Summary
Background
The importance of hyperphagia as a cause for energy imbalance in humans with Bardet-Biedl syndrome (BBS) has not been established. We therefore compared hyperphagic symptoms in patients with BBS versus controls.
Methods
We studied 13 patients with BBS and 23 nonsyndromic controls with similar age, sex, and BMI z-score. A 13-item hyperphagia questionnaire was completed by patients’ parents/guardians.
Results
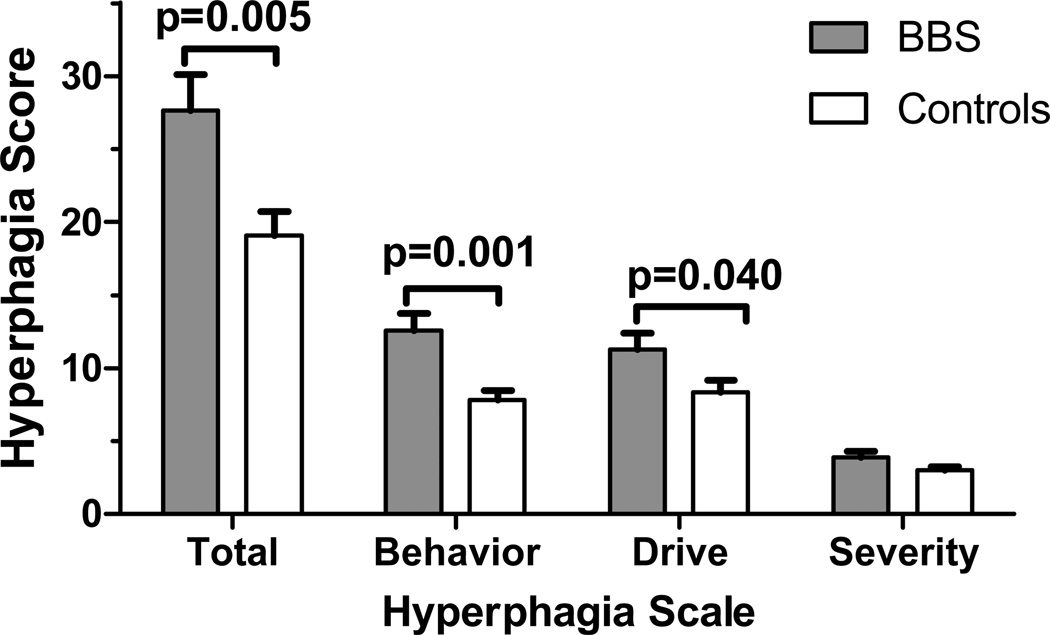
Total hyperphagia questionnaire score was higher in BBS than controls (27.6±9.0 vs. 19.1±7.9, p=0.005). Behavior and drive sub-scales were higher for BBS than controls (12.5±4.1 vs. 7.8±3.2, p=0.001, and 11.2±4.1 vs. 8.3±3.8, p=0.04, respectively); severity was not significantly different between groups (3.8±1.5 vs. 3.0±1.3, p=0.072). After adjustment for demographic variables and BMI-Z score, total and behavior subscale scores remained significantly different between groups, suggesting food-seeking activity, rather than preoccupation with food may be the main hyperphagic feature among patients with BBS.
Conclusion
Appetite dysregulation may contribute to obesity in BBS.
Keywords: hyperphagia, obesity, Bardet-Biedl syndrome, obesity syndromes, polyphagia, energy intake, questionnaire, child, adolescent
Bardet-Biedl syndrome (BBS) is a rare, genetically heterogeneous disorder (1, 2) with retinal dystrophy, post-axial polydactyly, developmental delay, renal abnormalities, and obesity in 70–90% (1, 3, 4). Genes involved in formation, stability, and function of cilia are implicated as causes of BBS (5–7). The high penetrance of the obesity phenotype in patients suggests that ciliary proteins play an important role in body energy balance and weight regulation. Several mouse models of BBS recapitulate features of the human phenotype and have altered protein/vesicle trafficking in leptin-sensing hypothalamic neurons, possibly inducing leptin resistance and hyperphagia, consistent with the observation that BBS knockout mice consume 20–30% more energy than controls (8, 9). Human studies characterizing the obesity phenotype of patients with BBS are few (10–12) and do not provide definitive data for the contributions of increased energy intake, decreased physical activity or altered metabolism to their obesity. A study of energy homeostasis in 20 BBS overweight or obese subjects and 20 controls reported no differences in energy intake or resting energy expenditure, but found physical activity (by actigraphy) was slightly, but significantly, lower among patients with BBS (10). This study, however, used a self-report 7-day food diary for energy intake assessment that, given the cognitive impairment of patients with BBS, may need to be interpreted cautiously.
There are several syndromes where obesity occurs together with cognitive dysfunction and the obesity is associated with hyperphagic symptoms (13–16). One example is the Prader-Willi syndrome (PWS) (13). Patients with PWS show defects in post-meal satiation and satiety in the laboratory setting (17, 18). They have many hyperphagic characteristics, in addition to overeating, that include preoccupation with food (hyperphagic drive), excessive food seeking such as waking up at night to seek food, foraging through trash, stealing and hiding food (hyperphagic behavior), and significant functional impairment and distress when denied food. To address the challenges of requiring intellectually-impaired participants to report their own behaviors, Dykens et al. (13) developed and validated in patients with PWS, a questionnaire that assesses hyperphagic symptoms by asking caregivers to report their observations. Given the convincing data for hyperphagia found in BBS mouse models (8, 9), we hypothesized that, if increased food intake was a cause of obesity in humans with BBS, the caregivers of patients with BBS would report greater hyperphagic symptoms than would caregivers of equally obese non-syndromic controls.
We recruited a convenient sample of 13 patients diagnosed with BBS using clinical criteria (19) from among participants in an observational study of the genetic and clinical characteristics of patients with BBS (http://clinicaltrials.gov/ct2/show/NCT00078091). Healthy control subjects (with no known syndrome associated with obesity or major illness affecting appetite) were selected from among participants in studies of eating behavior and metabolism (http://clinicaltrials.gov/ct2/show/NCT00758108, http://clinicaltrials.gov/ct2/show/NCT00631644, and http://clinicaltrials.gov/ct2/show/NCT01237041). The studies were approved by the NICHD or NHGRI IRBs. Participants provided consent/assent for participation. The Hyperphagia Questionnaire (13) is a 13-item questionnaire with 11 items rated on a 5-point Likert scale (from 1= not a problem at all, to 5= extremely problematic) that produce a total hyperphagic score (range 11–55) and three sub-scales: hyperphagic behavior (range 5–25), hyperphagic drive (range 4–20), and hyperphagic severity (range 2–10) (13). Questions 12 and 13 seek information on the age when a child’s interest in food increased and on how much the child’s interest in food changes from day to day. The Hyperphagia Questionnaire was completed by the parent/guardian who spent the most time with the participant. Using analyses of covariance (ANCOVA), we compared Hyperphagia Questionnaire scale scores for BBS vs. the control group, adjusting for age, sex, race, and BMI-Z score. Chi-square test was used to compare the two groups for the additional questions; day-to-day variability in behavior is not reported because of many missing responses in the control group.
Characteristics of study subjects are summarized in Table 1. The subjects in the two groups did not differ significantly in sex, age, race, or BMI-Z score, although height was significantly lower among BBS, which might be attributable to slower growth in adolescence (20). Among patients with BBS, genomic sequencing confirmed 1 case had mutations in BBS6, 1 in BBS7, and 6 in BBS10 (data not shown). In unadjusted comparisons, the total hyperphagia score (p=0.005), behavioral subscale (p=0.001), and drive subscale scores (p=0.040) were significantly greater in patients with BBS than controls (Figure 1). After adjustment for age, sex, race, and BMI-Z score, group differences remained significant for total hyperphagia score (p=0.032) and the behavioral subscale (p=0.003). Among those whose caregivers reported an age when they first observed increased interest in food, 91% (10/11) of patients with BBS, versus 42% (5/12) of controls reported increased interest in food before age 5 y (p=0.013). Each of the subscales and total hyperphagia score were highly correlated across the sample (r’s >0.85 for correlations between scores, all p<0.001).
Table 1.
Subject characteristics
| Characteristic | Patients with BBS (N=13) |
Controls (N=23) |
p-value† |
|---|---|---|---|
| Sex (% female) | 54 | 52 | 0.923 |
| Race (% Non-Hispanic White) | 85 | 52 | 0.052 |
| Age (y)* | 10.3 ±3.8 | 12.7±4.2 | 0.105 |
| Height (cm)* | 138.1 ± 23.1 | 154.7 ± 24.2 | 0.047 |
| Height-Z score* | −0.3±1.7 | 0.6±0.9 | 0.066 |
| Weight (kg)* | 65.3 ± 32.9 | 81.3 ± 32.4 | 0.172 |
| BMI (kg/m2)* | 32.4 ± 12.2 | 32.5 ± 8.3 | 0.973 |
| BMI-Z score* | 2.39 ± 0.58 | 2.30 ± 0.59 | 0.673 |
Mean ± SD
Chi square for sex and race; t-tests for other characteristics.
Figure 1.
Total hyperphagia score and sub-scale scores assessed with hyperphagia questionnaire. After adjustment for age, sex, race, and BMI-Z score, total score (p=0.032) and behavior subscale (p=0.003) remained significantly different.
Caregivers reported that patients with BBS displayed hyperphagic behaviors that started in early childhood. Our limited data do not allow us to determine if there are different developmental periods when hyperphagia is more evident, as is found in patients with PWS, whose extreme food seeking behaviors begin after age 2 y but subsequently subside (13, 21). Elevated scores among patients with BBS were comparable to reports from patients with PWS (total: 30.47±5.84, behavior: 13.57±4.52, drive: 12.29±3.32, severity: 4.61±1.63) or WAGR syndrome having BDNF haploinsufficiency (total: 26.37±7.32, behavior: 10.63±3.22, drive: 11.53±3.44, and severity: 4.21±1.70) (13, 14); however, the BBS group analyzed here did not have statistically greater hyperphagic drive or severity sub-scale scores compared to their matched controls. These findings are consistent with the clinical observation that food-seeking activity, rather than preoccupation with food or time spent thinking about food, may be the distinguishing hyperphagic feature in BBS. Patients with BBS typically do not become uncontrollably distressed and/or dysfunctional because of food-related behavior, but may still overeat, more so than individuals with nonsyndromic obesity, when afforded free access to food.
This investigation, the first to document hyperphagic behaviors among patients with BBS, is however limited by its small sample size and the absence of a criterion measure of body composition. Subgroup analyses of a range of age groups, as well as BBS genotypes, ideally in a larger sample, would give additional information, as would detailed studies of energy expenditures. Test meal studies conducted under standard conditions to measure energy intake, satiety, and satiation are warranted to confirm the role of higher intake in energy imbalance among patients with BBS.
Acknowledgments
This research was supported by the Intramural Research Programs of the NICHD, and NHGRI, NIH. J.A.Y. is a Commissioned Officer in the U.S. Public Health Service, Department of Health and Human Services. The funding organizations played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of data; or the preparation or review of the manuscript.
References
- 1.Hrynchak PK. Bardet-Biedl syndrome. Optometry and vision science : official publication of the American Academy of Optometry. 2000;77(5):236–243. doi: 10.1097/00006324-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Sheffield VC. The blind leading the obese: the molecular pathophysiology of a human obesity syndrome. Transactions of the American Clinical and Climatological Association. 2010;121:172–81. discussion 181-2. [PMC free article] [PubMed] [Google Scholar]
- 3.Waters AM, Beales PL. Bardet-Biedl Syndrome. 1993 [Google Scholar]
- 4.Moore SJ, Green JS, Fan Y, et al. Clinical and genetic epidemiology of Bardet-Biedl syndrome in Newfoundland: a 22-year prospective, population-based, cohort study. American journal of medical genetics. 2005;132(4):352–360. doi: 10.1002/ajmg.a.30406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox KF, Kerr NC, Kedrov M, et al. Phenotypic expression of Bardet-Biedl syndrome in patients homozygous for the common M390R mutation in the BBS1 gene. Vision Res. 2012 doi: 10.1016/j.visres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annual review of genomics and human genetics. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 7.Guo DF, Rahmouni K. Molecular basis of the obesity associated with Bardet-Biedl syndrome. Trends Endocrinol Metab. 2011;22(7):286–293. doi: 10.1016/j.tem.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahmouni K, Fath MA, Seo S, et al. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118(4):1458–1467. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18(7):1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grace C, Beales P, Summerbell C, et al. Energy metabolism in Bardet-Biedl syndrome. Int J Obes Relat Metab Disord. 2003;27(11):1319–1324. doi: 10.1038/sj.ijo.0802420. [DOI] [PubMed] [Google Scholar]
- 11.Daskalakis M, Till H, Kiess W, Weiner RA. Roux-en-Y gastric bypass in an adolescent patient with Bardet-Biedl syndrome, a monogenic obesity disorder. Obes Surg. 2010;20(1):121–125. doi: 10.1007/s11695-009-9915-6. [DOI] [PubMed] [Google Scholar]
- 12.Feuillan PP, Ng D, Han JC, et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J Clin Endocrinol Metab. 2011;96(3):E528–E535. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E. Assessment of hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring) 2007;15(7):1816–1826. doi: 10.1038/oby.2007.216. [DOI] [PubMed] [Google Scholar]
- 14.Han JC, Liu QR, Jones M, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359(9):918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray J, Yeo GS, Cox JJ, et al. Hyperphagia, Severe Obesity, Impaired Cognitive Function, and Hyperactivity Associated With Functional Loss of One Copy of the Brain-Derived Neurotrophic Factor (BDNF) Gene. Diabetes. 2006;55(12):3366–3371. doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo GS, Connie Hung CC, Rochford J, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7(11):1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 17.Zipf WB, Berntson GG. Characteristics of abnormal food-intake patterns in children with Prader-Willi syndrome and study of effects of naloxone. Am J Clin Nutr. 1987;46(2):277–281. doi: 10.1093/ajcn/46.2.277. [DOI] [PubMed] [Google Scholar]
- 18.Holland AJ, Treasure J, Coskeran P, Dallow J, Milton N, Hillhouse E. Measurement of excessive appetite and metabolic changes in Prader-Willi syndrome. Int J Obes Relat Metab Disord. 1993;17(9):527–532. [PubMed] [Google Scholar]
- 19.Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36(6):437–446. [PMC free article] [PubMed] [Google Scholar]
- 20.Feuillan PP, Ng D, Han JC, et al. Patients with Bardet-Biedl Syndrome Have Hyperleptinemia Suggestive of Leptin Resistance. J Clin Endocrinol Metab. 2011:2010–2290. doi: 10.1210/jc.2010-2290. jc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller JL, Lynn CH, Driscoll DC, et al. Nutritional phases in Prader-Willi syndrome. Am J Med Genet A. 2011;155A(5):1040–1049. doi: 10.1002/ajmg.a.33951. [DOI] [PMC free article] [PubMed] [Google Scholar]