Abstract
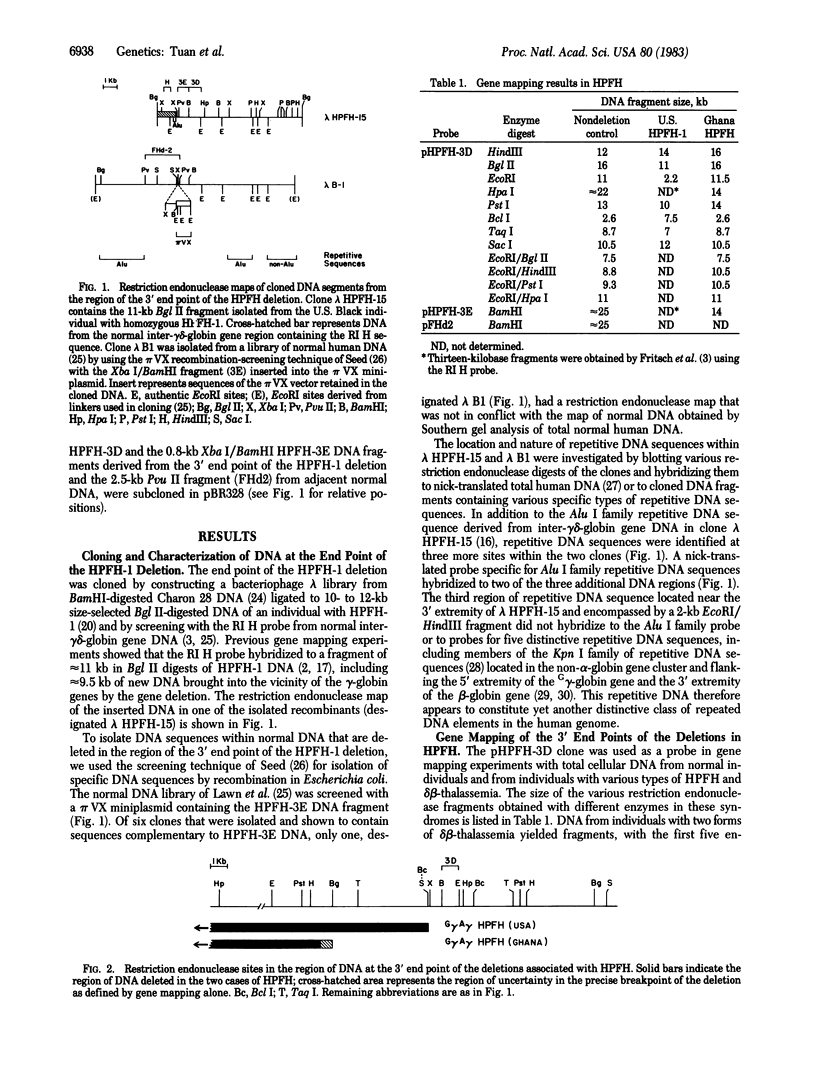
DNA at the end point of the gene deletion associated with one form of hereditary persistence of fetal hemoglobin (HPFH) was cloned and used as a probe in gene mapping experiments to analyze the extent and approximate 3' end points of various deletions associated with HPFH and delta beta-thalassemia. The deletions in the two known forms of deletion-type HPFH were shown to be considerably more extensive than in the two cases of delta beta-thalassemia studied. The overall extents of the deletions in the two types of HPFH were quite similar in both cases and the 3' end points were located at a minimum distance of approximately equal to 52 and 57 kilobases from the 3' extremity of the beta-globin gene. In contrast, the 3' end points of the deletions in the two forms of delta beta-thalassemia were located approximately equal to 5 and 10 kilobases to the 3' side of the beta-globin gene. The extent of these deletions and the nature of the DNA brought into the vicinity of the gamma-globin genes by the deletions may therefore be a more important influence on the phenotype of the deletions than the specific nature of the DNA sequences that are deleted within the non-alpha-globin gene cluster as a result of the mutations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Boehm C. D., Giardina P. J., Kazazian H. H., Jr Nonrandom association of polymorphic restriction sites in the beta-globin gene cluster. Proc Natl Acad Sci U S A. 1982 Jan;79(1):137–141. doi: 10.1073/pnas.79.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Flavell R. A. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res. 1980 Apr 11;8(7):1521–1534. doi: 10.1093/nar/8.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Kooter J. M., Flavell R. A. Physical mapping of the globin gene deletion in (delta beta (0)) -thalassaemia. Gene. 1979 Jul;6(3):265–280. doi: 10.1016/0378-1119(79)90062-3. [DOI] [PubMed] [Google Scholar]
- Boss M. A. Enhancer elements in immunoglobulin genes. Nature. 1983 May 26;303(5915):281–282. doi: 10.1038/303281a0. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Bernards R., Kooter J. M., de Boer E., Little P. F., Annison G., Williamson R. The structure of the human beta-globin gene in beta-thalassaemia. Nucleic Acids Res. 1979 Jun 25;6(8):2749–2760. doi: 10.1093/nar/6.8.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Kooter J. M., De Boer E., Little P. F., Williamson R. Analysis of the beta-delta-globin gene loci in normal and Hb Lepore DNA: direct determination of gene linkage and intergene distance. Cell. 1978 Sep;15(1):25–41. doi: 10.1016/0092-8674(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Hillman D. G., Lazarus H., Barell E. F., Benz ej J. R., Caskey C. T., Huisman T. H., Schroeder W. A., Housman D. Absence of messenger RNA and gene DNA for beta-globin chains in hereditary persistence of fetal hemoglobin. Cell. 1976 Mar;7(3):323–329. doi: 10.1016/0092-8674(76)90161-6. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Tuan D., Biro P. A., Jagadeeswaran P. O., Weissman S. M. Structural features of the DNA flanking the human non-alpha globin genes: implications in the control of fetal hemoglobin switching. Trans Assoc Am Physicians. 1981;94:204–210. [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Characterisation of deletions which affect the expression of fetal globin genes in man. Nature. 1979 Jun 14;279(5714):598–603. doi: 10.1038/279598a0. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Dahl H. H., de Boer E., Flavell R. A. Isolation of beta-globin-related genes from a human cosmid library. Gene. 1981 Apr;13(3):227–237. doi: 10.1016/0378-1119(81)90028-7. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Keys C., VarsanyiBreiner A., Kao F. T., Jones C., Puck T. T., Housman D. Isolation and localization of DNA segments from specific human chromosomes. Proc Natl Acad Sci U S A. 1980 May;77(5):2829–2833. doi: 10.1073/pnas.77.5.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung N., Roginski R. S., Henthorn P., Smithies O., Kucherlapati R., Skoultchi A. I. Introduction and expression of a fetal human globin gene in mouse fibroblasts. Mol Cell Biol. 1982 Apr;2(4):401–411. doi: 10.1128/mcb.2.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Efremov G. D., Duma H., Mladenovski B., Hyman C. B., Rachmilewitz E. A., Bouver N., Miller A., Brodie A. The present status of the heterogeneity of fetal hemoglobin in beta-thalassemia: an attempt to unify some observations in thalassemia and related conditions. Ann N Y Acad Sci. 1974;232(0):107–124. doi: 10.1111/j.1749-6632.1974.tb20576.x. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P., Tuan D., Forget B. G., Weissman S. M. A gene deletion ending at the midpoint of a repetitive DNA sequence in one form of hereditary persistence of fetal haemoglobin. Nature. 1982 Apr 1;296(5856):469–470. doi: 10.1038/296469a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J. DNA sequence variants in the G gamma-, A gamma-, delta- and beta-globin genes of man. Cell. 1979 Sep;18(1):1–10. doi: 10.1016/0092-8674(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Trent R. J., Clegg J. B., Weatherall D. J. Major rearrangement in the human beta-globin gene cluster. Nature. 1981 May 7;291(5810):39–44. doi: 10.1038/291039a0. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Trent R. J., Clegg J. B., Weatherall D. J. Restriction mapping of a new deletion responsible for G gamma (delta beta)o thalassemia. Nucleic Acids Res. 1981 Dec 21;9(24):6813–6825. doi: 10.1093/nar/9.24.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Charache S., Kazazian H. H. Deletion of the beta-globin structure gene in hereditary persistence of foetal haemoglobin. Nature. 1975 Nov 13;258(5531):162–163. doi: 10.1038/258162a0. [DOI] [PubMed] [Google Scholar]
- Kan Y. W., Lee K. Y., Furbetta M., Angius A., Cao A. Polymorphism of DNA sequence in the beta-globin gene region. Application to prenatal diagnosis of beta 0 thalassemia in Sardinia. N Engl J Med. 1980 Jan 24;302(4):185–188. doi: 10.1056/NEJM198001243020401. [DOI] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Nakamura F., Bloom A., Konotey-Ahulu F., Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. doi: 10.1073/pnas.75.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C. Deletion of the A gamma-globin gene in G gamma-delta beta-thalassemia. J Clin Invest. 1979 Sep;64(3):866–869. doi: 10.1172/JCI109535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C., Mahoney M. J., Lazarus H., Hobbins J. C., Nathan D. G. Application of endonuclease mapping to the analysis and prenatal diagnosis of thalassemias caused by globin-gene deletion. N Engl J Med. 1978 Jul 27;299(4):166–172. doi: 10.1056/NEJM197807272990403. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Old J. M., Weatherall D. J., Nathan D. G. Partial deletion of beta-globin gene DNA in certain patients with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1979 May;76(5):2400–2404. doi: 10.1073/pnas.76.5.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Comi P., Gianni A. M., Polli E., Acquaye C. T., Oldham J. H., Masera G. Globin gene deletion in HPFH, delta (o) beta (o) thalassaemia and Hb Lepore disease. Nature. 1979 Apr 12;278(5705):654–657. doi: 10.1038/278654a0. [DOI] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B., Taramelli R., Comi P., Mazza U., Saglio G., Camaschella C., Izzo P., Cao A., Galanello R. Molecular comparison of delta beta-thalassemia and hereditary persistence of fetal hemoglobin DNAs: evidence of a regulatory area? Proc Natl Acad Sci U S A. 1982 Apr;79(7):2347–2351. doi: 10.1073/pnas.79.7.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi S., Giglioni B. The deletion in a type of delta 0-beta 0-thalassaemia begins in an inverted AluI repeat. Nature. 1982 Dec 23;300(5894):770–771. doi: 10.1038/300770a0. [DOI] [PubMed] [Google Scholar]
- Seed B. Purification of genomic sequences from bacteriophage libraries by recombination and selection in vivo. Nucleic Acids Res. 1983 Apr 25;11(8):2427–2445. doi: 10.1093/nar/11.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafit-Zagardo B., Brown F. L., Maio J. J., Adams J. W. KpnI families of long, interspersed repetitive DNAs associated with the human beta-globin gene cluster. Gene. 1982 Dec;20(3):397–407. doi: 10.1016/0378-1119(82)90208-6. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Tuan D., Biro P. A., deRiel J. K., Lazarus H., Forget B. G. Restriction endonuclease mapping of the human gamma globin gene loci. Nucleic Acids Res. 1979 Jun 11;6(7):2519–2544. doi: 10.1093/nar/6.7.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Murnane M. J., deRiel J. L., Forget B. G. Heterogeneity in the molecular basis of hereditary persistence of fetal haemoglobin. Nature. 1980 May 29;285(5763):335–337. doi: 10.1038/285335a0. [DOI] [PubMed] [Google Scholar]





