Abstract
BACKGROUND
Burkitt’s lymphoma is an aggressive B-cell lymphoma that occurs in children and adults and is largely curable with the use of intensive and toxic chemotherapy. Current treatments are less effective and have more severe side effects in adults and patients with immunodeficiency than in children.
METHODS
We studied low-intensity treatment consisting of infused etoposide, doxorubicin, and cyclophosphamide with vincristine, prednisone, and rituximab (EPOCH-R) in patients with untreated Burkitt’s lymphoma. Two EPOCH-R regimens were tested: a standard dose-adjusted combination in human immunodeficiency virus (HIV)–negative patients (DA-EPOCH-R group) and a lower-dose short-course combination with a double dose of rituximab in HIV-positive patients (SC-EPOCH-RR group).
RESULTS
A total of 30 consecutive patients were treated; 19 patients were in the DA-EPOCH-R group, and 11 in the SC-EPOCH-RR group. The overall median age of the patients was 33 years, and 40% were 40 years of age or older; 73% of the patients had intermediate-risk disease, and 10% had high-risk disease. The principal toxic events, fever and neutropenia, were observed during 22% of the DA-EPOCH-R treatment cycles and 10% of the SC-EPOCH-RR treatment cycles. The tumor lysis syndrome developed in 1 patient; no treatment-related deaths occurred. The median cumulative doses of doxorubicin–etoposide and cyclophosphamide administered in the SC-EPOCH-RR group were 47% and 57% lower, respectively, than those administered in the DA-EPOCH-R group. With median follow-up times of 86 months in the DA-EPOCH-R group and 73 months in the SC-EPOCH-RR group, the rates of freedom from progression of disease and overall survival were, respectively, 95% and 100% with DA-EPOCH-R and 100% and 90% with SC-EPOCH-RR. None of the patients died from Burkitt’s lymphoma.
CONCLUSIONS
In this uncontrolled prospective study, low-intensity EPOCH-R–based treatment was highly effective in adults with sporadic or immunodeficiency-associated Burkitt’s lymphoma. (Funded by the National Cancer Institute; ClinicalTrials.gov numbers, NCT00001337 and NCT00006436.)
Burkitt’s lymphoma, first described by Denis Burkitt in African children, is a highly proliferative human cancer.1 Although rare, Burkitt’s lymphoma disproportionately affects children, accounting for 30 to 50% of pediatric lymphomas. Three major variants are recognized: endemic, which occurs in equatorial Africa; sporadic, which occurs worldwide; and immunodeficiency-associated, which occurs primarily in persons with human immunodefi-ciency virus (HIV) infection. Young patients with sporadic Burkitt’s lymphoma have a favorable outcome with intense short-cycle treatment, whereas adult patients and those with immunodeficiency have inferior outcomes.2–7
Burkitt’s lymphoma is derived from a germinal-center B cell and has distinct oncogenic pathways.8,9 A translocation between MYC and an immunoglobulin promoter leads to the constitutive expression of MYC and is found in all cases.9 Other genetic events occur with MYC to increase tumor proliferation and growth by means of the phosphatidylinositide 3-kinase pathway and cyclin-dependent kinases.8 Sporadic and immunodeficiency-associated variants of Burkitt’s lymphoma are pathogenetically similar.8
Recognition of the biologic overlap of Burkitt’s lymphoma and B-cell acute lymphoblastic leukemia led to the use of multiphase leukemia-based regimens in patients with Burkitt’s lymphoma.10 It is accepted that the high growth fraction and short doubling time of Burkitt’s lymphoma make intensive short-cycle chemotherapy a therapeutic necessity. However, such treatment has severe side effects in adults and patients with immunodeficiency and causes long-term morbidity in children.11 Efforts to substantially lower the treatment intensity while maintaining efficacy have not been very successful in adults, although risk-adapted treatment has reduced toxicity in children.3,5,12,13
We hypothesized that the exquisite sensitivity of Burkitt’s lymphoma cells to genotoxic stress makes prolonged exposure time, not increased dose, the important therapeutic strategy for maximizing the killing of tumor cells.14 This concept underlies the use of etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (EPOCH-R), a regimen of infused drugs based on in vitro studies showing enhanced tumor-cell killing with prolonged low-concentration drug exposure, as compared with brief, high-concentration exposure.15 We tested two EPOCH-R regimens in patients with untreated Burkitt’s lymphoma: a standard dose-adjusted regimen (DA-EPOCH-R), and a short-course regimen with a double dose of rituximab (SC-EPOCH- RR) to reduce toxicity.16–18 Here, we present the results of an uncontrolled prospective study of these two low-intensity EPOCH-R–based regimens in patients with sporadic or immunodeficiency- associated Burkitt’s lymphoma.
METHODS
STUDY DESIGN AND PARTICIPANTS
From November 2000 through December 2009, we enrolled 30 consecutive patients with untreated Burkitt’s lymphoma in a prospective study of DA-EPOCH-R for HIV-negative patients and SC- EPOCH-RR for HIV-positive patients. In both groups, the primary study end points were the rate of complete response, time to progression, and treatment toxicity; in addition, we attempted to determine whether the reduction in treatment in the SC-EPOCH-RR group was feasible.
Eligible patients had Burkitt’s lymphoma, had not received systemic chemotherapy previously, and had adequate organ function apart from organ function affected by disease; among women with childbearing potential, a negative pregnancy test was required. Evaluation included standard laboratory tests, computed tomographic scans of the whole body, and a bone marrow aspirate and biopsy. All the patients underwent cytologic and flow cytometric analysis of the cerebrospinal fluid and imaging of the brain. Standard criteria were used to assess tumor response.19
The institutional review board of the National Cancer Institute (NCI) approved the studies, and all the patients provided written informed consent. Filgrastim was provided to the NCI through an agreement with Amgen, which had no other role in the study. No other commercial support was provided. All the authors vouch for the completeness and accuracy of the data and analyses reported and for the fidelity of the study to the protocol.
TREATMENT
DA-EPOCH-R and SC-EPOCH-RR were administered as described in the Supplementary Appendix (available with the full text of this article at NEJM.org) and were usually administered in the outpatient setting.16,18,20 Patients in the DA-EPOCH-R group received two cycles after complete remission was established, for a total of six to eight cycles. Patients in the SC-EPOCH-RR group received one cycle after complete remission was established, for a minimum of three cycles and a maximum of six cycles. HIV-positive patients did not receive antiretroviral therapy during chemotherapy. Follow-up restaging was performed for 5 years in both studies.18,20 During the first cycle, patients received allopurinol at a dose of 300 mg and fluids. Patients were monitored for the tumor lysis syndrome.
DA-EPOCH-R was pharmacodynamically dose-adjusted on the basis of the neutrophil nadir.20 SC-EPOCH-RR was not dose-adjusted.18 Patients received filgrastim beginning 24 hours after the last dose of chemotherapy and continuing through the neutrophil nadir until absolute neutrophil recovery, defined as 5000 cells per cubic millimeter or more, was documented. Patients without evidence of cerebrospinal-fluid involvement received prophylactic intrathecal methotrexate at a dose of 12 mg. Intrathecal treatment was administered on days 1 and 5 every 3 weeks beginning in cycle 3 for eight doses with DA-EPOCH-R, and on days 1 and 5 every 3 weeks beginning in cycle 3 for six doses with SC-EPOCH-RR. Patients with evidence of cerebrospinal-fluid involvement received active treatment with methotrexate at a dose of 12 mg intrathecally or 6 mg by means of an Ommaya reservoir twice weekly for 4 weeks, then once weekly for 6 weeks, and then once monthly for 4 months.
PATHOLOGICAL AND PHARMACOKINETIC ANALYSES
Pathological findings were reviewed and confirmed by study pathologists, in compliance with the fourth edition of the World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues.21 Immunohistochemical studies and Epstein–Barr virus (EBV)–encoded RNA (EBER) in situ hybridization were performed as indicated.20 Fluorescence in situ hybridization or conventional karyotype analyses were performed to identify a translocation of MYC in available tissue samples.
Plasma concentrations of doxorubicin were analyzed at 0, 24, and 96 hours of infusion, as previously described.22 Data were fit to a curve for the 96-hour infusion and were combined with values from the literature to generate data for a two-compartment bolus simulation.23–25
STATISTICAL ANALYSIS
Overall survival was calculated from the date of study enrollment until death or the last follow-up visit. Freedom from progression of disease was calculated from the date of study enrollment until the date of progression or the last follow-up visit, with the use of the Kaplan–Meier method. The median potential follow-up was calculated from the period between the date of study enrollment for each patient and November 2012, the last follow-up date.
The difference in continuous variables between the two treatment groups was determined by means of an exact Wilcoxon rank-sum test. The difference in CD4+ T-cell paired values (change from baseline) was determined by means of the Wilcoxon signed-rank test. The differences in dichotomous variables between the two treatment groups were determined by means of Fisher’s exact test. The difference in categorical-risk groups between the two groups was determined with Mehta’s modification of Fisher’s exact test.26 All reported P values are two-tailed and are presented without any adjustment for multiple comparisons.
RESULTS
CHARACTERISTICS OF THE PATIENTS
A total of 30 consecutive, previously untreated patients with Burkitt’s lymphoma were enrolled in the study, including 17 patients with a sporadic variant and 13 with an immunodeficiency-associated variant (Table 1). Of the 13 patients with an immunodeficiency-associated variant, 11 were HIV-positive, and 2 had primary immunodeficiencies (the autoimmune lymphoproliferative syndrome in 1 patient and a deficiency of dedicator of cytokinesis 8 in 1). Overall, the median age of the patients was 33 years (range, 15 to 88), and 40% were 40 years of age or older. According to the Société Française d’Oncologie Pédiatrique lymphomes malins B (LMB) prognostic categories,13 17% of the patients had low-risk disease (defined as resected stage I or abdominal stage II cancer), 10% had high-risk disease (defined as central nervous system involvement, at least 25% blasts in bone marrow, or both characteristics), and 73% had intermediate-risk disease (i.e., were not in either of the other risk groups).
Table 1.
Characteristics of the Patients.*
| Characteristic | All Patients (N = 30) | DA-EPOCH-R (N = 19) | SC-EPOCH-RR (N = 11) | P Value |
|---|---|---|---|---|
| Male sex — no. (%) | 22 (73) | 13 (68) | 9 (82) | 0.67 |
| Age — yr | 0.03 | |||
| Median | 33 | 25 | 44 | |
| Range | 15–88 | 15–88 | 24–60 | |
| Age ≥40 yr — no. (%) | 12 (40) | 5 (26) | 7 (64) | 0.06 |
| Ann Arbor stage III or IV — no. (%) | 20 (67) | 11 (58) | 9 (82) | 0.25 |
| ECOG performance-status score ≥2 — no. (%)† | 9 (30) | 3 (16) | 6 (55) | 0.04 |
| Serum lactate dehydrogenase >ULN — no. (%) | 16 (53) | 7 (37) | 9 (82) | 0.03 |
| Extranodal site — no. (%)‡ | 19 (63) | 10 (53) | 9 (82) | 0.14 |
| Bowel | 15 (50) | 9 (47) | 6 (55) | 1.00 |
| Bone marrow or blood | 4 (13) | 3 (16) | 1 (9) | 1.00 |
| Central nervous system | 1 (3) | 1 (5) | 0 | 1.00 |
| LMB risk group — no. (%)§ | ||||
| A | 5 (17) | 5 (26) | 0 | 0.16 |
| B | 22 (73) | 12 (63) | 10 (91) | |
| C | 3 (10) | 2 (10) | 1 (9) | |
| Burkitt’s lymphoma variant — no. (%) | <0.001 | |||
| Sporadic | 17 (57) | 17 (89) | 0 | |
| Immunodeficiency-associated¶ | 13 (43) | 2 (11) | 11 (100) | |
| Secondary | 11 (37) | 0 | 11 (100) | |
| Primary | 2 (7) | 2 (11) | 0 | |
| Molecular marker — no./total no. (%) | ||||
| MYC rearrangement | 22/22 (100) | 14/14 (100) | 8/8 (100) | 1.00 |
| BCL6 protein expression | 24/24 (100) | 15/15 (100) | 9/9 (100) | 1.00 |
| BCL2 protein expression | 0/26 | 0/16 | 0/10 | 1.00 |
| EBER in situ hybridization | 6/21 (29) | 4/14 (29) | 2/7 (29) | 1.00 |
DA-EPOCH-R denotes dose-adjusted infusional therapy with etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, and rituximab; EBER Epstein–Barr virus–encoded RNA; SC-EPOCH-RR short-course infusional therapy with etoposide, doxorubicin, and vincristine with cyclophosphamide, prednisone, and a double dose of rituximab; and ULN upper limit of the normal range.
Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with 0 indicating no symptoms and full activity and higher scores indicating increasing levels of disability.
Patients may have had more than one extranodal site. In the SC-EPOCH-RR group, three patients had a site not listed in the table (liver, kidney, or other site).
Lymphomes malins B (LMB) risk groups are defined as follows: group A includes patients with low-risk disease (resected stage I or abdominal stage II cancer), group B includes those with intermediate-risk disease (patients not in group A or C), and group C includes those with high-risk disease (central nervous system involvement, at least 25% blasts in bone marrow, or both characteristics).13
Patients with the immunodeficiency-associated variant may have had HIV infection, the autoimmune lymphoproliferative syndrome, or a deficiency of dedicator of cytokinesis 8.
HIV-positive patients were older and had higher-risk disease than HIV-negative patients (Table 1). The HIV-positive patients had a median CD4+ T-cell count of 322 (range, 32 to 835) before treatment, and 4 of 11 patients (36%) had not received antiretroviral therapy previously.
Among the 22 patients who were assessed for MYC rearrangement, all had translocations involving an immunoglobulin gene (Table 1). All the cases analyzed were positive for BCL6 protein expression but negative for BCL2 protein expression, which is consistent with typical Burkitt’s lymphoma.27 EBV was detected by means of EBER in situ hybridization in 28% of HIV-negative patients and 28% of HIV-positive patients.
CLINICAL OUTCOME
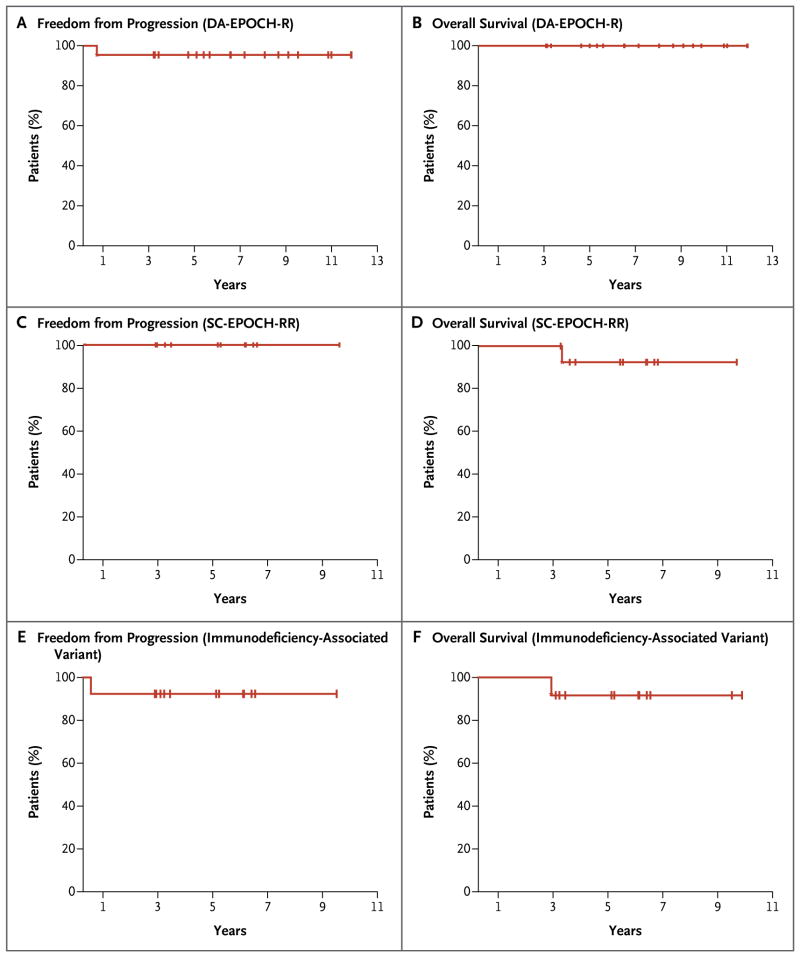
Patients who received DA-EPOCH-R were followed for a median of 86 months, and those who received SC-EPOCH-RR were followed for a median of 73 months. At the median follow-up, the DA-EPOCH-R group had a rate of freedom from progression of disease of 95% (95% confidence interval [CI], 75 to 99) and an overall survival rate of 100% (95% CI, 82 to 100), and the SC-EPOCH-RR group had a rate of freedom from progression of 100% (95% CI, 72 to 100) and an overall survival rate of 90% (95% CI, 60 to 98) (Fig. 1A through 1D). Among the patients with immuno-deficiency-associated Burkitt’s lymphoma, the rates of freedom from progression and overall survival were both 92% (Fig. 1E and 1F).
Figure 1. Kaplan–Meier Estimates of Freedom from Disease Progression and Overall Survival.
Panels A and B show the estimates of freedom from progression of disease and overall survival, respectively, among patients who received a dose-adjusted combination of etoposide, doxorubicin, and vincristine with cyclophosphamide, prednisone, and rituximab (DA-EPOCH-R). Panels C and D show the estimates of freedom from progression and overall survival, respectively, among patients who received a short course of the combination therapy with a double dose of rituximab in each cycle (SC-EPOCH-RR). Panels E and F show the estimates of freedom from progression and overall survival, respectively, among patients who had the immunodeficiency-associated variant of Burkitt’s lymphoma.
None of the patients in either group had a recurrence of disease or died from Burkitt’s lymphoma. However, one patient with primary immunodeficiency–associated Burkitt’s lymphoma did not have a pathological complete response and received localized radiotherapy. Acute myeloid leukemia developed in one HIV-positive patient 2.5 years after the completion of SC-EPOCH-RR, and the patient died 4 months later.
TREATMENT DOSE AND PHARMACOKINETICS
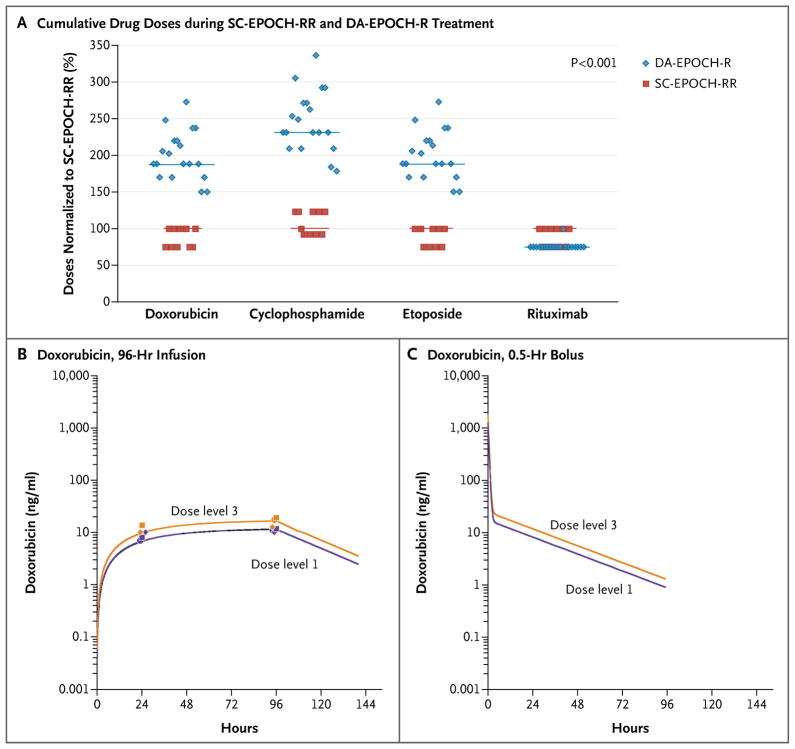
All but one patient in the DA-EPOCH-R group received six treatment cycles; one patient received eight cycles. With pharmacodynamic dose adjustment, 74% of the patients received at least dose level 3 and 37% received dose level 4 (for details of dose levels, see Fig. S3 in the Supplementary Appendix). Among patients who received SC-EPOCH-RR, which had been designed to minimize treatment, 45% received three treatment cycles and 55% received four treatment cycles, and doses were not escalated. To understand the role of dose intensity in the efficacy of EPOCH-R–based treatment, we looked at the cumulative doses of chemotherapy administered in the DA-EPOCH-R and SC-EPOCH-RR groups. The median cumulative doses of doxorubicin–etoposide and cyclophosphamide administered in the SC-EPOCH-RR group were 47% and 57% lower, respectively, than those administered in the DA-EPOCH-R group (Fig. 2A).
Figure 2. Cumulative Drug Doses and Infusional and Bolus Administrations of Doxorubicin.
The median cumulative doses of doxorubicin–etoposide and cyclophosphamide administered in the SC-EPOCH-RR group were 47% and 57% lower, respectively, than those administered in the DA-EPOCH-R group (Panel A). In a comparison of the maximal exposure of doxorubicin when administered at dose levels 1 and 3 in the DA-EPOCH-R group (see the Supplementary Appendix), we found a significant difference of 45% in the area under the concentration–time curves (1140 ng per milliliter per hour and 1650 ng per milliliter per hour, respectively) (Panel B). Purple symbols indicate drug concentrations at dose level 1, and yellow symbols drug concentrations at dose level 3. Circles indicate Patient 1, who only had a measurement for dose level 3 at 96 hours but had measurements for both dose levels at 24 hours; diamonds and squares, respectively, indicate Patients 2 and 3, who had measurements for both dose levels at 24 and 96 hours. In a simulation of a 0.5-hour bolus-administration schedule, bolus administration led to transient high peak concentrations (Panel C).
We analyzed the pharmacokinetics of doxorubicin in patients in the DA-EPOCH-R group who received treatment at dose levels 1 and 3 to investigate the differences in maximal exposure of the infused drugs that were commonly achieved with SC-EPOCH-RR and DA-EPOCH-R (Fig. 2B). We found a 45% difference in the area under the concentration-time curves of 1140 ng per milliliter per hour and 1650 ng per milliliter per hour for dose levels 1 and 3, respectively, indicating substantial differences in drug exposure.
Next, we simulated a 0.5-hour bolus-administration schedule, which was based on these pharmacokinetic results, to illustrate the effect of the administration schedule (Fig. 2C). Although the administration schedule has no effect on overall drug exposure, it does have a major effect on concentration-exposure kinetics. Bolus administration led to transient high-peak concentrations, whereas administration by infusion led to an extended exposure to steady-state concentrations (Fig. 2B and 2C). Our findings suggest that the length of exposure time that is above an effective threshold concentration (e.g., the steady-state concentration of 10 ng of doxorubicin per milliliter achieved at dose level 1), not the peak concentration, is the important therapeutic principle. In the present example, doxorubicin exposure above this steady-state concentration was approximately 2.5 times as long with the 96-hour administration time as it was with the 0.5-hour administration time (43 vs. 17 hours). This pharmacokinetic principle may explain why standard regimens in Burkitt’s lymphoma require dose-intense treatment to maximize the outcome of treatment.
TREATMENT TOXICITY
The toxic effects of DA-EPOCH-R and SC-EPOCH-RR were mainly grade 1 or 2 (Table 2). By design, DA-EPOCH-R was dose-adjusted to achieve a neutrophil nadir of less than 500 cells per cubic millimeter, which was observed during 52% of the cycles. Thrombocytopenia below 25,000 platelets per cubic millimeter occurred during only 2% of the cycles in either group. Fever and neutropenia were observed during 22% of the cycles in the DA-EPOCH-R group and were infrequent in patients 40 years of age or older.
Table 2.
Adverse Events.*
| Event | All Cycles (N = 155) | DA-EPOCH-R Cycles (N = 116) | SC-EPOCH-RR Cycles (N = 39) | P Value |
|---|---|---|---|---|
| Tumor lysis syndrome — no. of cycles (%) | 1 (1) | 0 | 1 (3) | NA |
| Absolute neutropenia — no. of cycles (%) | ||||
| Nadir <500 cells/mm3 | 72 (46) | 60 (52) | 12 (31) | 0.03 |
| Nadir <100 cells/mm3 | 26 (17) | 20 (17) | 6 (15) | 1.00 |
| Thrombocytopenia — no. of cycles (%) | ||||
| Nadir <50,000 platelets/mm3 | 12 (8) | 7 (6) | 5 (13) | 0.18 |
| Nadir <25,000 platelets/mm3 | 3 (2) | 2 (2) | 1 (3) | 1.00 |
| Fever and neutropenia necessitating hospital admission | ||||
| Any patient — no. of cycles (%) | 30 (19) | 26 (22) | 4 (10) | 0.11 |
| Patients ≥40 yr of age — no. of cycles/total no. (%) | 4/54 (7) | 2/30 (7) | 2/24 (8) | 1.00 |
| Gastrointestinal event — no. of cycles (%)† | ||||
| Mucositis | 8 (5) | 7 (6) | 1 (9) | 0.68 |
| Constipation | 2 (1) | 0 | 2 (5) | 0.06 |
| Ileus | 2 (1) | 2 (2) | 0 | 1.00 |
| Neurologic event — no. of patients/total no. (%)‡ | ||||
| Sensory impairment | 5/30 (17) | 4/19 (21) | 1/11 (9) | 0.63 |
| Motor impairment | 2/30 (7) | 2/19 (11) | 0/11 | 0.52 |
NA denotes not applicable.
All the gastrointestinal events were grade 3.
All the sensory-impairment events were grade 3, and all the motor-impairment events were grade 2.
SC-EPOCH-RR was associated with a lower incidence of neutropenia than DA-EPOCH-R (31% of the cycles vs. 52%). Hospital admission due to fever and neutropenia was observed during 10% of the SC-EPOCH-RR cycles. HIV-positive patients had a modest decline in the mean (±SE) CD4+ T-cell count before and immediately after treatment, from 325±75 cells per cubic millimeter to 219±4 cells per cubic millimeter (P = 0.03).
Nonhematopoietic toxic events with both regimens were similar to those in prior studies.16,18,20 The tumor lysis syndrome developed in one patient; no treatment-related deaths occurred. Overall, 18 of 155 cycles (12%) were administered in the hospital.
DISCUSSION
We present evidence from an uncontrolled prospective study that Burkitt’s lymphoma can be treated effectively with low-intensity therapy in an outpatient setting. Two dose-intensity variants of EPOCH-R–based treatment were tested in adults with sporadic or immunodeficiency-associated Burkitt’s lymphoma. Patients who were HIV-negative received standard DA-EPOCH-R, which was developed in studies of diffuse large B-cell lympho-ma.15,16,20 At a median follow-up of 86 months, 95% of patients in the DA-EPOCH-R group did not have progression of disease, and all were alive. A variant regimen with considerably lower treatment intensity, SC-EPOCH-RR, was tested in HIV-positive patients in order to reduce hematopoietic toxicity. Although patients in the SC-EPOCH-RR group had more advanced disease than those in the DA-EPOCH-R group, as well as immunodeficiency, they all had complete remissions that have been sustained without additional therapy.
The safety profile of both EPOCH-R regimens is similar to that in our previous studies of DA-EPOCH-R.16,20 Patients who received SC-EPOCH-RR had a lower incidence of neutropenia than those who received DA-EPOCH-R, which reflects the lower treatment intensity of SC-EPOCH-RR. Furthermore, the incidence of fever and neutropenia was lowest in patients who received SC-EPOCH-RR and in patients 40 years of age or older who received DA-EPOCH-R — the two patient groups most vulnerable to the toxicity of standard regimens for the treatment of Burkitt’s lymphoma.3,12,28,29 It is notable that the tumor lysis syndrome developed in only one patient. One HIV-positive patient died from acute myeloid leukemia. To assess whether EPOCH-R–based treatment is associated with secondary acute myeloid leukemia, we reviewed published data from all 360 patients with aggressive B-cell lymphoma who received treatment in our EPOCH-R–based studies and found two instances: one in an HIV-positive patient in the current study and one in a patient with a telomerase mutation. HIV infection and telomerase mutations are both risk factors for acute leukemia.15–18,20,30–32
The clinical outcome of EPOCH-R–based treatment in adults is similar to that of intensive short-cycle regimens for Burkitt’s lymphoma in children. In these regimens, patients receive intensive treatment with methotrexate, cytarabine, and cyclophosphamide, in addition to doxorubicin.10 The Berlin–Frankfurt–Münster (BFM) Group reported an event-free survival rate of 89% at 6 years among 266 children with Burkitt’s lymphoma who were treated in the Non-Hodgkin’s Lymphoma–BFM 90 trial.5 The Société Française d’Oncologie Pédiatrique group reported a similar result in their LMB89 study involving 420 children with Burkitt’s lymphoma, with an event-free survival rate of 92% at 5 years.4 When these regimens were modified for adults, the outcomes were less favorable, and the toxicity was very high.
A study of an adapted pediatric LMB protocol showed an event-free survival rate of 65% at 2 years among 72 adults with Burkitt’s lymphoma.12 An international study of modified CODOX-M–IVAC (cyclophosphamide, vincristine, doxorubicin, and methotrexate plus ifosfamide, etoposide, and cytarabine) in 52 adults with lymphoma also showed an event-free survival rate of 65% at 2 years.3,28 The distribution of patients according to risk factors in our study is similar to the distribution in these previous studies that involved adults.3,12,28 It is important to note that our treatment included rituximab, which is not a component of these other regimens for Burkitt’s lymphoma. However, recent studies suggest that rituximab provides only a modest survival benefit when added to standard regimens for Burkitt’s lymphoma.33–35
The toxicity of EPOCH-R–based treatment in Burkitt’s lymphoma is considerably less than that of other regimens that have been used in children or adults. Indeed, modified CODOX-M–IVAC had severe toxicity, including grade 4 neutropenia and thrombocytopenia in 90% and 58% of patients, respectively.3 Similar toxicity was observed with the adapted pediatric LMB protocol for adults, including three deaths that were considered to be related to treatment.12
Our case series study has some limitations. The small sample of adults with Burkitt’s lymphoma and the small number of patients with central nervous system involvement are major limitations, as compared with studies of pediatric case series.4,36 Like other case series studies involving adults, ours has wide confidence intervals.3,10,12,28,35 Unlike other studies, the present study included patients with immunodeficiency-associated Burkitt’s lymphoma, who are reported to have a worse outcome than those with other variants of the disease.37
Several lines of evidence indicate that the scheduling of EPOCH-R–based therapy overcomes the need for high-intensity treatment. The excellent efficacy of both DA-EPOCH-R and SC-EPOCH-RR, despite a difference of up to 57% in the cumulative drug dose between the two regimens, suggests that exposure-concentration time, not peak concentration, is important.
Mechanistically, prolonged exposure to doxorubicin, etoposide, and vincristine may inhibit DNA repair and favor apoptosis by enhancing genotoxic stress and impeding microtubule-dependent protein transport.38,39 On the other hand, even at high doses, standard regimens for Burkitt’s lymphoma may not achieve effective exposure kinetics, thereby limiting their therapeutic index. Other low-intensity regimens, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), CHOP-R (CHOP and rituximab), and CHOEP-R (CHOP-R and etoposide), which use similar drugs administered on a bolus schedule, are associated with low overall survival rates (<40%) among patients with Burkitt’s lymphoma.33,40 Furthermore, attempts to lower the incidence of toxic events among adults by reducing the drug doses in standard regimens for Burkitt’s lymphoma have led to less favorable outcomes than those with standard regimens.3,12,35
An important characteristic of EPOCH-R–based treatment is the absence of high-dose methotrexate and cytarabine, both of which increase the toxicity of treatment but have been considered essential for disease control.10 Our results indicate that these agents are not essential for the control of systemic disease with EPOCH-R–based treatment. The limited number of patients with active involvement of the central nervous system precludes a confident conclusion about efficacy in this subgroup. However, the single patient with active central nervous system disease had a durable remission with intrathecal methotrexate alone, and among the 25 patients with intermediate-risk or high-risk disease (according to the LMB classification) who were at risk for central nervous system disease, none had a recurrence.41
In conclusion, low-intensity EPOCH-R–based therapy appears to obviate the need for high-intensity treatment and markedly reduces treatment toxicity while achieving high rates of durable response. Two confirmatory trials of risk-stratified treatment that are based on the SC-EPOCH-RR and DA-EPOCH-R regimens are under way in adults (NCT01092182) and children (NCT01760226) with Burkitt’s lymphoma.
Supplementary Material
Acknowledgments
Supported by the National Cancer Institute.
We thank Nicole Grant and Therese White for research-nurse support; Alexandra Freeman for clinical support; and Maryalice Stetler-Stevenson, Diane Arthur, Mark Raffeld, and Svetlana Pack for laboratory support.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–23. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 2.Wang ES, Straus DJ, Teruya-Feldstein J, et al. Intensive chemotherapy with cyclophosphamide, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine (CODOX-M/IVAC) for human immunodeficiency virus-associated Burkitt lymphoma. Cancer. 2003;98:1196–205. doi: 10.1002/cncr.11628. [DOI] [PubMed] [Google Scholar]
- 3.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13:1264–74. doi: 10.1093/annonc/mdf253. [DOI] [PubMed] [Google Scholar]
- 4.Patte C, Auperin A, Michon J, et al. The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–9. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 5.Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 1999;94:3294–306. [PubMed] [Google Scholar]
- 6.Todeschini G, Bonifacio M, Tecchio C, et al. Intensive short-term chemotherapy regimen induces high remission rate (over 90%) and event-free survival both in children and adult patients with advanced sporadic Burkitt lymphoma/leukemia. Am J Hematol. 2012;87:22–5. doi: 10.1002/ajh.22189. [DOI] [PubMed] [Google Scholar]
- 7.Burkhardt B, Oschlies I, Klapper W, et al. Non-Hodgkin’s lymphoma in adolescents: experiences in 378 adolescent NHL patients treated according to pediatric NHL-BFM protocols. Leukemia. 2011;25:153–60. doi: 10.1038/leu.2010.245. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–20. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub R, Moulding C, Battey J, et al. Activation and somatic mutation of the translocated c-myc gene in Burkitt lymphoma cells. Cell. 1984;36:339–48. doi: 10.1016/0092-8674(84)90227-7. [DOI] [PubMed] [Google Scholar]
- 10.Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood. 2004;104:3009–20. doi: 10.1182/blood-2004-02-0405. [DOI] [PubMed] [Google Scholar]
- 11.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 12.Diviné M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005;16:1928–35. doi: 10.1093/annonc/mdi403. [DOI] [PubMed] [Google Scholar]
- 13.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–80. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connor PM, Wassermann K, Sarang M, Magrath I, Bohr VA, Kohn KW. Relationship between DNA cross-links, cell cycle, and apoptosis in Burkitt’s lymphoma cell lines differing in sensitivity to nitrogen mustard. Cancer Res. 1991;51:6550–7. [PubMed] [Google Scholar]
- 15.Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99:2685–93. doi: 10.1182/blood.v99.8.2685. [DOI] [PubMed] [Google Scholar]
- 16.Wilson WH, Jung SH, Porcu P, et al. A Cancer and Leukemia Group B multi-center study of DA-EPOCH-rituximab in untreated diffuse large B-cell lymphoma with analysis of outcome by molecular subtype. Haematologica. 2012;97:758–65. doi: 10.3324/haematol.2011.056531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368:1408–16. doi: 10.1056/NEJMoa1214561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood. 2010;115:3017–24. doi: 10.1182/blood-2009-11-253039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 20.Wilson WH, Dunleavy K, Pittaluga S, et al. Phase II study of dose-adjusted EPOCH and rituximab in untreated diffuse large B-cell lymphoma with analysis of germinal center and post-germinal center biomarkers. J Clin Oncol. 2008;26:2717–24. doi: 10.1200/JCO.2007.13.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swerdlow S, Campo E, Harris N, et al. WHO classification of tumors of haematopoietic and lymphoid tissues. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 22.Warren KE, Patel MC, McCully CM, Montuenga LM, Balis FM. Effect of P-gly-coprotein modulation with cyclosporin A on cerebrospinal fluid penetration of doxorubicin in non-human primates. Cancer Chemother Pharmacol. 2000;45:207–12. doi: 10.1007/s002800050031. [DOI] [PubMed] [Google Scholar]
- 23.Ratain MJ. Pharmacology of cancer chemotherapy. 6. Philadelphia: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- 24.Kontny NE, Würthwein G, Joachim B, et al. Population pharmacokinetics of doxorubicin: establishment of a NONMEM model for adults and children older than 3 years. Cancer Chemother Pharmacol. 2013;71:749–63. doi: 10.1007/s00280-013-2069-1. [DOI] [PubMed] [Google Scholar]
- 25.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. Multicompartment models. [Google Scholar]
- 26.Mehta CR. PNR: a network algorithm for performing Fisher’s exact test in r x c contingency tables. J Am Stat Assoc. 1983;78:427–34. [Google Scholar]
- 27.Swerdlow SHCE, Harris NL, Campo E, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4. Lyon, France: International Agency for Research on Cancer; 2008. [Google Scholar]
- 28.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol. 1996;14:925–34. doi: 10.1200/JCO.1996.14.3.925. [DOI] [PubMed] [Google Scholar]
- 29.Montoto S, Wilson J, Shaw K, et al. Excellent immunological recovery following CODOX-M/IVAC, an effective intensive chemotherapy for HIV-associated Burkitt’s lymphoma. AIDS. 2010;24:851–6. doi: 10.1097/QAD.0b013e3283301578. [DOI] [PubMed] [Google Scholar]
- 30.Kirwan M, Vulliamy T, Marrone A, et al. Defining the pathogenic role of telomerase mutations in myelodysplastic syndrome and acute myeloid leukemia. Hum Mutat. 2009;30:1567–73. doi: 10.1002/humu.21115. [DOI] [PubMed] [Google Scholar]
- 31.Sutton L, Guénel P, Tanguy ML, et al. Acute myeloid leukaemia in human immunodeficiency virus-infected adults: epidemiology, treatment feasibility and outcome. Br J Haematol. 2001;112:900–8. doi: 10.1046/j.1365-2141.2001.02661.x. [DOI] [PubMed] [Google Scholar]
- 32.Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood. 2003;101:4653–9. doi: 10.1182/blood-2002-11-3589. [DOI] [PubMed] [Google Scholar]
- 33.Wästerlid T, Brown PN, Hagberg O, et al. Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic Lymphoma Group. Ann Oncol. 2013;24:1879–86. doi: 10.1093/annonc/mdt058. [DOI] [PubMed] [Google Scholar]
- 34.Barnes JA, Lacasce AS, Feng Y, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol. 2011;22:1859–64. doi: 10.1093/annonc/mdq677. [DOI] [PubMed] [Google Scholar]
- 35.Kasamon YL, Brodsky RA, Borowitz MJ, et al. Brief intensive therapy for older adults with newly diagnosed Burkitt or atypical Burkitt lymphoma/leukemia. Leuk Lymphoma. 2013;54:483–90. doi: 10.3109/10428194.2012.715346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 2000;95:416–21. [PubMed] [Google Scholar]
- 37.Cortes J, Thomas D, Rios A, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone and highly active antiretroviral therapy for patients with acquired immunodeficiency syndrome-related Burkitt lymphoma/leukemia. Cancer. 2002;94:1492–9. doi: 10.1002/cncr.10365. [DOI] [PubMed] [Google Scholar]
- 38.Gundersen GG, Cook TA. Microtubules and signal transduction. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 39.Offer H, Erez N, Zurer I, et al. The onset of p53-dependent DNA repair or apoptosis is determined by the level of accumulated damaged DNA. Carcinogenesis. 2002;23:1025–32. doi: 10.1093/carcin/23.6.1025. [DOI] [PubMed] [Google Scholar]
- 40.Longo DL, Duffey PL, Jaffe ES, et al. Diffuse small noncleaved-cell, non-Burkitt’s lymphoma in adults: a high-grade lymphoma responsive to ProMACE-based combination chemotherapy. J Clin Oncol. 1994;12:2153–9. doi: 10.1200/JCO.1994.12.10.2153. Erratum, J Clin Oncol 1996;14:1969. [DOI] [PubMed] [Google Scholar]
- 41.Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–7. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.