Abstract
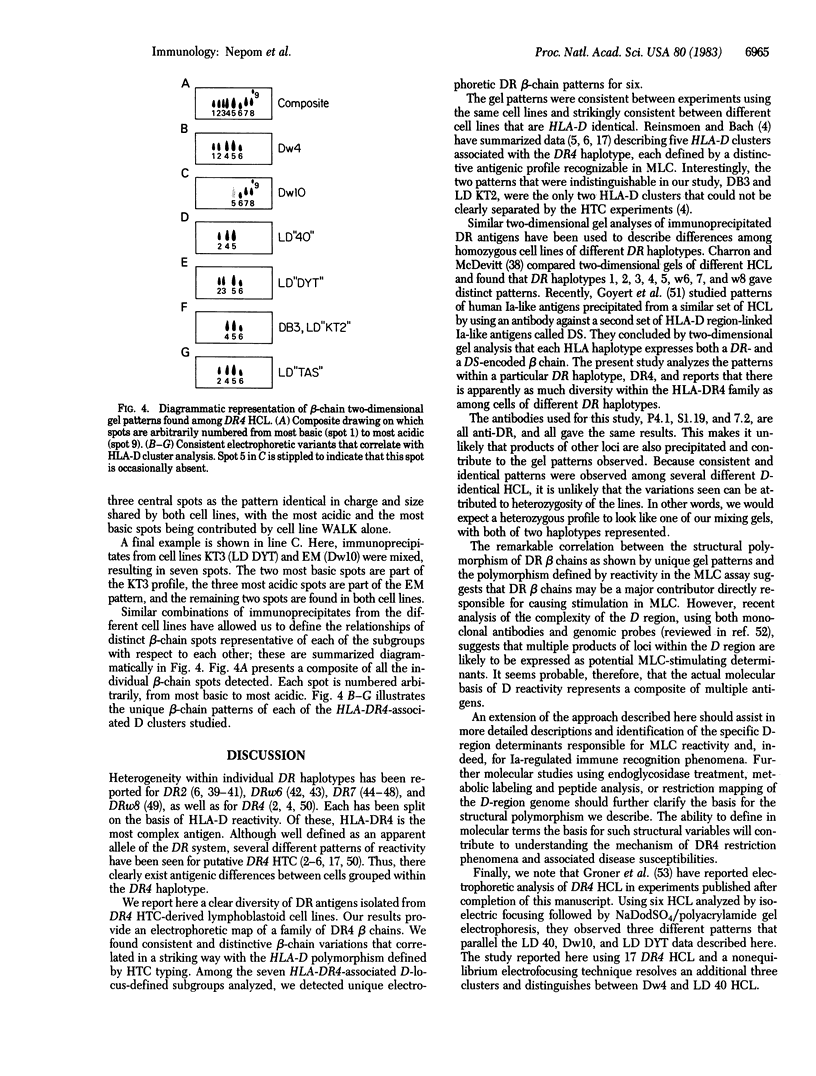
Two-dimensional gel electrophoresis of immunoprecipitated human HLA-DR antigens from cells expressing the HLA-DR4 haplotype shows distinct clustering of beta-chain patterns. Six unique electrophoretic variants were observed among 17 HLA-DR4 homozygous cell lines (HCL) analyzed. These patterns correlate precisely with the HLA-D phenotype of the HCL donor as determined by reactivity in mixed lymphocyte culture. All DR4 HCL that belong to one of the well-defined HLA-D antigen groups (Dw4, Dw10, LD "40", LD "DYT", LD "KT2", or LD "TAS") have identical DR beta-chain patterns; DR4 HCL belonging to different HLA-D antigen groups do not. The concordance of the functional expression in mixed lymphocyte culture of a specific D phenotype with a distinct DR beta-chain pattern on gel analysis provides a direct structural basis for understanding the genetic control of HLA-D polymorphisms; HLA-D specificities as revealed by T-cell recognition in mixed lymphocyte culture thus might be accounted for by DR beta-chain polymorphisms. The extent of this beta-chain diversity within a single DR haplotype may aid in understanding variations in Ia-regulated functions, such as Ir gene control and certain disease susceptibilities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amar A., Oksenberg J., Cohen N., Cohen I., Brautbar C. HLA-D locus in Israel. Characterization of 14 local HTC's and a population study. Tissue Antigens. 1982 Sep;20(3):198–207. doi: 10.1111/j.1399-0039.1982.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Bias W. B., Hsu S. H., Pollard M. K., Harvey J., Lotze M. T., Arnett F. C., Stevens M. B. HLA-DR characterization of a Chippewa Indian subpopulation with high prevalence of rheumatoid arthritis. Hum Immunol. 1981 Mar;2(2):155–163. doi: 10.1016/0198-8859(81)90062-8. [DOI] [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Characterization of HLA-D-region antigens by two-dimensional gel electrophoresis. Molecular-genotyping. J Exp Med. 1980 Aug 1;152(2 Pt 2):18s–36s. [PubMed] [Google Scholar]
- Dupont B., Hansen J. A., Whitsett C., Slater L., Lee T. D. Population genetics for five HLA-D determinants in caucasians (New York City). Transplant Proc. 1977 Mar;9(1 Suppl 1):67–72. [PubMed] [Google Scholar]
- Fuller T. C., Einarson M., Pinto C., Ahern A., Yunis E. J. Genetic evidence that HLA-DR (Ia) specificities include multiple HLA-D determinants on a single haplotype. Transplant Proc. 1978 Dec;10(4):781–784. [PubMed] [Google Scholar]
- Gladstone, Pious D. Stable variants affecting B cell alloantigens in human lymphoid cells. Nature. 1978 Feb 2;271(5644):459–461. doi: 10.1038/271459a0. [DOI] [PubMed] [Google Scholar]
- Goyert S. M., Shively J. E., Silver J. Biochemical characterization of a second family of human Ia molecules, HLA-DS, equivalent to murine I-A subregion molecules. J Exp Med. 1982 Aug 1;156(2):550–566. doi: 10.1084/jem.156.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyert S. M., Silver J. Further characterization of HLA-DS molecules: implications for studies assessing the role of human Ia molecules in cell interactions and disease susceptibility. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5719–5723. doi: 10.1073/pnas.80.18.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner J. P., Watson A. J., Bach F. H. Dw/LD-related molecular polymorphism of DR4 beta-chains. J Exp Med. 1983 May 1;157(5):1687–1691. doi: 10.1084/jem.157.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde H., Hauptmann G., Johannsen R., Netzel B., Rittner C., Ruppelt W. Population data on two new HLA-D determinants, EI and RE. Scand J Immunol. 1977;6(5):515–521. doi: 10.1111/j.1365-3083.1977.tb02114.x. [DOI] [PubMed] [Google Scholar]
- Hanrahan L. R., Jr, Mehta C., Reinsmoen N. L., Yunis E. J. Linkage disequilibrium between HLA-B13 and HLA-D. Transplant Proc. 1977 Dec;9(4):1729–1732. [PubMed] [Google Scholar]
- Hsu S. H., Lambert P., Scribner P. P., Hartzman R. J., Robbins F., Bias W. B. Primary and secondary MLR characterization of three allelic variants of HLA-Dw7 segregating in a single family. Hum Immunol. 1983 Mar;6(3):167–176. doi: 10.1016/0198-8859(83)90099-x. [DOI] [PubMed] [Google Scholar]
- Kaufman J. F., Strominger J. L. HLA-DR light chain has a polymorphic N-terminal region and a conserved immunoglobulin-like C-terminal region. Nature. 1982 Jun 24;297(5868):694–697. doi: 10.1038/297694a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lee D. R., Hansen T. H., Cullen S. E. Detection of an altered I-A beta polypeptide in the murine Ir mutant, B6.C-h-2bm12. J Immunol. 1982 Jul;129(1):245–251. [PubMed] [Google Scholar]
- Mickelson E. M., Nisperos B., Hansen J. A. Two homozygous typing cells defining a subgroup of HLA-Dw6. Hum Immunol. 1980 Dec;1(4):363–368. doi: 10.1016/0198-8859(80)90112-3. [DOI] [PubMed] [Google Scholar]
- Mickelson E. M., Nisperos B., Layrisse Z., Kim S. J., Thomas E. D., Hansen J. A. Analysis of the HLA-DRw8 haplotype: recognition by HTC typing of three distinct antigen complexes in Caucasians, Native Americans, and Orientals. Immunogenetics. 1983;17(4):399–410. doi: 10.1007/BF00372458. [DOI] [PubMed] [Google Scholar]
- Nose Y., Sato K., Nakagawa M., Kondoh K., Inouye H., Tsuji K. HLA-D clusters associated with DR4 in the Japanese population. Hum Immunol. 1982 Nov;5(3):199–203. doi: 10.1016/0198-8859(82)90132-x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Reinsmoen N. L., Bach F. H. Five HLA-D clusters associated with HLA-DR4. Hum Immunol. 1982 Jun;4(3):249–258. doi: 10.1016/0198-8859(82)90040-4. [DOI] [PubMed] [Google Scholar]
- Reinsmoen N. L., Sasazuki T., Kaneoka H., Ohta N., Noreen H. J., Greenberg L. J., Kersey J. H. Two distinct HLA-D specificities (DHO and Dw2) in linkage with HLA-DRw2 as defined in white and Japanese populations. Transplant Proc. 1978 Dec;10(4):789–791. [PubMed] [Google Scholar]
- Sasazuki T., McMichael A., Payne R., McDevitt H. O. HLA-D antigens in the Japanese population. Tissue Antigens. 1977 May;9(5):267–272. doi: 10.1111/j.1399-0039.1977.tb01117.x. [DOI] [PubMed] [Google Scholar]
- Schreuder G. M., Parlevliet J., Termijtelen A., van Rood J. J. Reanalysis of the HLA-DRw6 complex. Tissue Antigens. 1983 Jan;21(1):62–74. doi: 10.1111/j.1399-0039.1983.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Kaufman J. F., Korman A. J., Strominger J. L. HLA-DR antigens: structure, separation of subpopulations, gene cloning and function. Immunol Rev. 1982;66:133–187. doi: 10.1111/j.1600-065x.1982.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Lampson L. A., Strominger J. L. Analysis of HLA-DR antigens by using monoclonal antibodies: recognition of conformational differences in biosynthetic intermediates. J Immunol. 1981 Oct;127(4):1403–1410. [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Ferrone S. Structural polymorphism of human DR antigens. Nature. 1979 May 31;279(5712):436–437. doi: 10.1038/279436a0. [DOI] [PubMed] [Google Scholar]
- Silver J., Walker L. E., Reisfeld R. A., Pellegrino M. A., Ferrone S. Structural studies of murine I-E and human DR antigens. Mol Immunol. 1979 Jan;16(1):37–42. doi: 10.1016/0161-5890(79)90025-7. [DOI] [PubMed] [Google Scholar]
- Suciu-Foca N., Godfrey M., Khan G., Woodward K., Rohowsky C., Reed E., Hardy M., Reemtsma K. New HLA-D alleles associated with DR1 and DR2. Tissue Antigens. 1981 Mar;17(3):294–302. doi: 10.1111/j.1399-0039.1981.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Trucco M. M., Garotta G., Stocker J. W., Ceppellini R. Murine monoclonal antibodies against HLA structures. Immunol Rev. 1979;47:219–252. doi: 10.1111/j.1600-065x.1979.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Willkens R. F., Hansen J. A., Malmgren J. A., Nisperos B., Mickelson E. M., Watson M. A. HLA antigens in Yakima Indians with rheumatoid arthritis. Lack of association with HLA-Dw4 and HLA-DR4. Arthritis Rheum. 1982 Dec;25(12):1435–1439. doi: 10.1002/art.1780251208. [DOI] [PubMed] [Google Scholar]
- Yunis E. J., Amos D. B. Three closely linked genetic systems relevant to transplantation. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3031–3035. doi: 10.1073/pnas.68.12.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rood J. J., van Leeuwen A., Keuning J. J., van Oud Alblas A. B. The serological recognition of the human MLC determinants using a modified cytotoxicity technique. Tissue Antigens. 1975 Apr;5(2):73–79. doi: 10.1111/j.1399-0039.1975.tb00532.x. [DOI] [PubMed] [Google Scholar]