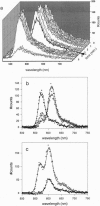
Abstract
We extend the sensitivity of fluorescence resonance energy transfer (FRET) to the single molecule level by measuring energy transfer between a single donor fluorophore and a single acceptor fluorophore. Near-field scanning optical microscopy (NSOM) is used to obtain simultaneous dual color images and emission spectra from donor and acceptor fluorophores linked by a short DNA molecule. Photodestruction dynamics of the donor or acceptor are used to determine the presence and efficiency of energy transfer. The classical equations used to measure energy transfer on ensembles of fluorophores are modified for single-molecule measurements. In contrast to ensemble measurements, dynamic events on a molecular scale are observable in single pair FRET measurements because they are not canceled out by random averaging. Monitoring conformational changes, such as rotations and distance changes on a nanometer scale, within single biological macromolecules, may be possible with single pair FRET.
Full text
PDF
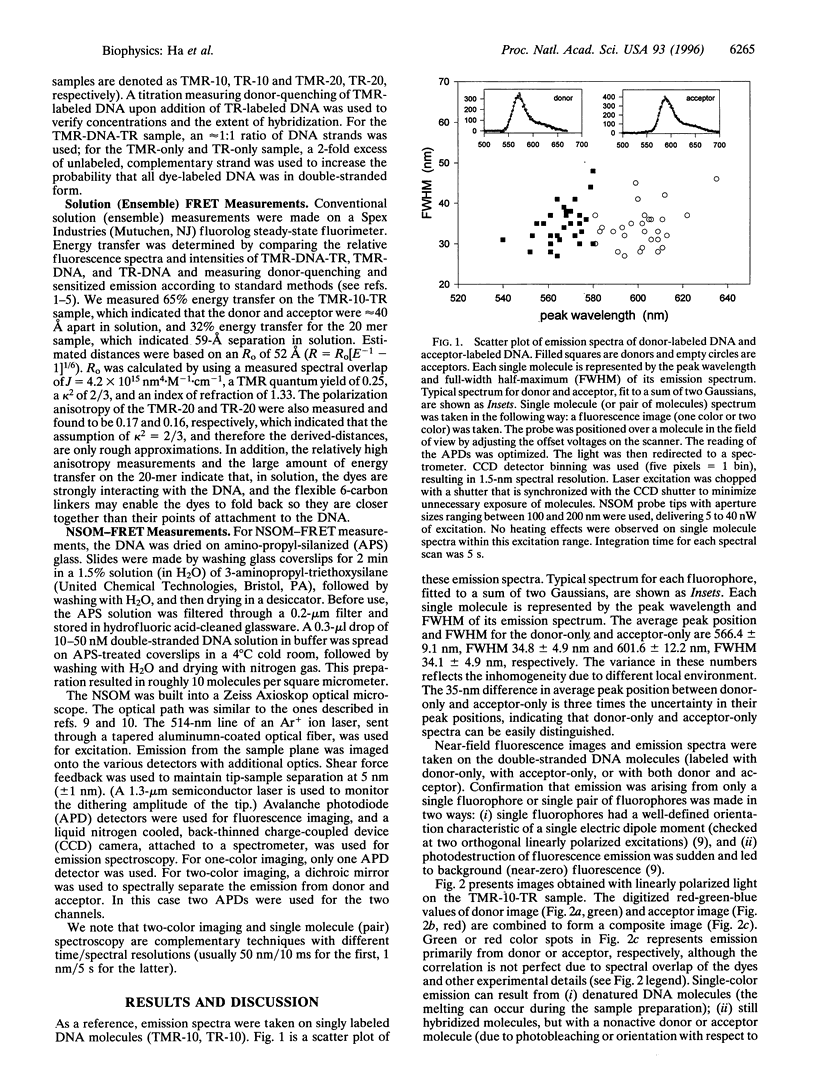
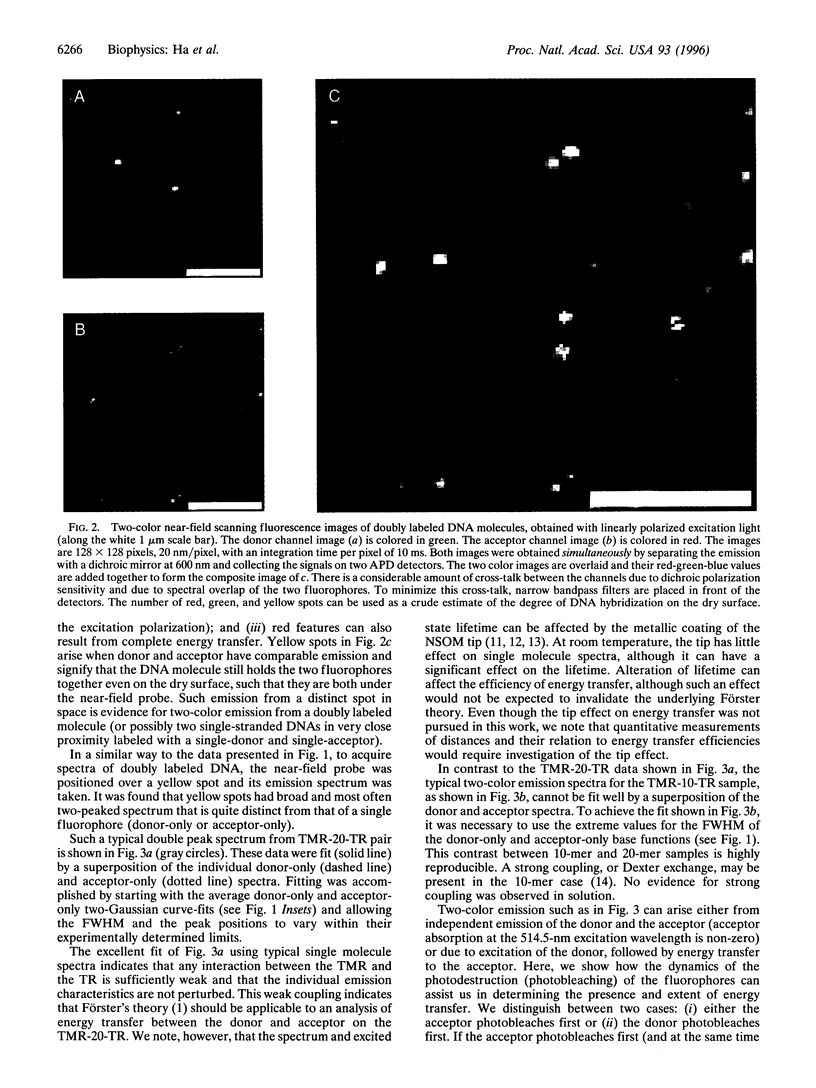
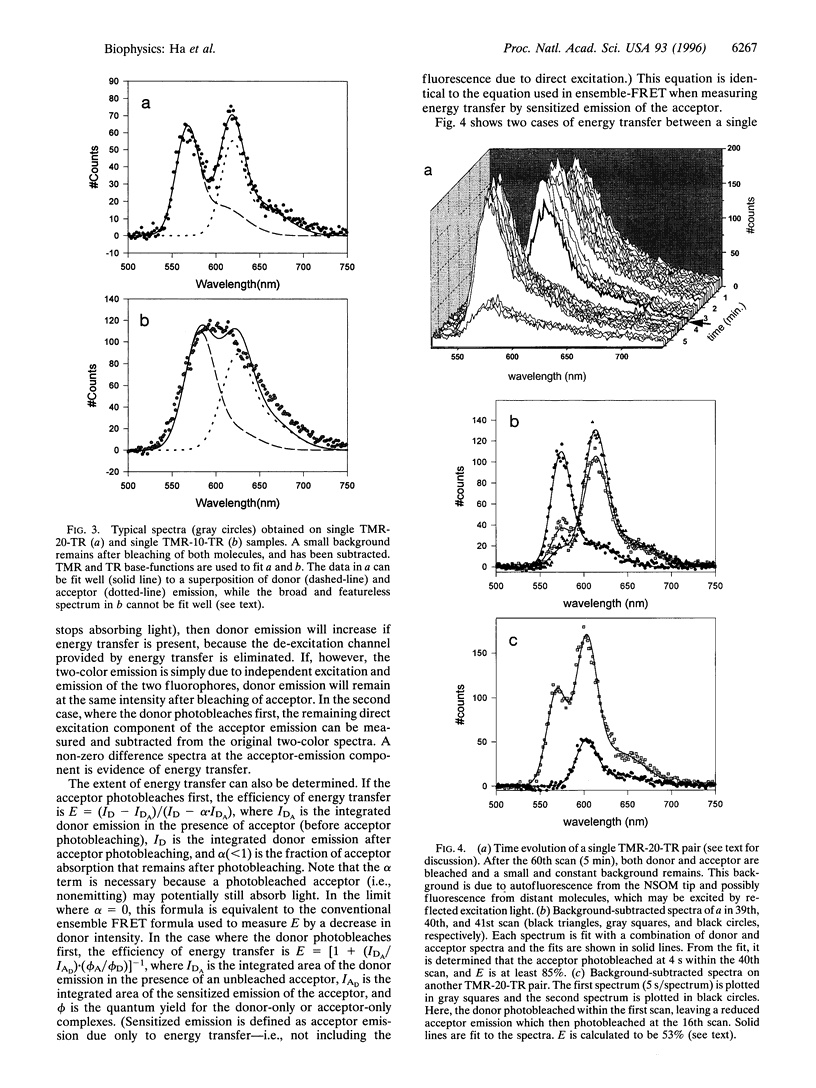

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose W. P., Goodwin P. M., Keller R. A., Martin J. C. Alterations of single molecule fluorescence lifetimes in near-field optical microscopy. Science. 1994 Jul 15;265(5170):364–367. doi: 10.1126/science.265.5170.364. [DOI] [PubMed] [Google Scholar]
- Betzig E., Chichester R. J. Single molecules observed by near-field scanning optical microscopy. Science. 1993 Nov 26;262(5138):1422–1425. doi: 10.1126/science.262.5138.1422. [DOI] [PubMed] [Google Scholar]
- Betzig E., Trautman J. K. Near-field optics: microscopy, spectroscopy, and surface modification beyond the diffraction limit. Science. 1992 Jul 10;257(5067):189–195. doi: 10.1126/science.257.5067.189. [DOI] [PubMed] [Google Scholar]
- Bian RX, Dunn RC, Xie XS, Leung PT. Single molecule emission characteristics in near-field microscopy. Phys Rev Lett. 1995 Dec 25;75(26):4772–4775. doi: 10.1103/PhysRevLett.75.4772. [DOI] [PubMed] [Google Scholar]
- Clegg R. M. Fluorescence resonance energy transfer and nucleic acids. Methods Enzymol. 1992;211:353–388. doi: 10.1016/0076-6879(92)11020-j. [DOI] [PubMed] [Google Scholar]
- Funatsu T., Harada Y., Tokunaga M., Saito K., Yanagida T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature. 1995 Apr 6;374(6522):555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- Sase I., Miyata H., Corrie J. E., Craik J. S., Kinosita K., Jr Real time imaging of single fluorophores on moving actin with an epifluorescence microscope. Biophys J. 1995 Aug;69(2):323–328. doi: 10.1016/S0006-3495(95)79937-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin P. R. Fluorescence resonance energy transfer. Methods Enzymol. 1995;246:300–334. doi: 10.1016/0076-6879(95)46015-2. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K., Block S. M. Biological applications of optical forces. Annu Rev Biophys Biomol Struct. 1994;23:247–285. doi: 10.1146/annurev.bb.23.060194.001335. [DOI] [PubMed] [Google Scholar]
- Xie X. S., Dunn R. C. Probing single molecule dynamics. Science. 1994 Jul 15;265(5170):361–364. doi: 10.1126/science.265.5170.361. [DOI] [PubMed] [Google Scholar]