed] [Google Scholar]
- 27. Katsura T., Gustafson C. E., Ausiello D. A., Brown D. (1997) Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am. J. Physiol. 272, F817–F822 [PubMed] [Google Scholar]
- 28. Leonard A. S., Davare M. A., Horne M. C., Garner C. C., Hell J. W. (1998) SAP97 is associated with the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J. Biol. Chem. 273, 19518–19524 [DOI] [PubMed] [Google Scholar]
- 29. Cai C., Coleman S. K., Niemi K., Keinänen K. (2002) Selective binding of synapse-associated protein 97 to GluR-A R-amino-5-hydroxy-3-methyl-4-isoxazole propionate receptor subunit is determined by a novel sequence motif. J. Biol. Chem. 277, 31484–31490 [DOI] [PubMed] [Google Scholar]
- 30. Zhou W., Zhang L., Guoxiang X., Mojsilovic-Petrovic J., Takamaya K., Sattler R., Huganir R., Kalb R. (2008) GluR1 controls dendrite growth through it binding partner, SAP97. J. Neurosci. 28, 10220–10233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanowitz M., von Zastrow M. (2003) A novel endocytic recycling signal that distinguishes the membrane trafficking of naturally occurring opioid receptors. J. Biol. Chem. 278, 45978–45986 [DOI] [PubMed] [Google Scholar]
- 32. Vargas G. A., Von Zastrow M. (2004) Identification of a novel endocytic recycling signal in the D1 dopamine receptor. J. Biol. Chem. 279, 37461–37469 [DOI] [PubMed] [Google Scholar]
- 33. Bredt D. S., Nicoll R. A. (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 [DOI] [PubMed] [Google Scholar]
- 34. Puthenveedu M. A., Lauffer B., Temkin P., Vistein R., Carlton P., Thorn K., Taunton J., Weiner O. D., Parton R. G., von Zastrow M. (2010) Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dunn H. A., Walther C., Godin C. M., Hall R. A., Ferguson S. S. (2013) Role of SAP97 protein in the regulation of corticotropin-releasing factor receptor 1 endocytosis and extracellular signal-regulated kinase 1/2 signaling. J. Biol. Chem. 288, 15023–15034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gardner L. A., Hajjhussein H., Frederick-Dyer K. C., Bahouth S. W. (2011) Rab11a and its binding partners regulate the recycling of the β1-adrenergic receptor. Cell. Signal. 23, 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Magalhaes A. C., Holmes K. D., Dale L. B., Comps-Agrar L., Lee D., Yadav P. N., Drysdale L., Poulter M. O., Roth B. L., Pin J. P., Anisman H., Ferguson S. S. (2010) CRF receptor 1 regulates anxiety behavior via sensitization of 5-HT2 receptor signaling. Nat. Neurosci. 13, 622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Valentine C. D., Haggie P. M. (2011) Confinement of β1 and β2-adrenergic receptors in the plasma membrane of cardiomyocyte-like H9c2 cells is mediated by selective interactions with PDZ domain and A-kinase anchoring proteins but not caveolae. Mol. Biol. Cell 22, 2970–2982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Naim E., Bernstein A., Bertram J. F., Caruana G. (2005) Mutagenesis of the epithelial polarity gene, discs large 1, perturbs nephrogenesis in the developing mouse kidney. Kidney Int. 68, 955–965 [DOI] [PubMed] [Google Scholar]
- 40. Maxfield F. R., McGraw T. E. (2004) Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5, 121–132 [DOI] [PubMed] [Google Scholar]
- 41. Marsh E. W., Leopold P. L., Jones N. L., Maxfield F. R. (1995) Oligomerized transferrin receptors are selectively retained by a lumenal sorting signal in a long-lived endocytic recycling compartment. J. Cell Biol. 129, 1509–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chu P., Murray S., Lissin D., von Zastrow M. (1997) δ and κ opioid receptors are differentially regulated by dynamin-dependent endocytosis when activated by the same alkaloid agonist. J. Biol. Chem. 272, 27124–27130 [DOI] [PubMed] [Google Scholar]
- 43. Hu L. A., Chen W., Premont R. T., Cong M., Lefkowitz R. J. (2002) G protein-coupled receptor kinase 5 regulates β1-adrenergic receptor association with PSD-95. J. Biol. Chem. 277, 1607–1613 [DOI] [PubMed] [Google Scholar]
- 44. Fredericks Z. L., Pitcher J. A., Lefkowitz R. J. (1996) Identification of the G protein-coupled receptor kinase phosphorylation sites in the human β2-adrenergic receptor. J. Biol. Chem. 271, 13796–13803 [DOI] [PubMed] [Google Scholar]
- 45. Nobles K. N., Xiao K., Ahn S., Shukla A. K., Lam C. M., Rajagopal S., Strachan R. T., Huang T. Y., Bressler E. A., Hara M. R., Shenoy S. K., Gygi S. P., Lefkowitz R. J. (2011) Distinct phosphorylation sites on the β2-adrenergic receptor establish a barcode that encodes differential functions of β-arrestin. Sci. Signal. 4, ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seibold A., Williams B., Huang Z. F., Friedman J., Moore R. H., Knoll B. J., Clark R. B. (2000) Localization of the sites mediating desensitization of the β2-adrenergic receptor by the GRK pathway. Mol. Pharmacol. 58, 1162–1173 [DOI] [PubMed] [Google Scholar]
- 47. Tran T. M., Friedman J., Qunaibi E., Baameur F., Moore R. H., Clark R. B. (2004) Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the β2-adrenergic receptor using phosphoserine-specific antibodies. Mol. Pharmacol. 65, 196–206 [DOI] [PubMed] [Google Scholar]
- 48. Vaughan D. J., Millman E. E., Godines V., Friedman J., Tran T. M., Dai W., Knoll B. J., Clark R. B., Moore R. H. (2006) Role of the G protein-coupled receptor kinase site serine cluster in β2-adrenergic receptor internalization, desensitization, and β-arrestin translocation. J. Biol. Chem. 281, 7684–7692 [DOI] [PubMed] [Google Scholar]
- 49. Tran T. M., Jorgensen R., Clark R. B. (2007) Phosphorylation of the β2-adrenergic receptor in plasma membranes by intrinsic GRK5. Biochemistry 46, 14438–14449 [DOI] [PubMed] [Google Scholar]
- 50. Irannejad R., Tomshine J. C., Tomshine J. R., Chevalier M., Mahoney J. P., Steyaert J., Rasmussen S. G., Sunahara R. K., El-Samad H., Huang B., von Zastrow M. (2013) Conformational biosensors reveal GPCR signalling from endosomes. Nature 495, 534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nisar S. P., Cunningham M., Saxena K., Pope R. J., Kelly E., Mundell S. J. (2012) Arrestin scaffolds NHERF1 to the P2Y12 receptor to regulate receptor internalization. J. Biol. Chem. 287, 24505–24515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Signoret N., Pelchen-Matthews A., Mack M., Proudfoot A. E., Marsh M. (2000) Endocytosis and recycling of the HIV coreceptor Ccr5. J. Cell Biol. 151, 1281–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]