Abstract
We report here on the discovery and preliminary evaluation of a novel non-macrocyclic low molecular weight quadruplex-stabilizing chemotype. The lead compounds, based on a furan core, show high G-quadruplex stabilisation and selectivity as well as potent in vitro anti-proliferative activity.
Quadruplexes (G4s) are higher-order nucleic acid arrangements involving a core of π-π stacked guanine-quartets (G-quartets) rather than the Watson-Crick base pairs of double-helical nucleic acids.1 G4-forming sequences are widely prevalent in eukaryotic telomeric sequences as well as being over-represented in other genomes2, notably promoter and 5'-UTR sequences of genes involved in cellular proliferation.3 The recent demonstration of the presence of G4s in human cells4 has added credence to the concept that G4s can be targets for therapeutic intervention, at the single gene or polygene levels.5 Appropriate small molecules can serve to stabilise G4s and the resulting complexes can then act as impediments to telomere maintenance, transcription or translation, depending on the nature of the quadruplex target site.6 These effects have been shown in several target genes of relevance to human cancer such as c-MYC7 and c-KIT.8
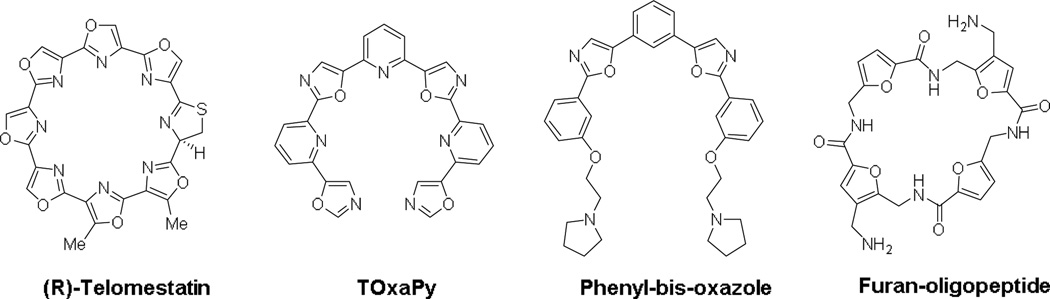
A large number of small molecule chemotypes have been reported as G4-binding ligands.6 The overwhelming majority are heteroaromatic with large flat surfaces, designed to complement the surface characteristics of a terminal G-quartet in a typical quadruplex structure. A second class of ligand is represented by the cyclic polyoxazole natural product telomestatin.9 A number of cyclic and acyclic analogues have been reported, some of which show potent biological activity.10 The acyclic compounds tend to be characterised by a crescent shape. For example, pyridostatin11 and several series of phenyl- and pyridyl-bis-oxazoles12–14 all selectively target G4s (Figure 1). A more general requirement of most G4- binding ligands is the possession of side-chains terminating in cationic charge.6
Figure 1.
Structures of various non-polycyclic G-quadruplex ligands
Few G4-binding small molecules have proceeded to in vivo evaluation in models of human cancer, and to date only one compound, Quarfloxin, has been evaluated in clinical trials.15 The perceived lack of drug-like characteristics in many G4-binding compounds may have hindered progress to the clinic. We report here on a study to discover novel ligands with M Wts <400 Da that could be suitable starting-points for future drug discovery efforts.
Thirty-eight representative members of a large chemical library from the anti-parasitic drug discovery programme at Georgia State University16,17 (several hundred compounds), with highly diverse scaffolds and functional groups, were screened using a high-throughput 96-well FRET (Fluorescence Resonance Energy Transfer) assay.18 G4 stabilisation was initially evaluated using dual-labelled F21T (human telomeric 21-mer) and c-KIT2 (a tyrosine kinase oncogene) G4s, as well as a duplex DNA sequence (T-loop). The ten most active compounds were subsequently screened against an expanded panel of fluorescently-labelled promoter G4-forming sequences, with HSP90A, HSP90B (heat shock protein 90 promoter sequences),12 k-RAS21 (in the promoter of the k-RAS oncogene)19 and AR, a G4 recently identified in the promoter of the androgen receptor (involved in prostate cancer development).20
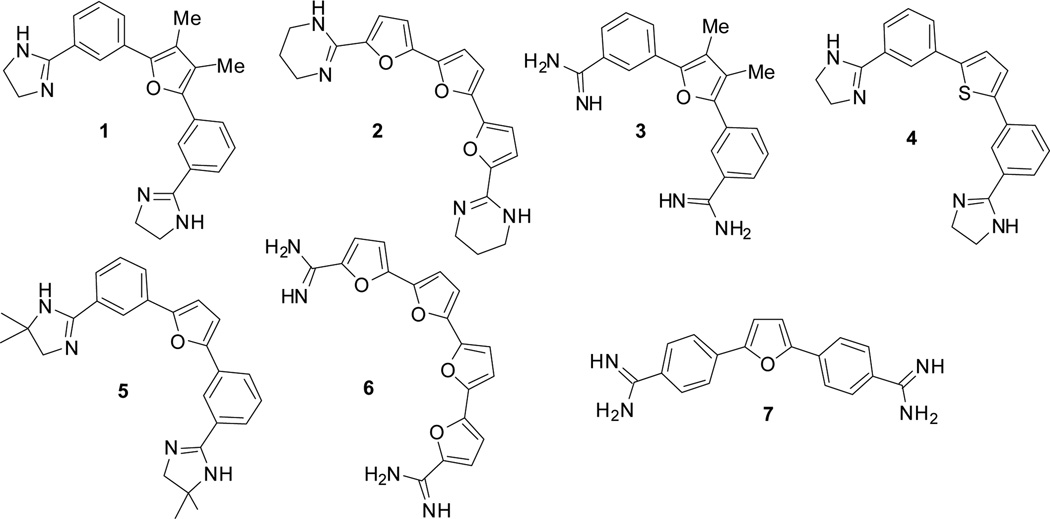
Six acyclic furan- and thiophene-based compounds (Figure 2: 1–6), representing two distinctive chemotypes were identified with high (>15 °C) ΔTm values. A competition assay using unlabelled calf thymus duplex DNA and compounds 1–6 examined the ability of these compounds to differentiate between duplex and the F21T G4 DNA at high duplex:G4 ratios. The compounds were also examined in a 96 hr short-term sulforhodamine B (SRB) assay, to determine their ability to inhibit cancer cell growth (Tables 1, 2). All six compounds showed potent G4 stabilising abilities, as judged by the large changes in ΔTm values for the selected G4s. In particular the bis-phenyl- mono-furan compounds 1 and 5 had especially high ΔTm values, broadly comparable to those for established high-affinity G4-binding compounds such as tetra-substituted naphthalene diimides.21 The tri-furan compound 2 is consistently more effective in stabilising the G4s than the tetrafuran compound 6. Compound 2 and 6, representatives of a tetrafuran second chemotype, were less selective at high duplex ratios and were inactive in the SRB assay, possibly because of aqueous solubility and cellular uptake issues. Switching from a furan (1, 5) to a thiophene (4), does slightly affect G4 stabilisation and selectivity vs duplex DNA, though not in vitro potency, which at least in the cell lines examined, is comparable to that of compounds 1 and 5.
Figure 2.
Structures of the lead compounds 1–6 identified in this study, together with a control compound 7.
Table 1.
FRET G4 stabilization (ΔTm at 1 µM in °C) and calf thymus DNA (CT) competition data (the latter showing % retention of the F21T ΔTm at 1 µM). <Esd> ± 0.5 °C, from triplicate measurements. n/a: indicates unsuccessful curve fitting to the melting data. Compound 7, a negative control, is a para analogue of the mono-furan compounds. Compound 8 is a tetra-substituted naphthalene diimide derivative21, used here as a G4 control.
| Cmpnd | Mol Wt |
F21T | c-KIT2 | HSP- 90A |
HSP- 90B |
k-RAS 21R |
AR | T- loop |
G4:CT 1:1 |
G4:CT 1:10 |
G4:CT 1:100 |
G4:CT 1:300 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 384.5 | 22.3 | 16.8 | 27.7 | 23.7 | 19.6 | 15.4 | <2 | 100 | 100 | 100 | 100 |
| 2 | 364.4 | 20.4 | 16.3 | 26.0 | 22.0 | 18.5 | 13.8 | <2 | 100 | 100 | 92.7 | 63.3 |
| 3 | 330.5 | 17.6 | 17.8 | 21.2 | 18.1 | 15.1 | 11.9 | 2.2 | 100 | 100 | 65.9 | 43.9 |
| 4 | 372.5 | 18.0 | 13.9 | 22.5 | 19.1 | 15.9 | 10.2 | <2 | 100 | 100 | 60.5 | 39.9 |
| 5 | 412.5 | 22.6 | 18.5 | 26.6 | 22.7 | 18.3 | 14.7 | <2 | 100 | 100 | 100 | n/a |
| 6 | 350.3 | 18.6 | n/a | 20.6 | 16.2 | 13.4 | 10.1 | <2 | 100 | 100 | 53.1 | 35.4 |
| 7 | 302.5 | 14.4 | 12.2 | 19.0 | 15.7 | 16.6 | 9.9 | 3.4 | 100 | 100 | 24.5 | 2.5 |
| 8 | 830.6 | 26.6 | 22.0 | 33.1 | 28.6 | n/a | 15.9 | 4.9 | 100 | 100 | n/a | 27.2 |
Table 2.
IC50 values of compounds 1–8 determined by a 96 hr SRB assay (see the Supplementary Information for further details). <Esd> ± 0.3 µM.
| A549 | MCF7 | ALT | Mia- PaCa2 |
Panc1 | RCC4 | 786-O | WI38 | |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.3 | 0.9 | 1.0 | 0.3 | 0.5 | 4.9 | 3.0 | 1.3 |
| 2 | >25 | >25 | >25 | >25 | >25 | >25 | >25 | >25 |
| 3 | 0.8 | 0.9 | 6.6 | 2.0 | 1.7 | >25 | >25 | >25 |
| 4 | 0.4 | 1.4 | 1.9 | 0.7 | 1.0 | 3.3 | 5.6 | 2.0 |
| 5 | 0.5 | 1.2 | 1.4 | 0.5 | 1.0 | 3.9 | 4.8 | 2.0 |
| 6 | 4.2 | 15.6 | 18.8 | 7.0 | >25 | >25 | 12.8 | >25 |
| 7 | 3.7 | 11.7 | >25 | 8.90 | 10.9 | >25 | >25 | >25 |
| 8 | 0.019 | 0.070 | 0.063 | 0.011 | 0.003 | 0.560 | 0.320 | 0.230 |
Overall, F21T and the two HSP90 G4s have been most stabilised by compounds 1–6. Comparison with the behaviour of a tetra-substituted naphthalenediimide compound previously examined by us20, shows that 1–6 exhibit only moderate ΔTm values with the AR G4, which are generally lower than with other G4s. Compounds 1–6 produced slightly reduced but still significant stabilisation with the c-KIT2 and k-RAS21 G4s, suggesting that these compounds have the ability to act simultaneously on multiple G4 targets (G4 poly-targeting). The stabilisation of a duplex DNA sequence (T-loop) was not significantly affected by any of the compounds at the biologically relevant concentration employed here (1 µM). Compounds 1 and 5 in particular are highly selective for G4 versus duplex DNA, as found in a series of competition assays, where the G4 stabilisation ability of both compounds is undiminished by adding calf thymus DNA in excess, at ratios up to 1:100/300. A control compound, 7, an established duplex DNA minor groove binder, also showed significant G4 stabilisation, albeit with greater effects on the duplex DNA used (with a sub-optimal sequence for this compound).
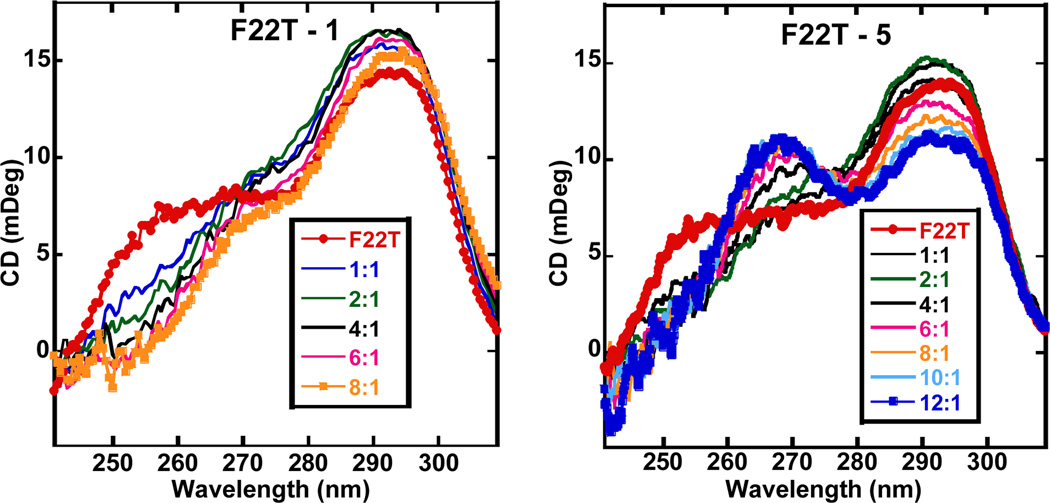
Circular dichroism (CD) was employed to qualitatively evaluate the binding mode of the lead compounds and to examine induced structural transitions in the telomeric G4. The CD spectra (Figure 3 and Supplementary Information) show that compounds 1 and 3–6 produce very small induced CD signals in a human telomeric quadruplex sequence. Such weak induced CD signals are characteristic of quadruplex end-stacking compounds and the small differences in the CD signal patterns for different compounds indicate minor differences in the stacking geometries of the ligands at the terminal G-quartets.
Figure 3.
Circular dichroism (CD) spectra for compounds 1 and 5 at differing ligand:G4 ratios. F22T is a 22-mer analogue of the F21T sequence.
All the compounds exhibiting weak induced CD signals that show a binding preference for an anti-parallel type quadruplex conformation as observed by decreases in the CD signal around 260 nm. Compound 5 however behaves differently, inducing a much larger conformational transition in the telomeric quadruplex upon complex formation. Upon titrating 5, the CD intensity around 290 nm decreases with a subsequent increase in the CD signal intensity around 260 nm. This is most likely due to an induced conformational transition from a hybrid to a more parallel-type G4 form.
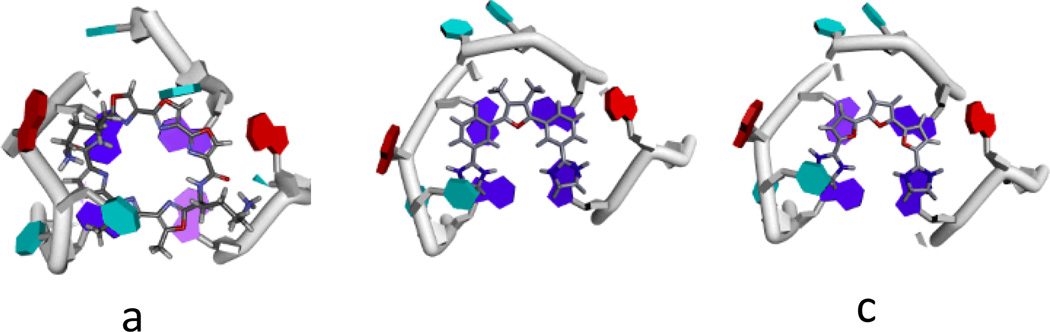
Analysis of the equilibrium conformation for compound 1 was undertaken with classical molecular mechanics (MM2 and AMBER) force fields and by ab initio (STO-3G) calculations. The MM2 analysis suggested that overall arrangement involved coplanar rings. This somewhat implausible conclusion was at variance with results from the AMBER force field and the ab initio calculations, which concurred in suggesting a twisted conformation due to the repulsive effects of the two furanoid methyl groups on the attached phenyl rings. The barrier between planar and twisted conformations is likely to be low so a qualitative analysis of plausible G4-bound conformations for compounds 1 and 2 has been undertaken. The NMR structure of an anti-parallel human telomeric G4 complexed with a telomestatin derivative has been used as a starting-point.22 Compounds 1 and 2 have similarities to the overall shape and curvature of telomestatin (Figure 4), albeit in an acyclic manner. The NMR structure shows that the telomestatin derivative is non-planar and its out-of-plane distortions complement the non-planarity of the G-quartet to which it is bound. The qualitative low-energy structures proposed for compounds 1 and 2 incorporate such distortions, which still enable effective π-π stacking onto the terminal G-quartet of this G4, as well as some additional stabilisation from a thymine. The modelling show that the meta position of the dihydroimidazole substituents in 1 and the tetrahydropyrimidine substituents in 2, is crucial for shape similarity to telomestatin. The para substitution in the control compound 7 enables it to effectively bind to the minor groove of duplex DNA, which is not possible for any of the six compounds arising from the screen.
Figure 4.
Plots showing the terminal G-quartet of anti-parallel G4 complexes, with guanines shown in dark blue, thymines in cyan and adenines in red. Ligands are shown in stick representation. (a). The telomestatin derivative complex22, (b) results of qualitative modelling with compound 1 and (c) with compound 2.
Several of the compounds showed low µM anti-proliferative activity (Table 2) in a cancer cell line panel [A549 (lung cancer), MCF7 (breast cancer), RCC4 and 786-O (renal cancer), Panc1 and Mia-PaCa2 (pancreatic cancer), ALT (transformed WI38 lung fibroblast cells characterised by Alternative Maintenance of Telomeres) and WI38 (non-transformed lung fibroblast cells)].
The two poly-furan compounds 2 and 6 have low anti-proliferative activity, even though both have G4-stabilising activity comparable to the other four compounds in the group. This may be due to cell uptake and nuclear localisation problems as well as limited aqueous solubility; the lack of observed precipitation during the SRB assay supports the former suggestions. Compounds from the mono-furan and mono-thiophene series on the other hand show activity in several cell lines in the low-µM range. The lung cancer cell line A549 is slightly more sensitive to these compounds, although we do not currently have a molecular explanation for this.
We cannot exclude the possibility of non-G4 targets being involved in these cellular effects: experiments are currently underway to examine links with G4 affinity for these compounds. G4 selectivity appears to be limited even though for the two lead compounds at least, duplex DNA is less likely to be a target. This suggests that they may be acting as poly-targeting agents, affecting a number of genes and oncogenes involved in cellular proliferation and also that the compounds do not have identical cellular targets. It is notable that compounds 3 and 6 have high selectivity for several of the cancer lines compared to the normal WI38 line and have some activity in the ALT line, which maintains telomere length by non-telomerase mechanisms. This suggests that telomere maintenance rather than telomerase per se, is being targeted.
Conclusions
We report here that screening putative ligands using a HTS-FRET assay against a panel of G4s with a duplex control sequence, has resulted in the discovery of meta-substituted bisphenylmonofurans as a novel G4 stabilizing chemotype. A similar chemotype, with a urea group replacing the furan ring, has been reported23 as having high G4 affinity. These compounds are structurally-simple, conformationally flexible and chemically readily accessible with M Wts <400 Da. They have G4 stabilisation ability comparable to those previously observed with polycyclic heteroaromatic compounds21 (cf compound 8 (4,9-bis((3-(4-methyl-piperazin-1-yl)propyl)amino)-2,7-bis(3-morpholinopropyl) benzo-[lmn] [3,8] phenanthroline-1,3,6,8(2H,7H)-tetraone) in Table 1), but with low duplex DNA affinity. They inhibit cancer cell growth at low µM/high nM levels, suggesting that these or related compounds may have potential as drug-like poly-quadruplex targeting agents.
Supplementary Material
Acknowledgments
Synthesis and biophysical studies at Georgia State University were supported by National Institutes of Health NIAID Grant AI-064200 (WDW, DWB). Work at UCL was supported by a MRC Confidence in Concept grant (SN).
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/c000000x/
References
- 1.Burge S, Parkinson GN, Hazel P, Todd AK, Neidle S. Nucleic Acids Res. 2006;34:5402. doi: 10.1093/nar/gkl655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huppert J, Balasubramanian S. Nucleic Acids Res. 2005;33:2908. doi: 10.1093/nar/gki609. [DOI] [PMC free article] [PubMed] [Google Scholar]; Todd A, Johnston M, Neidle S. Nucleic Acids Res. 2005;33:2901. doi: 10.1093/nar/gki553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huppert JL, Balasubramanian S. Nucleic Acids Res. 2007;35:406. doi: 10.1093/nar/gkl1057. [DOI] [PMC free article] [PubMed] [Google Scholar]; Huppert JL, Bugaut A, Kumari S, Balasubramanian S. Nucleic Acids Res. 2008;36:6260. doi: 10.1093/nar/gkn511. [DOI] [PMC free article] [PubMed] [Google Scholar]; Balasubramanian S, Hurley LH, Neidle S. Nature Rev. Drug Disc. 2011;10:261. doi: 10.1038/nrd3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffi G, Tannahill D, McCafferty J, Balasubramanian S. Nature Chem. 2013;5:182–186. doi: 10.1038/nchem.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekaran V, Soares J, Jarstfer MB. J. Med. Chem. 2013 doi: 10.1021/jm400528t. [DOI] [PubMed] [Google Scholar]
- 6.Monchaud D, Teulade-Fichou M-P. Org. Biomol. Chem. 2008;6:627. doi: 10.1039/b714772b. [DOI] [PubMed] [Google Scholar]; Ou TM, Lu YJ, Tan JH, Huang ZS, Wong KY, Gu LQ. Chem Med Chem. 2008;3:690. doi: 10.1002/cmdc.200700300. [DOI] [PubMed] [Google Scholar]; Yang DZ, Okamoto K. Future Med. Chem. 2010;2:619. doi: 10.4155/fmc.09.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai J, Carver M, Hurley LH, Yang D. J. Amer. Chem. Soc. 2011;133:17673. doi: 10.1021/ja205646q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rankin S, Reszka AP, Huppert J, Zloh M, Parkinson GN, Todd AK, Ladame S, Balasubramanian S, Neidle S. J. Amer. Chem. Soc. 2005;127:10584. doi: 10.1021/ja050823u. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fernando H, Reszka AP, Huppert J, Ladame S, Rankin S, Venkitaraman AR, Neidle S, Balasubramanian S. Biochemistry. 2006;45:7854. doi: 10.1021/bi0601510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen MC, Ulven T. Curr. Med. Chem. 2010;17:3438. doi: 10.2174/092986710793176320. [DOI] [PubMed] [Google Scholar]
- 10.Linder J, Garner TP, Williams HE, Searle MS, Moody CJ. J. Amer. Chem. Soc. 2011;133:1044. doi: 10.1021/ja109158k. [DOI] [PubMed] [Google Scholar]
- 11.Müller S, Sanders DA, Di Antonio M, Matsis S S, Riou J-F, Rodriguez R, Balasubramanian S. Org. Biomol. Chem. 2012;10:6537. doi: 10.1039/c2ob25830g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohnmacht SA, Micco M, Petrucci V, Todd AK, Reszka AP, Gunaratnam M, Carvalho MA, Zloh M, Neidle S. Bioorg. Med. Chem. Lett. 2012;22:5930. doi: 10.1016/j.bmcl.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 13.Ohnmacht SA, Ciancimino C, Vignaroli G, Gunaratnam M, Neidle S. Bioorg. Med. Chem. Lett. 2013;23:5351. doi: 10.1016/j.bmcl.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 14.Hamon F, Large E, Guédin-Beaurepaire A, Rouchon-Dagois M, Sidibe A, Monchaud D, Mergny J-L, Riou J-F, Nyugen C-H, Teulade-Fichou M-P. Angew Chem. Int. Ed. Engl. 2011;50:8745. doi: 10.1002/anie.201103422. [DOI] [PubMed] [Google Scholar]
- 15.Drygin D, Siddiqui-Jain A, O'Brien S, Schwaebe M, Lin A, Bliesath J, Ho CB, Proffitt C, Trent K, Whitten JP, Lim JK, Von Hoff D D, Anderes K, Rice WG. Cancer Res. 2009;69:7653. doi: 10.1158/0008-5472.CAN-09-1304. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen B, Tardy C, Bailly C, Colson P, Houssier C, Kumar A, Boykin DW, Wilson WD. Biopolymers. 2002;63:281. doi: 10.1002/bip.10073. [DOI] [PubMed] [Google Scholar]
- 17.Nanjunda R, Musetti C, Kumar A, Ismail MA, Farahat AA, Wang S, Sissi C, Palumbo M M, Boykin DW, Wilson WD. Curr. Pharm. Des. 2012;18:1934. doi: 10.2174/138161212799958422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.See Guyen B, Schultes CM, Hazel P, Mann J, Neidle S. Org. Biomol. Chem. 2004;2:981. doi: 10.1039/b316055f. and Supplementary Information for further details.
- 19.Cogoi S, Xodo LE. Nucleic Acids Res. 2006;34:2536. doi: 10.1093/nar/gkl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell T, Ramos-Montoya A, Di Antonio M, Murat P, Ohnmacht S, Micco M, Jurmeister S, Fryer L, Balasubramanian S, Neidle S, Neal DE. Biochemistry. 2013;26:1429. doi: 10.1021/bi301349c. [DOI] [PubMed] [Google Scholar]
- 21.Micco M, Collie GW, Dale AG, Ohnmacht SA, Pazitna I, Gunaratnam M, Reszka AP, Neidle S. J. Med. Chem. 2013;56:2959. doi: 10.1021/jm301899y. [DOI] [PubMed] [Google Scholar]
- 22.Chung WJ, Heddi B, Tera M, Iida K, Nagasawa K, Phan AT. J. Amer. Chem. Soc. 2013;135:13495. doi: 10.1021/ja405843r. [DOI] [PubMed] [Google Scholar]
- 23.Benz A, Singh V, Mayer TU, Hartig JS. Chem Bio Chem. 2011;12:1422. doi: 10.1002/cbic.201100094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.