Summary
OBJECTIVE
To evaluate the performance of a Verbal Autopsy (VA) expert algorithm (the InterVA model) for diagnosing AIDS mortality against a reference standard from hospital records that include HIV serostatus information in Addis Ababa, Ethiopia.
METHODS
Verbal autopsies were conducted for 193 individuals who visited a hospital under surveillance during terminal illness. Decedent admission diagnosis and HIV serostatus information is used to construct two reference standards (AIDS versus other causes of death and TB/AIDS versus other causes). The InterVA model is used to interpret the VA interviews, and the sensitivity, specificity, and cause-specific mortality fractions are calculated as indicators of the diagnostic accuracy of the InterVA model.
RESULTS
The sensitivity and specificity of the InterVA model for diagnosing AIDS are 0.82 (95%-CI: 0.74-0.89) and 0.76 (95%-CI: 0.64-0.86), respectively. The sensitivity and specificity for TB/AIDS are 0.91 (95%-CI: 0.85-0.96) and 0.78 (95%-CI: 0.63-0.89), respectively. The AIDS specific mortality fraction estimated by the model is 61.7% (95%-CI: 54%-69%), which is close to 64.7% (95%-CI: 57%-72%) in the reference standard. The TB/AIDS mortality fraction estimated by the model is 73.6% (95%-CI: 67%-80%), compared to 74.1% (95%-CI: 68%-81%) in the reference standard.
CONCLUSION
The InterVA model is an easy to use and cheap alternative to physician review for assessing AIDS mortality in countries without vital registration and medical certification of causes of death. The model seems to perform better when TB and AIDS are combined, but the sample is too small to statistically confirm that.
Keywords: mortality, surveillance, verbal autopsy, InterVA, cause of death, HIV/AIDS, Ethiopia
Introduction
Mortality statistics are an important resource for evaluating the effectiveness of antiretroviral therapy (ART) programmes (Diaz et al., 2005). In developing countries, and in Africa in particular, establishing the distribution of causes of death is often difficult due to incomplete vital registration and lack of medical certification (Mathers et al., 2005, Setel et al., 2007). Postmortem interviews with next of kin or other caregivers, also known as Verbal Autopsy (VA) interviews, are an alternative methodology for estimating the distribution of causes of death in a population (Chandramohan et al., 1994, Soleman et al., 2006). So far, the interpretation of VAs largely relied on physician reviews and it has an acceptable sensitivity and specificity for causes of death such as HIV/AIDS (Kahn et al., 2000, Quigley et al., 1999, Setel et al., 2006a). However, physician review has been criticized because the cause of death assignment is influenced by the local medical culture and may therefore not be suitable for comparisons across populations. In addition, the reliability of physician review is thought to be weak, time consuming, and not cost-effective (Murray et al., 2007, Soleman et al., 2006). Expert and data derived algorithms permit automation and standardization of the coding process and are cheaper than physician review but require validation (Quigley et al., 1999, Quigley et al., 2000, Reeves and Quigley, 1997).
The InterVA model is an expert opinion-based algorithm that has been applied in Ethiopia and Vietnam, but it has never been validated in a sample with a reliable reference or gold standard (Byass et al., 2006, Byass et al., 2003, Fantahun et al., 2006). For constructing a reference standard, VA validation studies typically rely on a sample of deaths that occurred at a health facility. These populations may be uncharacteristic in their cause of death distribution, but also in terms of the knowledge of caretakers concerning the illness and cause of death, and that may lead to the misspecification of the sensitivity and specificity of the VA tool (Lopman et al., 2006). In this study, we construct a reference standard for individuals who have ever visited a hospital during the course of their illness but did not necessarily die in a medical facility. In a different study, we established that 25% of patients in Addis Ababa died in a medical facility, but 87% had ever visited a medical facility prior to death (Reniers and Tesfai, 2009). Without arguing that our validation sample is necessarily representative, it is an improvement over a sample of exclusively hospital deaths. In addition, we use patients’ serostatus information for constructing the reference standard. Such a reference standard will more accurately distinguish AIDS from non-AIDS deaths than more commonly used reference standards in VA validation studies such as the retrospective review of medical records (Chandramohan et al., 1998, Kahn et al., 2000, Setel et al., 2006a, Setel et al., 2006b) or the physician review ascertained cause of death (Byass et al., 2003, Fantahun et al., 2006, Byass et al., 2006, Quigley et al., 1996, Quigley et al., 1999, Quigley et al., 2000).
Data and methods
The study is conducted in Addis Ababa, Ethiopia. The first diagnoses of AIDS in Ethiopia date back to 1986 (Lester et al., 1988). By 2005, HIV prevalence in the age group 15-49 had grown to 7.1% according to the Ministry of Health, an estimate that lies between those of the 2005 Demographic and Health Survey (5.0%) (CSA and ORC Macro, 2006), and earlier extrapolations from antenatal clinic data (11.7%) (MOH and NHAPCO, 2006). The impact of HIV on mortality is substantial: estimates for 2001 attribute between 54.7% and 62.4% of adult deaths (ages 20-64, both sexes) to AIDS (Reniers et al., 2006).
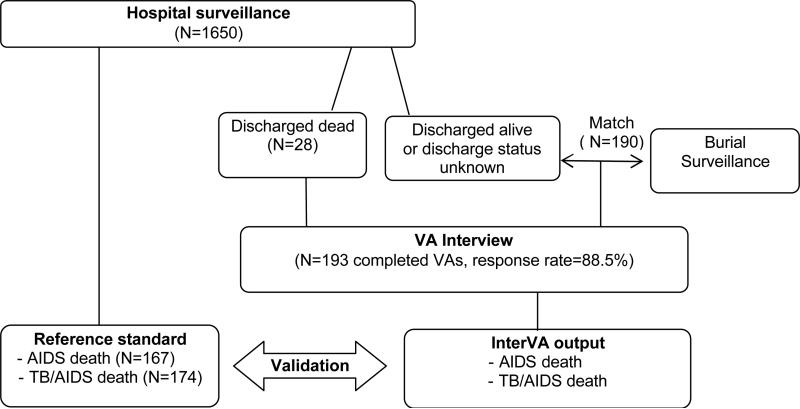
Data for this study come from hospital surveillance and a set of verbal autopsy interviews (Figure 1). The reference standard is constructed from data collected during a nine-month prospective surveillance in the Zewditu Memorial Hospital, a large governmental hospital in the center of Addis Ababa. The surveillance started in May 2003 and covered the TB-HIV clinic (serves outpatients), and the medical emergency, internal medicine, gynecology, surgical, and pediatric wards. A ward nurse collected basic background characteristics, admission and discharge diagnosis for each patient.
Figure 1.
Study protocol flow chart
A Determine rapid HIV1-2 test was carried out on the blood sample and post-test counseling was conducted by a VCT nurse. Free and informed consent was obtained either from the patient or their guardians. Capillus™ HIV-1/HIV-2 confirmatory tests were performed on positive samples. In the event that the results from the two tests were discrepant a Uni-Gold™ HIV test was done as a tie breaker. All tests were offered free of charge. An HIV test was administered for 1,332 of 1,650 registered patients. Non-response on the HIV test is due to both refusals and discharge prior to administering the test. The surveillance protocol and a discussion of the impact of non-response on HIV-1 status is given elsewhere (Reniers et al., 2009a). We also retrieved the patient card information for 38% of the decedents (the others were not found). A brief history of the decedent, physical examination, lab investigation, final diagnosis, course of treatment, condition of discharge and immediate cause of death were summarized from the patient card. Patient card information, if available, was used in conjunction with the admission diagnosis and the HIV serostatus to determine the cause of death in the reference standard.
The verbal autopsy interviews were administered for patients who died in the hospital under surveillance (N=28), and for those who died elsewhere, but whose hospital record matched with a record from an ongoing burial surveillance (N=190) (see also Figure 1). The burial surveillance was initiated in February 2001 at all known cemeteries in Addis Ababa and has been described elsewhere (Reniers et al., 2009b, Sanders et al., 2003). Verbal autopsy interviews were conducted between two and nine months (mean = 4.7) after the death by a pair of community health workers. The VA interviewers received one-week training in the administration of VAs, including fieldwork practice. The VAs are being conducted for monitoring all causes of death, so there is no specific focus on HIV/AIDS in the questionnaires, during the training or data collection The VA response rate was 88.5%. In the interview debriefing section of the VA questionnaire, the interviewers reported on the cooperativeness of the respondent and the perceived truthfulness of his or her answers. In three cases the cooperation and perceived truthfulness were low, and these cases are included in the analysis that follows.
The verbal autopsy questionnaire is based on the standard VA questionnaire developed by the INDEPTH Network (http://www.indepth-network.org/), and includes a section wherein respondents give a brief account of the illness, and a section with closed-ended questions wherein respondents are prompted for the presence of particular symptoms. Important symptoms that were not included in the structured part of the questionnaire (i.e., oral candidiasis, drowsiness, excessive food or water intake, and any prior diagnosis of a stroke) are included in the analysis if they were reported in the open-ended narrative part of the questionnaire. Bilingual Amharic-English questionnaires were developed, but the interviews were almost exclusively conducted in Amharic, the lingua franca in Ethiopia.
Reference standard
The reference standard is constructed using information from the admission diagnosis (or discharge diagnosis if available) and HIV status (Table 1). All cases with a negative HIV test result are classified as non-AIDS deaths. Among those with a positive HIV test result, deaths are classified as AIDS related if the admission diagnosis is indicative of an opportunistic infection. Two cases of HIV positive patients are coded as undetermined because the admission diagnosis did not refer to an opportunistic infection (i.e., diabetes mellitus, fever of unknown origin).
Table 1.
Definition of AIDS death in the reference standard using admission diagnosis (ICD-10), and HIV status
| HIV test Result | Cause of death | ||
|---|---|---|---|
| AIDS death | Non-AIDS death | Not classified | |
| Positive | A09, A15.0, A16.2, A18.2, A19.9, A41.9, B20.0, B24, B29, B37.0, B54, B58.2, B58.9, B59, C53.9, G05.2, I95.2, J18.9, J93.8, J98.4, K30, K60.3, K76.6, L51.1, R40.2, R50.9, R57.9, R69 | E14.0, R50.9 | |
| (N=99) | (N=0) | (N=2) | |
| Negative | A09, A16.2, A18.3, A41.9, D64.9, I10, I31.3, I50.9, I63.3, J18.9, K27, K29.7, K56.2, K56.3, K56.6, K65.0, K67.3, K75.9, K76.9, K80.1, K83.1, K93.0, N18.9, N20.0, N81.9, O03.4, R10.0, R11, R50.9, T65.9 | R50.9, R58 | |
| (N=0) | (N=54) | (N=2) | |
| Not available | A16.2, B20.0, B58.9, B59, I95.2 | A19.9, A35, I10, J18.9, K72.9, & died within seven days of admission | A09, A16.2, A19.9, A41.9, A68.9, D64.9, E16.2, I10, I95.2, J18.9, K29.7, K38.8, N81.9, O05, R57.1, R69 |
| (N=9) | (N=5) | (N=22) | |
Notes: when HIV status was not measured, the classification of cases by cause of death relied heavily on the information on the patient card. Patient card information is not summarized in the table above. The table simply classifies admission diagnoses against cause of death
The HIV status is unknown for 36 individuals. For most of these we could not establish whether the cause of death was AIDS related or not (N=22). Cases with an undetermined or missing cause of death in the reference standard are excluded from further analysis. Patients without HIV serostatus record, but with very specific AIDS related admission or patient card information (e.g., toxoplasmosis, AIDS) are classified as AIDS deaths (N=9). Five patients who were admitted for reasons other than HIV/AIDS and died within seven days of admission were classified as non-AIDS deaths.
Because TB and AIDS often occur in the same subjects, share many symptoms and are difficult to distinguish (Quigley et al., 1999), we also developed a reference standard whereby TB/AIDS cases are contrasted against non-TB/AIDS cases. The only difference with the AIDS reference standard is the reclassification of 21 TB cases (ICD10 of A15-A19) in those with an unknown or negative HIV status.
InterVA model outputs and validation
The InterVA model is a Bayesian probability theory-based expert algorithm. Bayes’ theorem defines the conditional probability of a cause in the presence of a particular indicator (Byass et al., 2003, Fantahun et al., 2006, Byass et al., 2006). The first version of the InterVA model was designed for the identification of 25 causes of death based on 66 indicators. In building the model, prior probabilities were assigned by expert panels using a semi-qualitative scale for each indicator and cause of death. The likelihood of each cause of death is calculated by applying the aforementioned theorem, taking into account each pertinent indicator. Up to three causes of death, with associated likelihoods are reported for each case. The likelihood of any cause reported by the model has to exceed the square root of the prior probability for that cause, and any second or third likely cause has to reach a likelihood of at least 50% of the most likely cause (Byass et al., 2003). For this study we used InterVA-3, which distinguishes between 35 causes of death based on 104 indicators. The model requires a local setting for the prevalence of malaria and HIV in the population (Byass et al., 2006). In the case of Addis Ababa, we specified low malaria (the city is located at high altitude) and high HIV prevalence conditions.
We define an AIDS (or TB/AIDS) death as a case where any of the InterVA assigned causes is indicative of AIDS (or TB/AIDS) and compare these with the reference standard by means of standard diagnostic statistics (sensitivity, specificity and positive predictive value (PPV)). We use receiver operating characteristic (ROC) curves to determine the likelihood cut-off point that maximizes the diagnostic accuracy of the InterVA model. Using those optimal cut-off points, we compare cause-specific mortality fractions by age from the model and reference standard.
Results
One hundred ninety three VA's were conducted for adults (aged range 14-85) with a matched hospital record. The male to female ratio of deaths in the sample is 0.95, and 90.7% of the cases pertain to adults in the 20-64 age range. Close to 82% of the decedents were tested for HIV with an HIV prevalence of 63.5%. Because of missing HIV serostatus information in the reference standard, only 167 out of 193 cases could be classified as either AIDS or non-AIDS (Table 1). The TB/AIDS reference standard is defined for 174 cases. In the reference standard, the AIDS-specific mortality fraction is 64.7% (108 out of 167 cases, 95%-CI: 57%-72%), and the TB/AIDS mortality fraction is 74.1% (129 out of 174 cases, 95%-CI: 68%-81%).
In our sample, the InterVA model provided up to two underlying causes of death, each with an assigned likelihood (Table 2). Based on the most likely cause of death, the most prominent underlying cause of death is HIV/AIDS (54.9%) followed by liver disease (15.0%) and pulmonary tuberculosis (14.5%). Close to 14% of the cases had a second cause of death assigned. The majority of those are classified as HIV/AIDS (44.4%), followed by tuberculosis (22.0%). No cases were attributed three causes of death. For making comparisons with the reference standard, we dichotomize the model outputs into AIDS versus non-AIDS and TB/AIDS versus non-TB/AIDS, and, in doing so, we consider the first as well as second cause of death.
Table 2.
Distribution of the major underlying causes of deaths using the InterVA model
| Likelihood | ||||||
|---|---|---|---|---|---|---|
| n | % | Minimum | Maximum | Mean | ||
| First cause of death | HIV/AIDS | 106 | 54.9 | 42 | 100 | 86 |
| Liver disease | 29 | 15.0 | 35 | 100 | 84 | |
| Tuberculosis | 28 | 14.5 | 55 | 98 | 81 | |
| Pneumonia/Sepsis | 9 | 4.7 | 33 | 94 | 66 | |
| Kidney or urinary disease | 3 | 1.6 | 45 | 96 | 63 | |
| Meningitis | 3 | 1.6 | 61 | 100 | 86 | |
| Stroke | 4 | 2.1 | 30 | 84 | 59 | |
| Bloody Diarrhea | 2 | 1.0 | 92 | 93 | 93 | |
| Other digestive disease | 2 | 1.0 | 51 | 72 | 62 | |
| Other | 7 | 3.6 | 24 | 93 | 59 | |
| Total | 193 | 100.0 | ||||
| Second cause of death | HIV/AIDS | 12 | 44.4 | 32 | 47 | 41 |
| Tuberculosis | 6 | 22.2 | 33 | 50 | 44 | |
| Pneumonia/Sepsis | 2 | 7.4 | 47 | 47 | 47 | |
| Liver disease | 3 | 11.1 | 30 | 37 | 34 | |
| Other | 4 | 14.8 | 24 | 44 | 34 | |
| Total | 27 | 100.0 | ||||
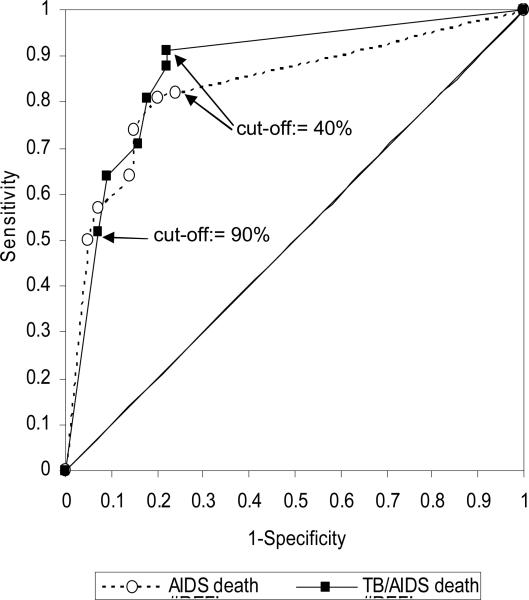
The InterVA model also provides an estimate of the likelihood with which causes of death are assigned. The likelihood of the first cause of death ranges from 24% to 100% with an average of 82.1%. The mean likelihood of the second cause of death is 40.4% varying from 24% to 50%. The likelihoods with which AIDS and TB/AIDS cases can be established are generally higher (Table 2). In Figure 2, we use those likelihoods as cut-off points (first and second most likely causes combined) to identify the likelihood level at which the area under the ROC curve is maximized. The highest likelihood cut-off point produces the lowest sensitivity and vice versa. In addition, a reduction in the likelihood cut-off point results in large gains in sensitivity relatively small losses in specificity. In case of the AIDS reference standard a maximum is reached at a likelihood cut-off point of 50%. The corresponding sensitivity and specificity are 0.81 (95%-CI: 0.73-0.88) and 0.80 (95%-CI: 0.67-0.89), respectively. For the TB/AIDS reference standard, a maximum is reached at the lowest observed likelihood cut-off point (40%). The sensitivity and specificity under that condition are 0.91 (95%-CI: 0.86-0.97) and 0.78 (95%-CI: 0.63-0.89), respectively. The optimal area under the ROC curve is 85% (95%-CI: 78%-91%) for the TB/AIDS reference standard compared to 81% (95%-CI: 74%-87%) for the AIDS standard confirming that the model performs better against a reference standard whereby TB and AIDS are combined. These differences are, however, not statistically significant. The individual-level agreement of the reference standards and model outputs are confirmed by Kappa statistics: these are 0.58 (95%-CI: 0.50-0.65) and 0.69 (95%-CI: 0.61-0.76) for the AIDS and TB/AIDS reference standards respectively.
Figure 2.
Receiver operator characteristics curves for the AIDS and TB/AIDS references standards: sensitivity plotted against specificity at different likelihood cut-off points
Note: points with the highest likelihood cut-off point have the lowest sensitivity and highest specificity, and vice versa
At the lowest likelihood cut-off point (i.e., any diagnosis of AIDS or TB/AIDS irrespective of the likelihood), and accounting for both the first and second assigned cause of death, the AIDS specific mortality fraction estimated by the InterVA model is 61.7% (103 out of 167 cases, 95%-CI: 54%-69%). This is very close to the 64.7% (108 out of 167 cases, 95%-CI: 57%-72%) in the reference standard. The TB/AIDS mortality fraction estimated by the model is even closer to the observed value: 73.6% (128 out of 174 cases, 95%-CI: 67%-80%) compared to the 74.1% (129 out of 174 cases, 95%-CI: 68%-81%) in the reference standard. Figure 3 further illustrates that the InterVA model reproduces the age pattern of AIDS and TB/AIDS mortality fairly well. Again, that is particularly the case for TB/AIDS mortality. The InterVA estimates of the AIDS mortality fraction, on the other hand, tend to be lower than in the reference standard in mid to late adulthood.
Figure 3.
AIDS and TB/AIDS specific mortality fractions by age
Discussion
The InterVA model is an easy to use and cheap alternative to the review of VA questionnaires by physicians for assigning causes of death. In this study, we confirm that it is fairly accurate in identifying AIDS and TB/AIDS mortality in individual cases, and also in determining cause-specific mortality fractions (by age). At the optimal likelihood cut-off point, the sensitivity and specificity of the model for identifying AIDS mortality are 0.81 and 0.80 respectively. The model appears to perform better when TB and AIDS mortality are grouped: the sensitivity and specificity under these circumstances are 0.92 and 0.78, respectively. Similarly, the estimated cause-specific mortality fraction of TB/AIDS (73.6%) is closer to the value in the reference standard (74.1%), than is the case for AIDS only (model estimate of 61.7% versus a value of 64.7% in the reference standard). Compared to other studies (Chandramohan et al., 1998, Lopman et al., 2006, Quigley et al., 2000) the sensitivity of the InterVA model for identifying AIDS or TB/AIDS mortality tends to be high while the specificity is relatively lower. However, should a higher level specificity level be desirable for any particular application, it is simply a matter of increasing the likelihood cut-off point.
In conclusion, this validation exercise confirms that the InterVA model is a useful tool for monitoring TB/AIDS mortality in resource constrained settings with routine VA collection, and could be valuable for assessing the impact of antiretroviral therapy programs. This study thus confirms the promise shown in earlier applications of the model (Byass et al., 2006, Byass et al., 2003, Fantahun et al., 2006). Because of the relatively small validation sample, our appraisal is restricted to adult deaths and the most important cause of death in adults, namely TB/AIDS mortality. Unlike previous studies, we were in a position to construct a more reliable reference standard because we also had the decedent's serostatus information.
Ethics
Data for this study have been collected with ethical clearance from the Addis Ababa University Faculty of Medicine (Faculty Research and Publications Committee), the Ethiopian Science and Technology Agency, the Institutional Review Boards of the University of Pennsylvania and the Centers for Disease Control and Prevention, the Research Ethics Review Committee of the WHO, and the Human Research Committee of the University of Colorado at Boulder.
Acknowledgements
The Addis Ababa Mortality Surveillance Program has been made possible with financial support from the AIDS Foundation of Amsterdam (grant nr. 7022), the World Health Organization (WHO/Second Generation Surveillance on HIV/AIDS, Contract No. SANTE/2004/089-735), the Centers for Disease Control and Prevention (EPHA-CDC Cooperative Agreement No. 5U22/PS022179_05), a Mellon Foundation pilot project grant to the Population Studies Center of the University of Pennsylvania, a Hewlett Foundation grant to the University of Colorado at Boulder for the African Population Studies Research and Training Program. The project receives institutional support from the Faculty of Medicine of Addis Ababa University and the Ethiopian Public Health Association. Religious leaders and the Addis Ababa Labour and Social Affairs Bureau facilitated our access to the burial sites. The content of this publication is the sole responsibility of the authors and does not necessarily represent the official views of any of the supporting institutions.
References
- Byass P, Fottrell E, Dao LH, Berhane Y, Corrah T, Kahn K, Muhe L, Do DV. Refining a probabilistic model for interpreting verbal autopsy data. Scandinavian Journal of Public Health. 2006;34:26–31. doi: 10.1080/14034940510032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byass P, Huong DL, Minh HV. A probabilistic approach to interpreting verbal autopsies: methodology and preliminary validation in Vietnam. Scandinavian Journal of Public Health. 2003;62:32–7. doi: 10.1080/14034950310015086. [DOI] [PubMed] [Google Scholar]
- Chandramohan D, Maude GH, Rodrigues LC, Hayes RJ. Verbal autopsies for adult deaths: issues in their development and validation. International Journal of Epidemiology. 1994;23:213–22. doi: 10.1093/ije/23.2.213. [DOI] [PubMed] [Google Scholar]
- Chandramohan D, Maude GH, Rodrigues LC, Hayes RJ. Verbal autopsies for adult deaths: their development and validation in a multicentre study. Tropical Medicine and International Health. 1998;3:436–46. doi: 10.1046/j.1365-3156.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- CSA & ORC Macro Ethiopia Demographic and Health Survey 2005. Central Statistical Authority (CSA) and ORC Macro, Addis Ababa and Calverton MA. 2006 [Google Scholar]
- Diaz T, Loth G, Whitworth J, Sutherland D. Surveillance methods to monitor the impact of HIV therapy programmes in resource-constrained countries. AIDS. 2005;19:S2, 31–37. doi: 10.1097/01.aids.0000172875.67262.21. [DOI] [PubMed] [Google Scholar]
- Fantahun M, Fottrell E, Berhane Y, Wall S, Hogberg U, Byass P. Assessing a new approach to verbal autopsy interpretation in a rural Ethiopian community: the InterVA model. Bulletin of the World Health Organization. 2006;84:204–10. doi: 10.2471/blt.05.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn K, Tollman SM, Garenne M, Gear JS. Validation and application of verbal autopsies in a rural area of South Africa. Tropical Medicine and International Health. 2000;5:824–31. doi: 10.1046/j.1365-3156.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- Lester FT, Ayehunie S, Zewdie D. Acquired immunodeficiency syndrome: seven cases in Addis Ababa hospital. Ethiopian Medical Journal. 1988;26:139–145. [PubMed] [Google Scholar]
- Lopman BA, Barnabas RV, Boerma JT, Chawira G, Gaitskell K, Harrop T, Mason P, Donnelly CA, Garnett GP, Nyamukapa C, Gregson S. Creating and validating an algorithm to measure AIDS mortality in the adult population using verbal autopsy. PLoS Medicine. 2006;3:e312. doi: 10.1371/journal.pmed.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bulletin of the World Health Organization. 2005;83:171–7. [PMC free article] [PubMed] [Google Scholar]
- MOH . Single point HIV prevalence estimate. Federal Ministry of Health; Addis Ababa: 2007. [Google Scholar]
- MOH & NHAPCO . AIDS in Ethiopia: Sixth Report. Federal Ministry of Health and National HIV/AIDS Prevention and Control Office; 2006. [Google Scholar]
- Murray CJ, Lopez AD, Feehan DM, Peter ST, Yang G. Validation of the symptom pattern method for analyzing verbal autopsy data. PLoS Medicine. 2007;4:e327. doi: 10.1371/journal.pmed.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley MA, Armstrong Schellenberg JR, Snow RW. Algorithms for verbal autopsies: a validation study in Kenyan children. Vol. 74. Bulletin of the World Health Organization; 1996. pp. 147–54. [PMC free article] [PubMed] [Google Scholar]
- Quigley MA, Chandramohan D, Rodrigues LC. Diagnostic accuracy of physician review, expert algorithms and data-derived algorithms in adult verbal autopsies. International Journal of Epidemiology. 1999;28:1081–7. doi: 10.1093/ije/28.6.1081. [DOI] [PubMed] [Google Scholar]
- Quigley MA, Chandramohan D, Setel P, Binka F, Rodrigues LC. Validity of data-derived algorithms for ascertaining causes of adult death in two African sites using verbal autopsy. Tropical Medicine and International Health. 2000;5:33–9. doi: 10.1046/j.1365-3156.2000.00517.x. [DOI] [PubMed] [Google Scholar]
- Reeves BC, Quigley MA. A review of data-derived methods for assigning causes of death from verbal autopsy data. International Journal of Epidemiology. 1997;26:1080–9. doi: 10.1093/ije/26.5.1080. [DOI] [PubMed] [Google Scholar]
- Reniers G, Araya T, Berhane Y, Davey G, Sanders EJ. Implications of the HIV testing protocol for non-response bias in seroprevalence surveys. BMC Public Health. 2009a;9:163. doi: 10.1186/1471-2458-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniers G, Araya T, Davey G, Nagelkerke N, Berhane Y, Coutinho R, Sanders EJ. Steep declines in AIDS mortality following the introduction of antiretroviral therapy in Addis Ababa, Ethiopia. AIDS. 2009b;23:511–518. doi: 10.1097/QAD.0b013e32832403d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reniers G, Araya T, Sanders EJ. Life table estimates of adult HIV/AIDS mortality in Addis Ababa. Ethiopian Journal of Health Development. 2006;20:3–9. [Google Scholar]
- Reniers G, Tesfai R. Health services utilization in terminal illness in Addis Ababa, Ethiopia. Health Policy and Planning. 2009;24:312–319. doi: 10.1093/heapol/czp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders EJ, Araya T, Kebede D, Schaap AJ, Nagelkerke ND, Coutinho RA. Mortality impact of AIDS in Addis Ababa, Ethiopia. AIDS. 2003;17:1209–1216. doi: 10.1097/00002030-200305230-00013. [DOI] [PubMed] [Google Scholar]
- Setel PW, Macfarlane SB, Szreter S, Mikkelsen L, Jha P, Stout S, AbouZahr C. A scandal of invisibility: making everyone count by counting everyone. Lancet. 2007;370:1569–77. doi: 10.1016/S0140-6736(07)61307-5. [DOI] [PubMed] [Google Scholar]
- Setel PW, Rao C, Hemed Y, Whiting DR, Yang G, Chandramohan D, Alberti KG, Lopez AD. Core verbal autopsy procedures with comparative validation results from two countries. PLoS Medicine. 2006a;3:e268. doi: 10.1371/journal.pmed.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setel PW, Whiting DR, Hemed Y, Chandramohan D, Wolfson LJ, Alberti KG, Lopez AD. Validity of verbal autopsy procedures for determining cause of death in Tanzania. Tropical Medicine and International Health. 2006b;11:681–96. doi: 10.1111/j.1365-3156.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- Soleman N, Chandramohan D, Shibuya K. Verbal autopsy: current practices and challenges. Bulletin of the World Health Organization. 2006;84:239–45. doi: 10.2471/blt.05.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]