Abstract
Glutamate receptors in the basolateral complex of the amygdala (BLA) are essential for the acquisition, expression and extinction of Pavlovian fear conditioning in rats. Recent work has revealed that glutamate receptors in the central nucleus of the amygdala (CEA) are also involved in the acquisition of conditional fear, but it is not known whether they play a role in fear extinction. Here we examine this issue by infusing glutamate receptor antagonists into the BLA or CEA prior to the extinction of fear to an auditory conditioned stimulus (CS) in rats. Infusion of the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptor antagonist, 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), into either the CEA or BLA impaired the expression of conditioned freezing to the auditory CS, but did not impair the formation of a long-term extinction memory to that CS. In contrast, infusion of the N-methyl-d-aspartate (NMDA) receptor antagonist, d,l-2-amino-5-phosphonopentanoic acid (APV), into the amygdala, spared the expression of fear to the CS during extinction training, but impaired the acquisition of a long-term extinction memory. Importantly, only APV infusions into the BLA impaired extinction memory. These results reveal that AMPA and NMDA receptors within the amygdala make dissociable contributions to the expression and extinction of conditioned fear, respectively. Moreover, they indicate that NMDA receptor-dependent processes involved in extinction learning are localized to the BLA. Together with previous work, these results reveal that NMDA receptors in the CEA have a selective role acquisition of fear memory.
Keywords: AMPA, amygdala, extinction, NMDA, Pavlovian fear conditioning, rats
Introduction
Pavlovian fear conditioning is an important behavioral paradigm used to study the neurobiological mechanisms of emotional learning and memory (Davis, 1992; Fendt & Fanselow, 1999; LeDoux, 2000; Maren, 2001, 2005a). In this form of conditioning, an animal learns that a neutral conditioned stimulus (CS), such as a tone, predicts an aversive unconditioned stimulus (US), such as a footshock. After conditioning, the CS alone elicits a variety of conditioned fear responses (CRs), including increases in blood pressure, potentiated acoustic startle and freezing behavior. Degrading the relationship between the CS and the US by presenting the CS alone numerous times results in an extinction of fear to the CS. During extinction, animals learn a new inhibitory memory that suppresses fear. This suppression is labile, however, and fear CRs may return with changes in context (renewal), for example (Maren, 2005a; Bouton et al., 2006).
In recent years, there has been a growing interest in understanding the neurobiological mechanisms of fear extinction (Maren & Quirk, 2004; Bouton et al., 2006; Corcoran & Quirk, 2007; Myers & Davis, 2007; Quirk & Mueller, 2008). It is now well established that the basolateral complex of the amygdala (BLA) is crucial for the acquisition, expression and extinction of conditioned fear (Fendt & Fanselow, 1999; LeDoux, 2000; Davis & Whalen, 2001; Maren, 2001). Within the BLA, considerable work has revealed an important role for glutamate receptors in these processes. Specifically, infusions of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptor (AMPAR) or N-methyl-d-aspartate receptor (NMDAR) antagonists into the BLA impair the expression of conditioned fear (Miserendino et al., 1990; Campeau et al., 1992; Maren et al., 1996; Lee & Kim, 1998; Fendt, 2001; Lee et al., 2001; Rodrigues et al., 2001; Goosens & Maren, 2004; Walker et al., 2005), whereas NMDAR antagonists prevent the acquisition and extinction of fear (Miserendino et al., 1990; Campeau et al., 1992; Falls et al., 1992; Cox & Westbrook, 1994; Fanselow & Kim, 1994; Maren et al., 1996; Lee & Kim, 1998; Rodrigues et al., 2001; Santini et al., 2001; Lin et al., 2003; Goosens & Maren, 2004; Maren & Quirk, 2004; Walker et al., 2005). Interestingly, infusions of NMDAR agonists into the BLA facilitate extinction (Walker et al., 2002).
In addition to the BLA, there is a growing appreciation for the role played by the central nucleus of the amygdala (CEA) in Pavlovian fear conditioning. For example, recent work has shown that lesions, temporary inactivation or NMDAR antagonism of the CEA block the acquisition of conditioned fear (Goosens & Maren, 2003; Wilensky et al., 2006; Zimmerman et al., 2007). Furthermore, protein synthesis inhibition within the CEA immediately after the acquisition of conditioned fear blocks the consolidation of the fear memory (Wilensky et al., 2006). Consistent with the role of the CEA in the acquisition of fear, Samson & Pare (2005) have demonstrated NMDAR-dependent plasticity within the CEA in vitro. These findings suggest the possibility that CEA glutamate receptors, and NMDARs in particular, have a role in the extinction of fear. Here we address this issue by comparing the effects of glutamate receptor antagonism in the BLA and CEA on the extinction of fear to an auditory CS in rats.
Materials and methods
Experiment 1: AMPAR antagonism in the BLA or CEA and fear extinction
Subjects
The subjects were 53 male Long–Evans rats (200–224 g; Blue Spruce) obtained from a commercial supplier (Harlan Sprague Dawley, Indianapolis, IN, USA). After arrival, the animals were individually housed in clear plastic cages hanging from a standard stainless-steel rack. The vivarium lights were on a 14/10 h light/dark cycle (lights on at 07.00 h), and the rats had free access to food and tap water. After housing, the rats were handled (15–20 s each) for 5 days to acclimate them to the experimenter. All experiments were carried out in accordance with guidelines of the NIH and approved by the University of Michigan University Committee on Use and Care of Animals.
Behavioral apparatus
Eight identical observation chambers (30 × 24 × 21 cm; Med-Associates, St Albans, VT, USA) were used for all phases of training and testing. The chambers were constructed from aluminum (two side walls) and Plexiglas (rear wall, ceiling and hinged front door), and were situated in sound-attenuating chests located in an isolated room. The floor of each chamber consisted of 19 stainless-steel rods (4 mm diameter) spaced 1.5 cm apart (center to center). The rods were wired to a shock source and solid-state grid scrambler (Med-Associates) for delivery of the footshock US (1.0 mA, 2 s). For ‘context A’ (used for conditioning), background noise (65 dB) was provided by ventilation fans built into the chests, house lights within the chambers and fluorescent lights within the room provided illumination, the chest doors were left open, the chambers were cleaned with a 1% ammonium hydroxide solution, and the rats were transported in black carriers. For ‘context B’ (used for Drug and Drug-Free Extinction), illumination was provided by incandescent red lights, the chest doors were closed, the ventilation fans were inactive, the chambers were cleaned with a 1% acetic acid solution, the floors were covered with black plastic panels, and the rats were transported in white 5-gallon buckets. Stainless-steel pans containing a thin film of the corresponding cleaning solutions were placed underneath the grid floors before the animals were placed inside the boxes.
Each conditioning chamber rested on a load cell platform that was used to record chamber displacement in response to each rats’ motor activity. To ensure interchamber reliability, each load cell amplifier was calibrated to a fixed chamber displacement. The output of the load cell of each chamber was set to a gain that was optimized for detecting freezing behavior. Load cell amplifier output from each chamber was digitized and acquired on-line using Threshold Activity software (Med-Associates).
Surgery
After handling for at least 5 days, rats were treated with atropine sulfate (0.4 mg/kg body weight, i.p.) and sodium pentobarbital (65 mg/kg body weight, i.p.), and mounted in stereotaxic apparatus (David Kopf instruments, Tujunga, CA, USA). The scalp was incised and retracted, and head position was adjusted to place bregma and lambda in the same horizontal plane. Small burr holes were drilled bilaterally in the skull for the placement of 26-gage guide cannula (cut at 11 mm below the pedestal; Plastics One, Roanoke, VA, USA) in the BLA (2.8 mm posterior to bregma, 5.0 mm lateral to the midline, 6.3 mm ventral to dura) or CEA (2.5 mm posterior to bregma, 4.3 mm lateral to the midline, 6.9 mm ventral to the skull surface), and three small screws. Following implantation dental acrylic was applied to the skull to hold the cannula in place. After surgery, dummy cannulae (33-gage, 16 mm; Plastics One) were inserted into the guide cannula, and the rats were allowed to recover from the anesthesia before being returned to their home cages. The dummy cannulae were replaced every other day during the week of recovery.
Procedure
After at least 7 days recovery from surgery, rats were acclimated to the infusion procedure by transporting them to the infusion room in identical white 5-gallon buckets in squads of eight (counterbalanced for each squad and group). Their dummy cannulas were replaced and the infusion pumps (Harvard Apparatus, South Natick, MA, USA) were activated. After 5 min, the pumps were stopped and the animals were returned to their home cages. Twenty-four hours after acclimation, on the conditioning day, the rats were transported to the laboratory in squads of eight and placed in the conditioning chambers. The chamber position was counterbalanced for each squad and group. The rats received five tone (80 dB, 10 s, 2 kHz)–shock (1.0 mA, 2.0 s) pairings (70-s intertrial interval) beginning 3 min after being placed in the chamber and ending 60 s after the final shock (context A). The rats were then transported back to their home cages. Twenty-four hours after training, the rats were transported to the infusion room as described above and infused with the AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione [NBQX; 2.5 µg in 0.25 µL of 100 mm phosphate-buffered saline (PBS) for the CEA or 5.0 µg in 0.5 µL of 100 mm PBS for the BLA at 0.1 µL/min] or 100 mm PBS (VEH; 0.25 µL for the CEA or 0.5 µL for the BLA at 0.1 µL/min). After the infusion, 1 min was allowed for diffusion before removing the internal cannula. After removing the internal cannulae, clean dummy cannulae were inserted into the guide cannula and rats were immediately transported to the conditioning chambers for Drug Extinction. Extinction consisted of 45 CS-alone presentations (80 dB, 10 s, 2 kHz) with a 30-s intertrial interval beginning 3 min after being placed in the chamber and ending 3 min after the final CS presentation for rats in the extinction groups (BLA-NBQX-E, CEA-NBQX-E, VEH-E; context B). During the Drug Extinction session, rats in the no-extinction group (VEH-NE) were placed in the conditioning chamber for the same amount of time as the extinction groups in the absence of any CS presentations (context B). Forty-eight hours after training the rats were transported to the conditioning chambers for Drug-Free Extinction (context B). Drug-Free Extinction was identical to the Drug Extinction performed 24 h prior (all groups received CS presentations).
During the training and extinction sessions, each rat’s activity was monitored continuously using the data acquisition software described above. For each chamber, load cell activity was digitized at 5 Hz, yielding one observation per rat every 200 ms (300 observations/rat/min). Load cell values ranged between 0 and 100, and this value was used to quantify locomotor activity. Freezing was quantified by computing the number of observations for each rat that had a load cell value less than the freezing threshold (threshold = 10). The freezing threshold was determined in a separate group of pilot animals by comparing load cell output with an observer’s rating of freezing behavior. To avoid counting momentary inactivity as freezing, an observation was only scored as freezing if it fell within a contiguous group of at least five observations that were all less than the freezing threshold. Thus, freezing was only scored if the rat was immobile for at least 1 s. For each session, the freezing observations were transformed to a percentage of total observations. In the present experiment, freezing was quantified before footshock during the pretrial period and after footshock offset on the conditioning day, and throughout the entirety of the extinction tests.
Histology
Histological verification of cannula placements was performed after behavioral testing. Rats were killed with CO2 asphyxiation followed by decapitation. After extraction from the skull, the brains were fixed in 10% formalin for at least 2 days, followed by 10% formalin and 30% sucrose until sectioning. Coronal sections (45 µm thick, taken every 135 µm) were cut on a cryostat (−20 °C) and wet-mounted on glass microscope slides with 70% ethanol. After drying, the sections were stained with 0.25% thionin to visualize neuronal cell bodies. Placements were verified by visual inspection of the stained brain sections.
Data analysis
For each session, the freezing data were transformed to a percentage of total observations, a probability estimate that is amenable to analysis with parametric statistics. These probability estimates of freezing were analysed using analysis of variance (anova). Post hoc comparisons in the form of Fisher’s PLSD tests were performed after a significant overall F-ratio. All data are represented as means ± SEMs.
Experiment 2: NMDAR antagonism in the BLA or CEA and fear extinction
Subjects
The subjects were 60 male Long–Evans rats (200–224 g; Blue Spruce) obtained and housed as described in Experiment 1.
Apparatus, surgery, procedure, histology and data analysis
All materials and methods are as described in Experiment 1, except that rats were infused with the NMDAR antagonist d,l-2-amino-5-phosphonopentanoic acid (APV; 2.5 µg in 0.25 µL of 100 mm PBS for the CEA or 5.0 µg in 0.5 µL of 100 mm PBS for the BLA at 0.1 µL/min) or 100 mm PBS (VEH; 0.25 µL for the CEA or 0.5 µL for the BLA at 0.1 µL/min).
Results
Experiment 1: AMPAR antagonism in the BLA or CEA prevents the expression, but not extinction, of conditioned fear
Histology
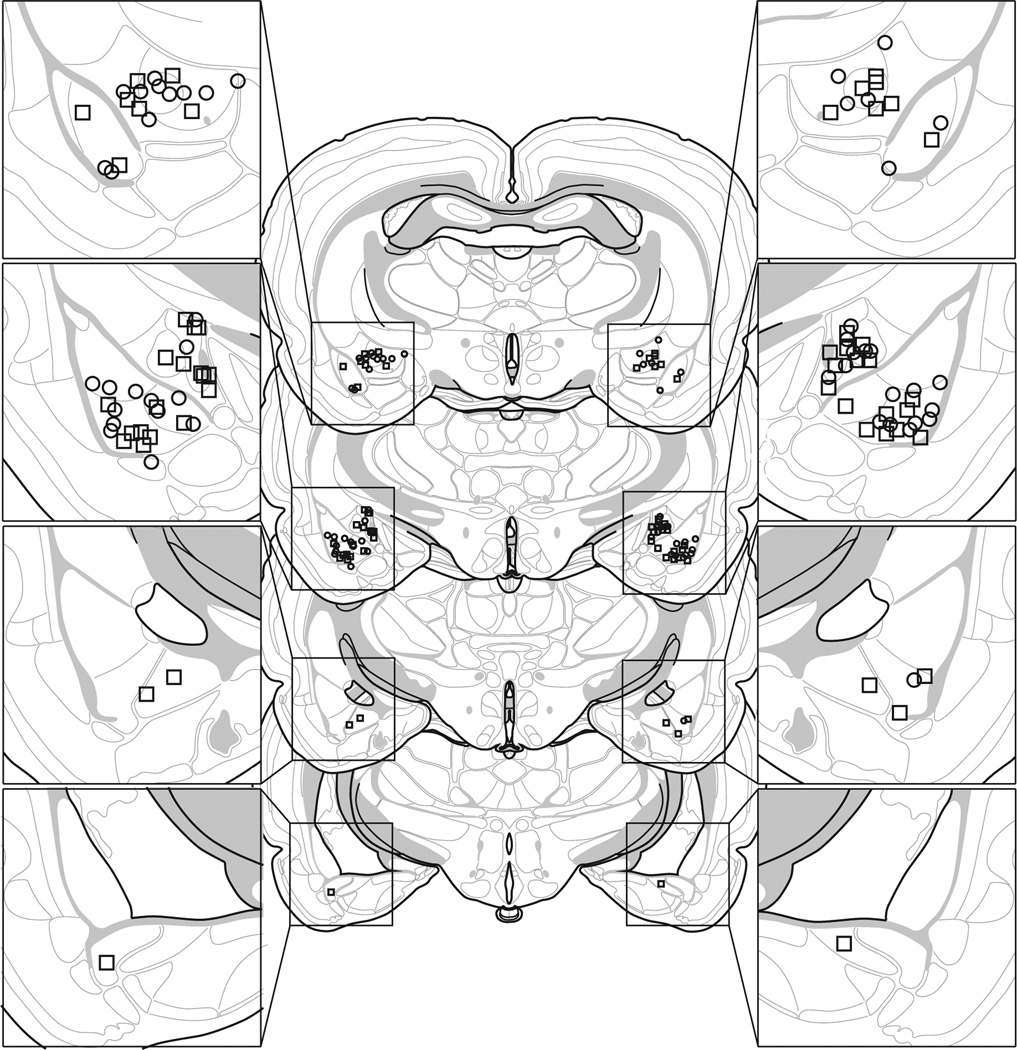
Based on histological results, 53 rats were included in this experiment. Rats were excluded if their guide cannulae were located outside the intended structure. This yielded the following group sizes: rats receiving NBQX in the BLA during extinction (BLA-NBQX-E; n = 12), rats receiving NBQX in the CEA during extinction (CEA-NBQX-E; n = 12), rats receiving PBS in the BLA or CEA during extinction, were not statistically different and were collapsed into a single group (VEH-E; n = 18), and rats receiving PBS in the BLA or CEA that did not receive extinction, were not statistically different and were collapsed into a single group (VEH-NE; n = 11). BLA and CEA cannula placements for rats included in the analysis are depicted in Fig. 1. All cannula placements were located within the intended structures (BLA or CEA).
Fig. 1.
Schematic representation showing the discrete locations of the internal cannula used to infuse saline (squares) or drug (APV or NBQX; circles). Coronal brain section images adapted from Swanson (1992).
Behavior
Post-shock freezing during the conditioning session is shown in Fig. 2A. Freezing was not statistically different across groups. The data were analysed using two-way anova with variables of group (BLA-NBQX-E, CEA-NBQX-E, VEH-E, VEH-NE) and trial (1–5). During the pre-trial period rats displayed minimal levels of freezing (< 10%). After the onset of conditioning rats displayed increased levels of freezing. The anova revealed no main effect of group (F3,49 = 0.97; P = 0.42) or a group × trial interaction (F12,196 = 1.11; P = 0.35). Additionally, the anova revealed a main effect of trial (F4,196 = 12.38; P < 0.0001). This indicates that the average level of freezing across the training session was not significantly different between the groups. However, the groups increased their freezing as the training session proceeded.
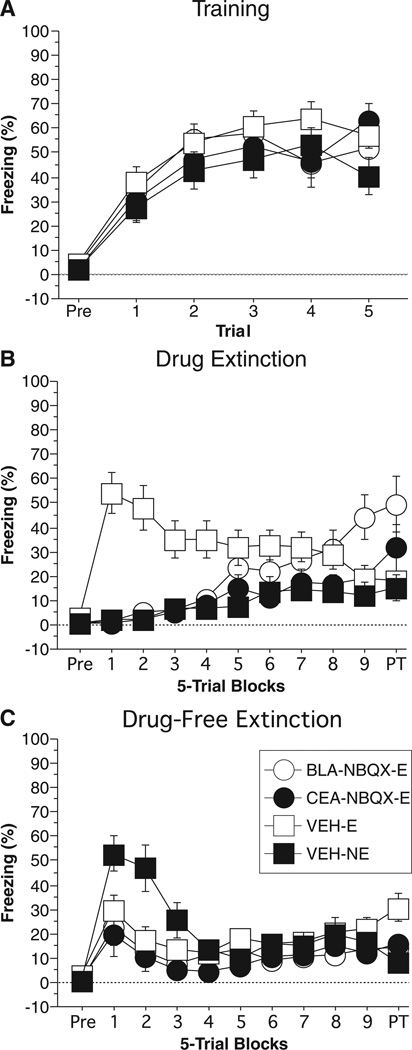
Fig. 2.
Conditioned freezing in rats receiving AMPAR inactivation during extinction (Experiment 1). (A) Mean percentage of freezing (±SEM) during the five-trial training session (data are displayed with a 3-min pre-trial period followed by five tone–shock pairings). Freezing was quantified before the first conditioning trial (Pre) and during the 1-min period after each conditioning trial. (B) Mean percentage of freezing (±SEM) during the drug extinction session immediately after drug infusions. Data are displayed with a 3-min pre-trial period followed by nine bins consisting of five CS alone presentations and a 2-min post-trial period. Data were quantified before the first CS presentation (Pre), during each subsequent trial consisting of the 10-s tone presentation and 30-s inter-trial interval, and during the 2-min post-trial period. Rats in the no-extinction group (NE) were placed in the chambers for the same time period as all other rats, however, received no CS presentations. (C) Mean percentage of freezing (±SEM) during the drug-free extinction session. Data are displayed and quantified as described in (B), with the exception of the NE rats, which received the same CS presentation as all other rats. Data are shown for rats receiving 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX) in the basolateral complex of the amygdala (BLA) and CS presentations during the drug extinction session (open circle), rats receiving NBQX in the central amygdala (CEA) and CS presentation during the drug extinction session (closed circle), rats receiving VEH in the BLA or CEA and CS presentations during the drug extinction session (open square), and rats receiving VEH in the BLA or CEA and no CS presentations during drug extinction (closed square).
Twenty-four hours after training, rats were infused with either VEH or NBQX immediately before Drug Extinction. Freezing during the Drug Extinction test is shown in Fig. 2B. Before CS onset, all groups showed low levels of freezing (similar to those seen during the pre-period of training). A two-way anova with variables of group (BLA-NBQX-E, CEA-NBQX-E, VEH-E, VEH-NE) and trial (1–45) revealed a significant main effect of group (F3,49 = 6.89; P = 0.0006), trial (F44,2156 = 2.80; P < 0.0001), and a group × trial interaction (F132,2156 = 5.02; P < 0.0001). Post hoc analysis of the main effect of group revealed that rats receiving NBQX in the BLA or CEA (BLA-APV-E, CEA-NBQX-E) froze significantly less than the rats receiving VEH during extinction (VEH-E; P < 0.02 for both comparisons). Additionally, rats receiving VEH before extinction (VEH-E) froze significantly more than rats receiving VEH without extinction (VEH-NE; P = 0.0003). There were no significant differences between rats receiving NBQX in the BLA (BLA-NBQX-E), rats receiving NBQX in the CEA (CEA-NBQX-E) or rats receiving vehicle without extinction (VEH-NE). Importantly, these results demonstrate that rats receiving NBQX in the BLA or CEA were unable to express conditional fear to the auditory CS earned 24 h earlier.
The long-term extinction memory acquired during the Drug Extinction session was tested 24 h later by exposing the rats to a second Drug-Free Extinction session. The results from the Drug-Free Extinction session are shown in Fig. 2C. A two-way anova with variables of group (BLA-NBQX-E, CEA-NBQX-E, VEH-E, VEH-NE) and trial (1–45) revealed a significant main effect of group (F3,49 = 3.13; P < 0.04), trial (F44,2156 = 7.06; P < 0.0001), and group × trial interaction (F132,2156 = 2.12; P < 0.0001). Post hoc analysis of the main effect of group revealed that rats receiving NBQX in the BLA (BLA-NBQX-E) or CEA (CEA-NBQX-E) froze significantly less than rats receiving VEH without extinction (VEH-NE; P < 0.03 for both comparisons). Further analysis of the first 10 trials of the Drug-Free Extinction session via two-way anova with variables of group (BLA-APV-E, CEA-APV-E, VEH-E, VEH-NE) and trial (1–10) revealed a significant main effect of group (F3,49 = 4.56; P < 0.007) and trial (F9,441 = 8.04; P < 0.0001). The group × trial interaction was not significant (F27,441 = 1.36; P = 0.11). Post hoc analysis of the main effect of group revealed that rats that did not receive extinction during the Drug Extinction session (VEH-NE) froze significantly more than all other groups (P = 0.008 for all comparisons). These results indicate that while rats receiving NBQX in the BLA or CEA were unable to express freezing during the Drug Extinction session (Fig. 2B), they were still able to acquire an extinction memory as tested during the Drug-Free Extinction session (Fig. 2C).
Experiment 2: NMDAR antagonism in the BLA, but not the CEA, prevents the extinction of conditioned fear
Histology
Based on histological results, 60 rats were included in this experiment. Rats were excluded if their guide cannulae were located outside the intended structure. This yielded the following group sizes: rats receiving APV in the BLA during extinction (BLA-APV-E; n = 9), rats receiving APV in the CEA during extinction (CEA-APV-E; n = 10), rats receiving PBS in the BLA or CEA during extinction, were not statistically different and were collapsed into a single group (VEH-E; n = 23), and rats receiving PBS in the BLA or CEA that did not receive extinction, were not statistically different and were collapsed into a single group (VEH-NE; n = 18). BLA and CEA cannula placements for rats included in the analysis are depicted in Fig. 1. All cannula placements were located within the intended structures (BLA or CEA).
Behavior
Post-shock freezing during the conditioning session is shown in Fig. 3A. Freezing was not statistically different across groups. The data were analysed using two-way anova with variables of group (BLA-APV-E, CEA-APV-E, VEH-E, VEH-NE) and trial (1–5). During the pre-trial period rats displayed minimal levels of freezing (< 10%). After the onset of conditioning rats displayed potentiated freezing. The anova revealed no main effect of group (F3,56 = 1.9; P = 0.14). Additionally, the anova revealed a main effect of trial (F4,224 = 20.1; P < 0.0001), and a group × trial interaction (F12,224 = 2.25; P = 0.01). This indicates that the average level of freezing across the training session was not significantly different between the groups. However, the groups increased their freezing as the training session proceeded and did so at different rates.
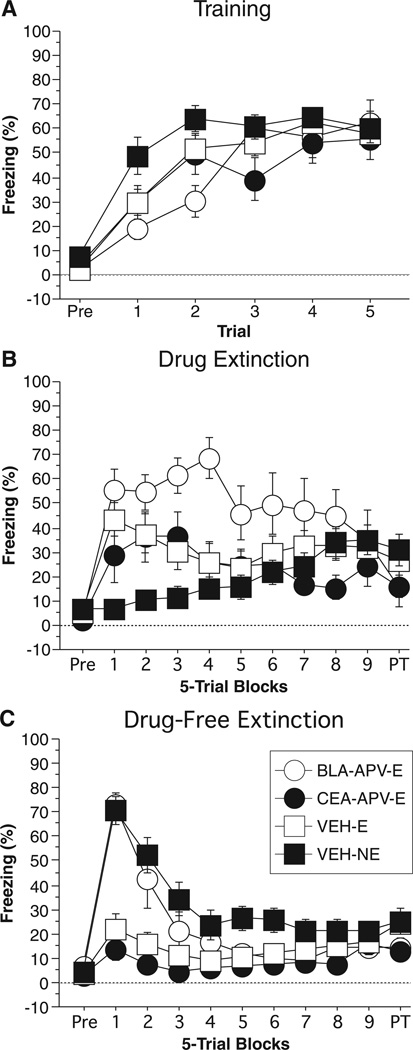
Fig. 3.
Conditioned freezing in rats receiving NMDAR inactivation during extinction (Experiment 2). (A) Mean percentage of freezing (±SEM) during the five-trial training session (data are displayed with a 3-min pre-trial period followed by five tone–shock pairings). Freezing was quantified before the first conditioning trial (Pre) and during the 1-min period after each conditioning trial. (B) Mean percentage of freezing (±SEM) during the drug extinction session immediately after drug infusions. Data are displayed with a 3-min pre-trial period followed by nine bins consisting of five CS alone presentations and a 2-min post-trial period. Data were quantified before the first CS presentation (Pre), during each subsequent trial consisting of the 10-s tone presentation and 30-s inter-trial interval, and during the 2-min post-trial period. Rats in the no-extinction group (NE) were placed in the chambers for the same time period as all other rats, however, received no CS presentations. (C) Mean percentage of freezing (±SEM) during the drug-free extinction session. Data are displayed and quantified as described in (B), with the exception of the NE rats, which received the same CS presentation as all other rats. Data are shown for rats receiving d,l-2-amino-5-phosphonopentanoic acid (APV) in the basolateral complex of the amygdala (BLA) and CS presentations during the drug extinction session (open circle), rats receiving APV in the central amygdala (CEA) and CS presentation during the drug extinction session (closed circle), rats receiving VEH in the BLA or CEA and CS presentations during the drug extinction session (open square), and rats receiving VEH in the BLA or CEA and no CS presentations during drug extinction (closed square).
Twenty-four hours after training rats were infused with either VEH or APV immediately before Drug Extinction. Freezing during the Drug Extinction test is shown in Fig. 3B. Before CS onset, all groups showed low levels of freezing (similar to those seen during the pre-period of training). A two-way anova with variables of group (BLA-APV-E, CEA-APV-E, VEH-E, VEH-NE) and trial (1–45) revealed a significant main effect of group (F3,56 = 4.42; P < 0.008), trial (F44,2464 = 1.58; P < 0.01), and a group × trial interaction (F132,2464 = 2.41; P < 0.0001). Post hoc analysis of the main effect of group revealed that rats receiving APV in the BLA (BLA-APV-E) froze significantly more than all other groups (P < 0.03 for all comparisons). There were no significant differences between the other groups.
The long-term extinction memory acquired during the Drug Extinction session was tested 24 h later by exposing the rats to a second Drug-Free Extinction session. The Drug-Free Extinction session is shown in Fig. 3C. A two-way anova with variables of group (BLA-APV-E, CEA-APV-E, VEH-E, VEH-NE) and trial (1–45) revealed a significant main effect of group (F3,56 = 10.23; P < 0.0001), trial (F44,2464 = 18.07; P < 0.0001), and group × trial interaction (F132,2464 = 4.67; P < 0.0001). Post hoc analysis of the main effect of group revealed that rats in the BLA-APV-E group froze significantly more than rats in the CEA-APV-E group (P < 0.02). Additionally, rats in the CEA-APV-E and VEH-E groups froze significantly less than rats in the VEH-NE group (P < 0.0001 for both comparisons). Further analysis of the first 10 trials of the Drug-Free Extinction session via two-way anova with variables of group (BLA-APV-E, CEA-APV-E, VEH-E, VEH-NE) and trial (1–10) revealed a significant main effect of group (F3,56 = 17.74; P < 0.0001), trial (F9,504 = 13.43; P < 0.0001), and a group × trial interaction (F27,504 = 2.87; P < 0.0001). Post hoc analysis of the main effect of group revealed that rats in the BLA-APV-E and VEH-NE groups froze significantly more than rats in the CEA-APV-E and VEH-E groups (P < 0.0001 for all comparisons). Importantly, rats in the BLA-APV-E group and VEH-NE group were not significantly different (P = 0.71), indicating that rats with BLA NMDAR antagonism had no memory of the extinction session that occurred 24 h earlier. Additionally, rats in the CEA-APV-E groups and VEH-E were not significantly different (P = 0.37) from one another. This indicates that NMDAR antagonism within the CEA had no effect on the formation of a long-term memory for extinction.
Discussion
The present experiments demonstrate distinct roles for AMPARs and NMDARs in the BLA and CEA in the expression and extinction of conditioned fear. We show that AMPARs within both the BLA and CEA are necessary for the expression of conditioned fear, but are not required for fear extinction. In contrast, NMDAR antagonism in the amygdala did not influence the expression of fear but did impair the acquisition of extinction. Importantly, the effect of NMDAR antagonism on extinction learning was only obtained with intra-BLA infusions of APV; antagonism of CEA NMDARs did not affect the expression or extinction of fear.
Consistent with previous reports, we found that AMPARs in the BLA are involved in the expression of conditional fear (Falls et al., 1992; Kim et al., 1993; Walker et al., 2005). We now show that AMPARs in the CEA are also involved in the expression of conditioned freezing. Interestingly, NMDARs in the BLA and CEA were not involved in the expression of conditioned fear. This is consistent with other studies in which normal fear responses were reported after NMDAR antagonism in the BLA (Miserendino et al., 1990; Campeau et al., 1992; Rodrigues et al., 2001; Walker et al., 2005). However, these data stand in contrast to several reports, including an earlier report from our laboratory, that NMDAR antagonism in the BLA prevents the expression of fear (Maren et al., 1996; Lee & Kim, 1998; Fendt, 2001; Lee et al., 2001; Goosens & Maren, 2004). In our earlier report, we used a contextual conditioning paradigm, whereas in the present study we assessed fear to an auditory CS. It is possible that amygdala NMDARs are differently involved in the expression of fear to contexts and cues.
Alternatively differences in the contribution of NMDAR subtypes to fear expression (Walker & Davis, 2008) and the influence of different APV enantiomers on these subtypes (Matus-Amat et al., 2007) might contribute to the variable effects of NMDAR antagonists in the expression of fear. While both the BLA and CEA contain NMDARs, the NMDAR subunit composition within these areas is different and therefore differentially susceptible to various NMDAR antagonists. NMDARs form heteromultimers containing an NR1 subunit and a combination of NR2A and/or NR2B subunits (Cull-Candy et al., 2001; Prybylowski & Wenthold, 2004). A recent in vitro electrophysiological study of NMDARs in the BLA and CEA reveals that the NMDAR-mediated currents in CEA neurons have slow kinetics and are blocked by NR2B-specific antagonists, suggesting that they are composed of the NR1/NR2B subunits (Lopez de Armentia & Sah, 2003). In contrast, NMDAR currents in BLA neurons demonstrate much faster kinetics and are less sensitive to NR2B-specific antagonists. This suggests that they are composed mostly of the NR1/NR2A subunits (Lopez de Armentia & Sah, 2003). Consistent with this, Walker & Davis (2008) have demonstrated that infusions of an NR2A antagonist (NVP-AAM077) into the BLA blocked both fear conditioning and expression, whereas an NR2B antagonist (CP101, 606) disrupted conditioning but not expression. It is therefore possible that a more selective NR2B-specific antagonist, such as ifenprodil, would lead to an extinction impairment when infused into the CEA.
Although APV did not impair the expression of fear, it did produce a robust attenuation of extinction learning when infused into the BLA. This outcome confirms numerous reports indicating the importance of BLA NMDARs in extinction learning (Falls et al., 1992; Cox & Westbrook, 1994; Santini et al., 2001; Walker et al., 2002; Lin et al., 2003; Maren & Quirk, 2004; Sotres-Bayon et al., 2007). Together, these data provide strong support to the view that NMDAR-dependent plasticity in the BLA is involved in both the acquisition and extinction of fear conditioning (Davis, 2002; Maren, 2005b; Quirk & Mueller, 2008). To our surprise, however, APV infusions into the CEA did not impair extinction learning, even though they severely attenuate fear conditioning when infused prior to training (Goosens & Maren, 2003). This finding provides unique insight into the specific neurocircuitry underlying extinction and draws a stark contrast between the role of NMDARs in the BLA and CEA in conditioning and extinction. Indeed, our findings here suggest a dissociation between the role of the CEA in the acquisition and extinction of fear. While NMDAR antagonism within CEA blocks the acquisition of conditioned fear (Maren et al., 1996; Goosens & Maren, 2003, 2004), it had no effect on the acquisition of extinction (Experiment 2). While this result is surprising, it is not without precedent. Bahar et al. (2003) found that infusion of a protein synthesis inhibitor into the CEA blocked the acquisition of conditioned taste aversion (CTA), while having no effect on the extinction of CTA. Collectively, these data suggest that NMDAR-dependent plasticity in the CEA (Wilensky et al., 2006) has a selective role in fear acquisition, whereas BLA plasticity has a broader role in acquiring both fear and extinction memories.
Although NMDAR-dependent plasticity in the CEA is not involved in extinction learning, there is considerable evidence that the regulation of neuronal activity in the CEA is importantly involved in the expression of extinction. Indeed, recent data indicate that a network of γ-aminobutyric acid (GABA)ergic inhibitory interneurons in the amygdala are involved in the expression of extinction (Likhtik et al., 2008). These intercalated neurons (ITC) receive input from both the lateral amygdala and the medial prefrontal cortex, and strongly inhibit the CEA. Given the evidence linking the medial prefrontal cortex to the expression of extinction (Quirk et al., 2000; Santini et al., 2001, 2004; Maren & Quirk, 2004; Pare et al., 2004; Sotres-Bayon et al., 2007; Quirk & Mueller, 2008; Knapska & Maren, 2009), it is widely believed that medial prefrontal cortical projections to the ITC and consequent inhibition of CEA activity is involved in the expression of extinction.
In conclusion, while both the CEA and BLA are necessary for the acquisition and expression of Pavlovian conditioned fear (Wilensky et al., 2006; Zimmerman et al., 2007), we now show distinct roles for AMPARs and NMDARs within the BLA and CEA in the expression of conditional fear and the acquisition of extinction. AMPARs in both the BLA and CEA are involved in the expression of fear, but are not required for fear extinction. In contrast, NMDARs are necessary for the extinction, but not expression, of fear. Importantly, only BLA NMDARs are involved in extinction learning. These findings provide important insight into the molecular mechanisms that underlie extinction and help to further refine the intra-amygdaloid circuitry that underlies conditioned fear.
Acknowledgement
This work was supported by a grant from NIMH (RO1MH073655) to S.M.
Abbreviations
- AMPAR
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor
- APV
d,l-2-amino-5-phosphonopentanoic acid
- BLA
basolateral complex of the amygdala
- CEA
central amygdala
- CR
conditioned response
- CS
conditioned stimulus
- ITC
intercalated neurons
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione
- NMDAR
N-methyl-d-aspartate receptor
- PBS
phosphate-buffered saline
- US
unconditioned stimulus
References
- Bahar A, Samuel A, Hazvi S, Dudai Y. The amygdalar circuit that acquires taste aversion memory differs from the circuit that extinguishes it. Eur. J. Neurosci. 2003;17:1527–1530. doi: 10.1046/j.1460-9568.2003.02551.x. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol. Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav. Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectr. 2007;12:200–206. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Cox J, Westbrook RF. The NMDA receptor antagonist MK-801 blocks acquisition and extinction of conditioned hypoalgesic responses in the rat. Q. J. Exp. Psychol. B. 1994;47:187–210. [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Ann. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. Eur. J. Neurosci. 2002;16:395–398. doi: 10.1046/j.1460-9568.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav. Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Fendt M. Injections of the NMDA receptor antagonist aminophosphonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. J. Neurosci. 2001;21:4111–4115. doi: 10.1523/JNEUROSCI.21-11-04111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci. Biobehav. Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav. Neurosci. 2003;117:738–750. doi: 10.1037/0735-7044.117.4.738. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur. J. Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Campeau S, Falls WA, Davis M. Infusion of the non-NMDA receptor antagonist CNQX into the amygdala blocks the expression of fear-potentiated startle. Behav. Neural Biol. 1993;59:5–8. doi: 10.1016/0163-1047(93)91075-x. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn. Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J. Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi JS, Brown TH, Kim JJ. Amygdalar nmda receptors are critical for the expression of multiple conditioned fear responses. J. Neurosci. 2001;21:4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J. Neurosci. 2003;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J. Neurosci. 2003;23:6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Building and Burying Fear Memories in the Brain. Neuroscientist. 2005a;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005b;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-d-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav. Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behav. Neurosci. 2007;121:721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol. Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Prybylowski K, Wenthold RJ. N-methyl-d-aspartate receptors: subunit assembly and trafficking to the synapse. J. Biol. Chem. 2004;279:9673–9676. doi: 10.1074/jbc.R300029200. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J. Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RD, Pare D. Activity-dependent synaptic plasticity in the central nucleus of the amygdala. J. Neurosci. 2005;25:1847–1855. doi: 10.1523/JNEUROSCI.3713-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J. Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Swanson L. Brain Maps: Structure of the Rat Brain. New York: Elsevier; 1992. [Google Scholar]
- Walker DL, Davis M. Amygdala infusions of an NR2B-selective or an NR2A-preferring NMDA receptor antagonist differentially influence fear conditioning and expression in the fear-potentiated startle test. Learn. Mem. 2008;15:67–74. doi: 10.1101/lm.798908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn. Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the Fear Circuit: The Central Nucleus of the Amygdala Is Required for the Acquisition, Consolidation, and Expression of Pavlovian Fear Conditioning. J. Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Rabinak CA, McLachlan IG, Maren S. The central nucleus of the amygdala is essential for acquiring and expressing conditional fear after overtraining. Learn. Mem. 2007;14:634–644. doi: 10.1101/lm.607207. [DOI] [PMC free article] [PubMed] [Google Scholar]