Abstract
Mast cells are important sentinels guarding the interface between the environment and the body: a breach in the integrity of this interface can lead to the release of a plethora of mediators which engage the foreign agent, recruit leukocytes, and initiate adaptive physiological changes in the organism. While these capabilities make mast cells critical players in immune defense, it also makes them important contributors to the pathogenesis of diseases such as asthma. Mast cell mediators induce dramatic changes in smooth muscle physiology, and the expression of receptors for these factors by smooth muscle suggests that they act directly to initiate constriction. Contrary to this view, we show here that mast cell-mediated bronchoconstriction is observed only in animals with intact innervation of the lung and that serotonin release alone is required for this action. While ablation of sensory neurons does not limit bronchoconstriction, constriction after antigen challenge is absent in mice in which the cholinergic pathways are compromised. Linking mast cell function to the cholinergic system likely provides an important means of modulating the function of these resident immune cells to physiology of the lung, but may also provide a safeguard against life-threatening anaphylaxis during mast cell degranulation.
Keywords: Rodent, Mast Cells, Allergy, Lung
Introduction
IgE-dependent mast cell activation is the central mechanism underlying immediate allergic reaction (1). Cross-linking of IgE bound by Fc receptors on mast cells leads to release of preformed mediators stored in the mast cell granules, as well as production of lipid mediators and up-regulation of cytokine synthesis (1). These mediators can profoundly influence the physiology of the organism and thus systemic release of mast cell mediators can result in life threatening anaphylaxis. Mast cells can also contribute to chronic inflammatory diseases, including asthma, where activation by environmental allergens contributes to inflammation and reversible airway constriction, a hallmark of this disease. Consistent with a role for mast cells in this aspect of asthma, an increase in airway mast cells has been reported in asthmatic patients compared to healthy individuals (2).
The mechanism(s) through which mast cell mediators contribute to the airway obstruction is not yet completely understood. However, airway smooth muscle (ASM) expresses many receptors for mediators produced by mast cells (3). Thus, in the simplest model, mast cell mediators, including cysteinyl leukotrienes (cysLTs), histamine and serotonin (5-HT) act directly on smooth muscle, triggering increases in intracellular Ca2+ followed by assembly of the contractile apparatus. Direct ability of several mast cell mediators to elicit Ca2+ flux in muscle cells and constriction of tracheal rings further supports this model (4–6).
In a number of organ systems, close interaction of mast cells with both sensory and parasympathetic neurons has been demonstrated, suggesting that mast cells may induce bronchoconstriction by altering the activity of the neuronal pathways which function to determine ASM tone. For example, histamine released by mast cells is reported to stimulate parasympathetic neurons in the guinea pig heart (7), while antigen challenge in sensitized rats results in the activation of afferent nerve fibers in the gut mediated by both histamine and serotonin (8). Consistent with this, mucosal mast cell mediators from patients with irritable bowl syndrome were reported to excite rat nociceptive visceral sensory nerves (9). Additionally, a number of studies suggest that this relationship is also present in other organs including the lung. For example it has been reported that substance P containing nerves in the rat trachea interact with mast cells to cause antigen-specific, and dependent, changes in lung solute clearance and epithelial chloride ion secretion (10–12).
In humans, the cholinergic parasympathetic nervous system represents the predominate bronchoconstrictor pathway in the airways. Efferent cholinergic nerve fibers run through the vagus nerve and synapse in small ganglia within the airway wall. These nerve fibers release acetylcholine (ACh), which binds nicotinic acetylcholine receptors (nAChRs) on the parasympathetic ganglion, which activates postganglionic fibers innervating ASM and submucosal glands. These postganglionic fibers release ACh, which binds to muscarinic acetylcholine receptors (mAChRs) directly on ASM. The activity of the parasympathetic pathway is modulated by sensory neurons present throughout the airway. Supporting a role for the mast cell interaction with the autonomic nervous system, mast cells have been shown to be closely associated with airway nerves (13), and receptors for many mast cells mediators, including histamine and serotonin, are expressed by neurons (14–16). In the studies detailed here we demonstrate that in allergic mice, antigen challenge induces constriction of the central airways, and this constriction is dependant not only on mast cells but also on an intact parasympathetic system.
Materials and methods
Experimental animals
All studies were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee (IACUC) guidelines of the University of North Carolina at Chapel Hill. Mast cell deficient C57BL/6KitW-sh/KitW-sh, PAR2−/− (17) and their wild-type C57BL/6J controls were purchased from Jackson Laboratory. M1 and M3 mAChR-deficient mice and their controls were bred as previously described (18, 19). The generation of congenic 5HTT-deficient mice has been previously described (20). All experiments were carried out using 8–12 week old mice, with the exception of the 5-HTT mice. Both control and experimental animals were 6 months of age.
Airway measurements in intubated mice
Mechanical ventilation and airway measurements were carried out as previously described (21). After baseline measurements, serotonin (6.25 µg/ml, Sigma Chemical) was administered i.v. through a jugular catheter in increasing doses (0.3, 0.6, and 1.2 pg/ml). Single doses of DNP (250 µl, 5 mg/ml, Sigma Chemical) or OVA (50 µl, 10 mg/ml, Grade V, Sigma Chemical) were also delivered i.v. through a jugular catheter. After administration, airway mechanics were determined using the Forced Oscillatory Technique (FOT) every 10 s for 3 min. The resultant pressure and flow data were fit to a constant phase model as previously described (22). Similar to other studies assessing FOT, we confined our analysis to Raw (Rn; Newtonian resistance), which assesses the flow resistance of the conducting airways, and G (tissue damping), which reflects tissue resistance in the peripheral airways (23). Total lung resistance (RL) was calculated from the FOT data by adding tissue resistance (Rti) to Raw as previously described (23, 24). Where Rti is given by:
where f is the frequency in Hz and α = (2/π) tan−1(H/G). All data are represented as percent baseline RL, Raw or G.
To determine the contribution of various receptors in airway responses, mice received a pre-treatment i.p. injection of pharmacological antagonist before exogenous administration of mediators or antigen. To asses the role of mAChRs and nAChRs to airway responses, mice received a pre-treatment i.p. injection of atropine sulfate (10µM/kg, American Pharmaceutical Partners) or mecamylamine (4mg/kg, Sigma Chemical), respectively. Methiothepin (2mg/kg, i.p.), ketanserin (12 mg/kg, i.p.), and ondansetron (1 mg/kg, i.p.) were administered to block serotonin receptors. Methiothepin is a nonspecific antagonist, whereas ketanserin and ondansetron are specific antagonists for 5-HT2A and 5-HT3 receptors, respectively.
Anaphylaxis protocols
IgE-dependent passive anaphylaxis
Mice were sensitized with 20 µg mouse monoclonal anti-DNP IgE (1.0 mg/ml, Sigma Chemical; 200 µl total volume in saline) or an equal volume PBS i.v. through the tail vein. Approximately 19–24 hours after anti-DNP IgE injection, mice were anesthetized, catheterized, tracheostomized and mechanically ventilated as described above. Immediately following the baseline measurements, 0.25 ml of 5 mg/ml DNP (or an equal volume of PBS) was injected into the jugular and airway parameters were measured every 10 s for 3 min immediately following anaphylaxis induction. Bar graphs represent the area under the curve.
Active systemic anaphylaxis to OVA
Mice received a single i.p. injection of 0.2 ml sterile 0.9% NaCl containing 20 µg OVA emulsified in 2.25 mg aluminum hydroxide gel (Sigma Chemical). 18–21 days after OVA immunization, mice were anesthetized, placed on a ventilator, challenged with a single i.v. infusion (via a jugular catheter) of 500 µg of OVA in 50 µl of sterile 0.9% NaCl and airway measurements were taken every 10 s for 3 min (25). Control mice were challenged with an equal volume of sterile 0.9% NaCl. Bar graphs represent the area under the curve.
Acute anaphylaxis to OVA following induction of allergic airway disease
Mice were sensitized by i.p. injection of 20 µg OVA emulsified in 2.25 mg aluminum hydroxide gel in a total volume of 200 µl on days 1 and 14. Mice were challenged (45 min) via the airways with OVA (1% in saline) for 5 days (days 21 – 25) using an ultrasonic nebulizer (DeVillbiss Health Care, Somerset, PA). On day 26 mice were anesthetized, placed on a ventilator, challenged with a single iv infusion (via a jugular catheter) of 500 µg of OVA in 50 µl of sterile 0.9 % NaCl. Airway measurements were taken every 10 s for 3 min. Bar graphs represent the area under the curve.
After airway measurements, mice were sacrificed and 0.5–1.0 ml of blood was collected by cardiac puncture. Following coagulation, serum was collected. Total IgE levels in the serum were measured by ELISA (R&D systems). BAL was performed successively with 1.0 ml of sterile HBSS five times. The recovered BAL fluid was centrifuged to remove cells. Histamine levels were measured in the cell-free supernatant by ELISA (Beckman Coulter).
Inhibition of serotonin with 4-Chloro-DL-phenylalanine (PCPA)
Mice received a single i.p. injection of 0.2 ml sterile 0.9% NaCl containing 20 µg OVA emulsified in 2.25 mg aluminum hydroxide gel. 18–21 days after OVA immunization, mice were treated with PCPA (150 mg/kg, i.p.; 25 mg/ml in 1N HCl) and immediately challenged with 250 µg of OVA in 50 µl of sterile 0.9% NaCl (i.v.) to deplete serotonin stored in mast cells. 24 hours later, mice received a second treatment of PCPA (150 mg/kg, i.p.) and were then anesthetized, placed on a ventilator, and challenged with a single i.v. infusion (via a jugular catheter) of 500 µg of OVA in 50 µl of sterile 0.9% NaCl and airway measurements taken every 10 s for 3 min. Control mice were challenged with an equal volume of sterile 0.9% NaCl.
After airway measurements, mice were sacrificed and 0.5–1.0 ml of blood collected by cardiac puncture with a syringe containing 20 µl 100 U/ml heparin. The blood was then centrifuged at 12000 rpm for 5 min to collect plasma. Serotonin levels were measured in the plasma by ELISA (Beckman Coulter).
Sensory ablation
Adults
Capsaicin was administered to ablate sensory neurons as previously described (26, 27). For pretreatment, mice received a s.c. dose of 25 mg/kg capsaicin at 0 hrs and a second s.c. dose of 75mg/kg capsaicin at 24 hrs (5 and 15 mg/ml capsaicin dissolved in 1:1:8 ethanol:Tween80:saline, respectively). To minimize the respiratory effects associated with capsaicin injection, animals were first anesthetized with avertin (250 mg/kg, i.p.) and then treated with 10 mg/kg theophylline (s.c., 5 mg/ml in distilled water, Sigma Chemical) and 0.1 mg/kg terbutaline (i.p., 0.05 mg/ml in saline, Sigma Chemical). Airway assessment was conducted 10–12 days after the final capsaicin treatment.
Neonates
Pups were injected with 50 mg/kg capsaicin (15 mg/ml, s.c.) at day 2 to 3 after birth to degrade sensory neurons. Animals were aged to 8 weeks of age before conducting studies.
To check for effectiveness of the treatment, a drop of 0.1 mg/ml capsaicin was instilled into one eye of each mouse and wiping movements were counted as previously described (28, 29). For substance P measurements, lungs were lavaged with 0.4 ml chilled PBS containing 500 KIU/ml aprotinin (Sigma Chemical). Substance P was measured from BAL by ELISA (R&D systems).
Vagotomy
A surgical vagotomy was conducted to assess the contribution of intact parasympathetic innervation as previously described (30). Briefly, the right and left vagus nerves running parallel to the trachea were isolated and a piece of surgical string was passed underneath to facilitate cutting during mechanical ventilation. In experimental animals, basal lung mechanics were established before severing of both vagus nerves. Post vagotomy baseline was then reassessed prior to i.v. challenge. Control animals received a sham operation which involved simply lifting and releasing of the surgical string along with the nerve fibers.
Statistical analysis
Data are represented as means ± SEM. Analysis Of Variance (ANOVA) followed by Tukey-Kramer Honestly Significant Difference for multiple comparisons was performed on complex data sets. Statistical significance for single data points was assessed by Student’s two-tailed t-test. A P value of <0.05 was considered statistically significant.
Results
Change in airway resistance during anaphylaxis is dependant on mast cells, primarily those located in the central airways
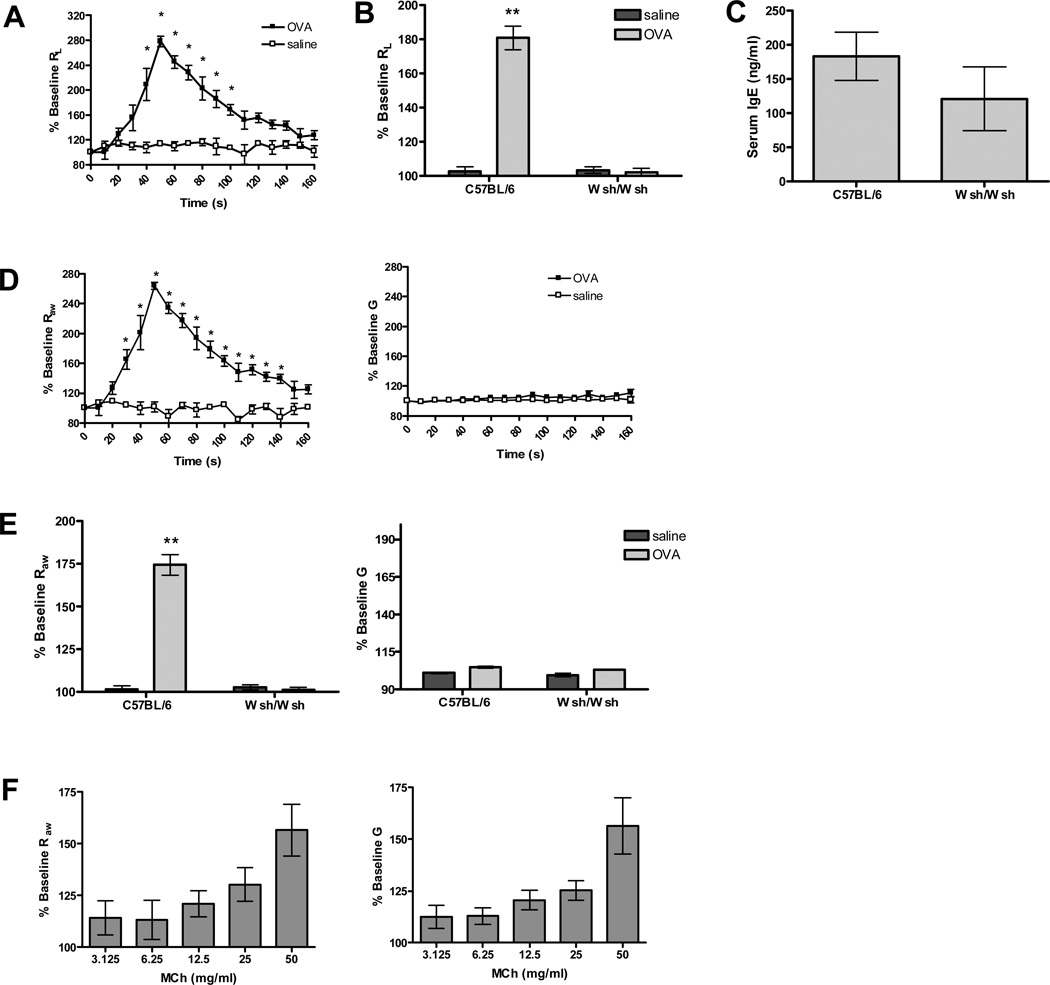
We first determined the contribution of mast cells to changes in airway mechanics. Previous studies have shown that in the mouse, antigen treatment following sensitization results in rapid decrease in lung conductance (GL) (25), which is the inverse of lung resistance (RL). Similarly, we found that sensitization of wild-type C57BL/6 (B6) mice with OVA plus adjuvant, followed by exposure to antigen, resulted in a rapid and dramatic increase in RL (Figure 1 A). Similar immunization and challenge of C57BL/6 KitW-sh/KitW-sh (Wsh/Wsh) mice, which lack mast cells, failed to induce changes in lung mechanics, indicating that this response was dependent on the normal development of this cell population (Figure 1 B). To verify that the difference in RL was not due to a reduction in overall IgE production in the mast cell deficient animals, we compared total serum IgE levels in the B6 and Wsh/Wsh congenic pairs (Figure 1 C). There was no difference in IgE production between the two groups.
Figure 1. Changes in airway physiology of wild-type and mast cell deficient mice in response to OVA and MCh.
(A) OVA challenge in sensitized mice (n=5) induced an immediate increase in RL that gradually returned to baseline. Control animals (n=4) sensitized with OVA but challenged with saline had no change in RL over baseline (*P<0.05 compared to OVA/saline). (B) Increases in RL are dependent on mast cells, as there was no response in mast cell deficient Wsh/Wsh mice (n=8) after antigen challenge (**P<0.001 compared to all other groups). (C) IgE levels were comparable between wild-type (n=7) and mast cell deficient (n=7) mice sensitized and challenged with OVA. (D) FOT measurement of airway mechanics after antigen challenge. OVA challenge in sensitized mice (n=5) resulted in a significant increase in Raw but no change in G compared to controls (n=4) (*P<0.05 compared to saline). (E) Increases in Raw in sensitized mice are dependent on mast cells. Sensitized Wsh/Wsh mice (n=8) had no response to antigen challenge (**P<0.001 compared to all other groups). (F) Aerosol methacholine (MCh) challenge in B6 mice (n=13) results in a dose dependent increase in both Raw and G.
Mast cells are located throughout the airways including the trachea, bronchus, and sporadically in the parenchyma (31), yet it is unclear whether all of these cells contribute to changes in lung physiology during anaphylaxis. To examine this point more closely, we monitored changes in airway mechanics after sensitization and exposure to OVA using FOT. Interestingly, using this method we found that the changes in lung mechanics were largely limited to a parameter termed ‘airway resistance’ or Raw, which a number of theoretical and experimental studies suggest is sensitive primarily to changes in the central airways (32). As can be seen in figure 1 D, antigen induced a significant and robust change in Raw in B6 mice previously sensitized to OVA plus adjuvant and little to no change in G, a parameter termed ‘tissue damping’ that is associated with changes in the distal lung. In contrast, no change in Raw was observed in mast cell deficient Wsh/Wsh mice after antigen challenge or in mice treated with vehicle (Figure 1 E). To further verify these findings, we examined changes in airway mechanics in a mouse model of passive anaphylaxis. Mice received i.v. monoclonal anti-DNP IgE, and 24 hours later, after establishment of baseline lung mechanics, mast cell degranulation was induced by i.v. delivery of antigen (DNP). DNP induced similar increases in Raw as those seen during active anaphylaxis to OVA (data not shown). Unlike antigen induced constriction, airway constriction induced with methacholine (MCh), a stable acetylcholine analog, resulted in both changes in Raw and G consistent with both changes in the central and distal airways in response to this neurotransmitter (Figure 1 F). Similar responses to both antigen and MCh challenge were also observed in the BALB/c and 129/SvEv strains (data not shown). This suggests that although the ability of methacholine to induce airflow obstruction is distributed throughout the lung, mast cell degranulation results in only central airway constriction, despite the presence of these cells in peripheral airways.
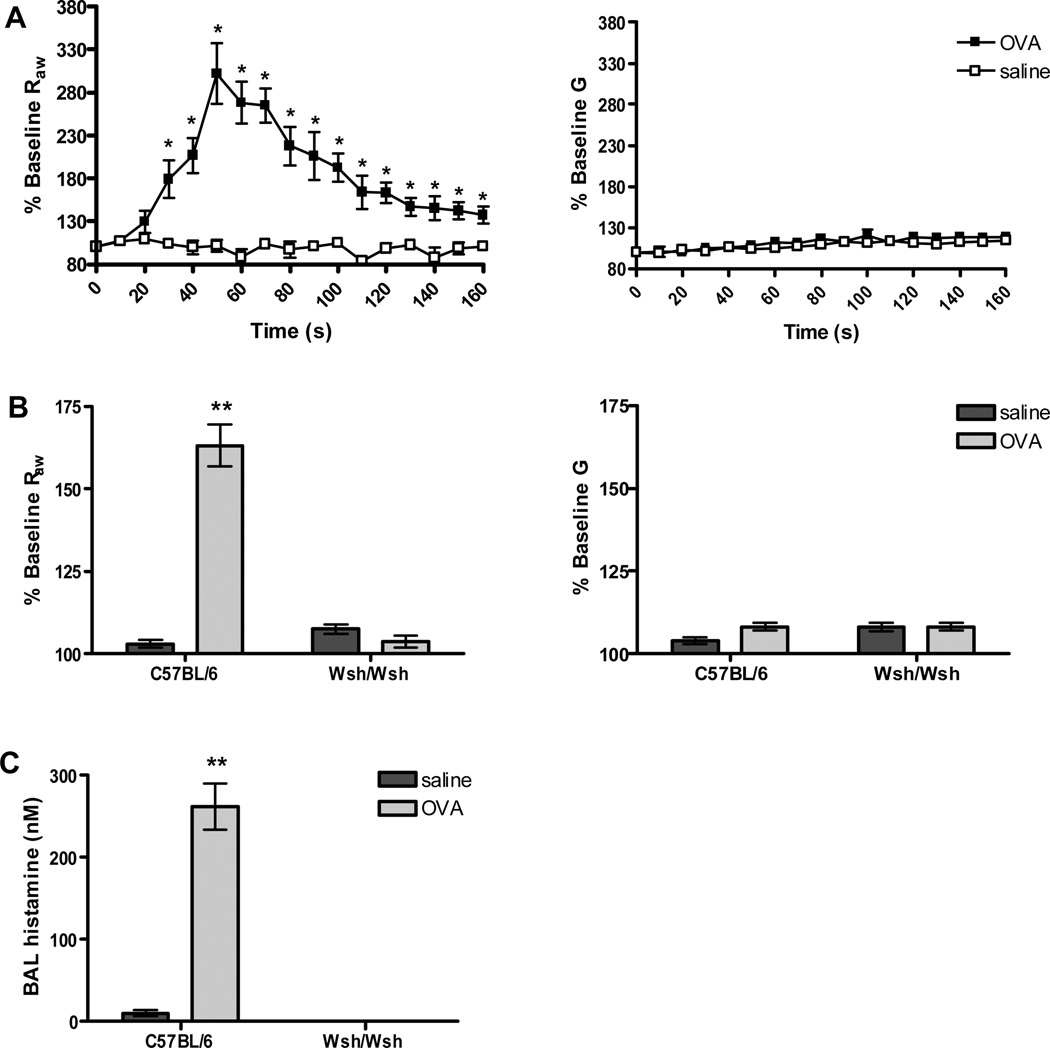
We next asked whether this is also the case in mice with inflamed airways, particularly in those with allergic lung disease. To address this question we induced allergic lung disease in B6 and Wsh/Wsh mice by sensitization and challenge with OVA using an immunization protocol previously shown to induce similar levels of inflammation in both lines (33). As expected, the absence of mast cells in the Wsh/Wsh mice did not significantly alter any of the disease parameters including IgE production, cellularity of the BAL fluid, mucus cell hyperplasia or the induction of the Th2 pathway (Supplemantal Figure S1). Twenty-four hours after the last OVA challenge, changes in airway resistance in response to antigen were measured using FOT, monitoring both Raw and G changes in lung mechanics in the OVA treated mice. Despite robust inflammation in the central and distal lung, changes in airway mechanics were limited to parameters associated with the central airways. A significant increase in Raw was observed; however, no significant increase in G was observed after infusion of antigen into mice with inflamed lungs (Figure 2 A). Similar to actively sensitized animals, the increase in Raw after OVA challenge in mice with allergic airway disease was dependent on mast cells; Wsh/Wsh mice failed to respond to antigen challenge Figure 2 B). As would be expected given the absence of mast cells in the Wsh/Wsh mice, no histamine was detected in the Wsh/Wsh animals, while this mediator could easily be detected in the BAL from B6 mice following antigen challenge Figure 2 C). Similar to the naïve airway, antigen induced constriction appears to be located within the central airways.
Figure 2. Anaphylactic bronchoconstriction in wild-type and mast cell deficient mice with allergic lung disease.
(A) Allergic lung disease was induced by sensitization and repeated aerosol with OVA. B6 mice with inflamed airways (n=5) exhibited a significant increase in Raw after antigen challenge but no change in G (*P<0.05 compared to saline). Saline challenged controls (n=3) had no response. (B) Mast cell deficient Wsh/Wsh mice with an inflamed airway (n=7) failed to respond to antigen challenge (**P<0.001 compared to all other groups). (C) Histamine levels in B6 mice with inflamed airways were significantly increased after antigen injection (n=6) compared to levels after saline injection (n=3) (**P<0.001 compared to saline). Histamine was undetectable in Wsh/Wsh mice (n=7).
Mast cell release of serotonin alone mediates airway constriction during anaphylaxis
To address the contribution of the various mast cell mediators to allergen-induced bronchoconstriction we took advantage of a number of pharmacological and genetic tools to examine the impact of loss of various pathways on the changes in Raw during anaphylaxis (Supplemental Methods; Figure S2). Anaphylaxis results in a measurable increase of leukotriene (LT) C4 in the BAL (34). To examine the contribution of LTC4 to anaphylactic bronchoconstriction, we utilized animals lacking the enzyme 5-lipoxygenase (5LO), which is required for the synthesis of all leukotrienes. The inability of the mast cells to produce this lipid mediator did not alter bronchoconstriction after induction of passive anaphylaxis in 5LO−/− mice (Figure S2 C). Although prostaglandin (PG) D2 has been shown to be synthesized and released during IgE-dependent activation of mouse bone marrow-derived mast cells (35), no attenuation of airway resistance was noted in mice in which the production of prostaglandins was inhibited by treatment with the NSAID indomethacin (Figure S2 B). Similarly, antagonists which block the H1 and H2 histamine receptors did not significantly alter anaphylactic bronchoconstriction (Figure S2 A). The most abundant mediator found in human mucosal mast cells, tryptase, has the ability to activate protease activated receptor 2 (PAR2) receptors, which can mediate airway constriction under some experimental conditions (36). To determine whether this pathway was involved in bronchoconstriction associated with anaphylaxis, we examined this response in PAR2−/− mice (17); no difference in Raw was noted between the mutant and control animals (Figure S2 D).
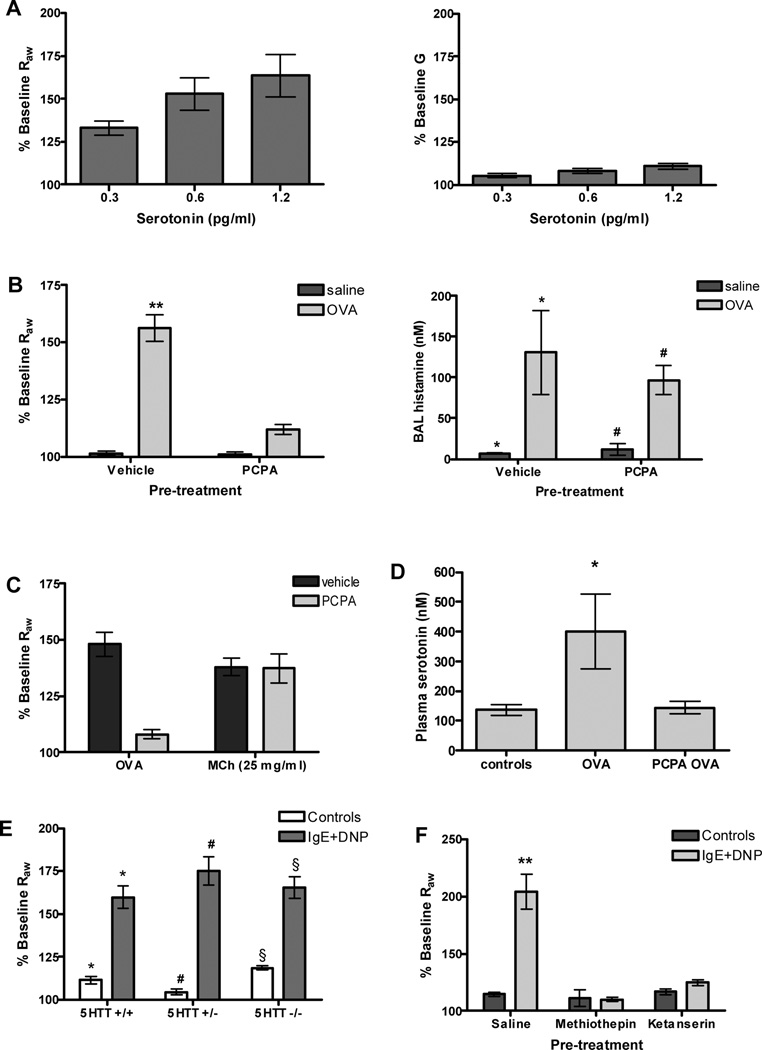
Mast cells express the enzyme required for the production of serotonin (37), and previous studies have indicated that serotonin can mediate changes in lung resistance (38). We therefore first asked whether the change in airway mechanics mediated by serotonin were also limited to the central airways. As can be seen in figure 3 A, exogenous serotonin induced a dose-dependent increase in Raw with little change in G: a pattern that is similarly observed after mast cell degranulation. To address the role of serotonin in mast cell mediated bronchoconstriction more directly, we examined the impact of PCPA on airway resistance. PCPA inhibits the activity of tryptophan hydroxylase, an enzyme which carries out the essential step in the synthesis of serotonin. Mice were sensitized with OVA and three weeks later treated with either vehicle or PCPA prior to the induction of anaphylaxis by injection of antigen. Antigen exposure resulted in a robust increase in airway resistance in the vehicle treated animals. In contrast, airway resistance was abolished in mice treated with PCPA, despite the normal degranulation of mast cells in these animals; histamine levels in the BAL collected immediately after induction of anaphylaxis were not significantly different between the two groups (Figure 3 B). Furthermore, no difference was observed between the PCPA and control animals in the ability of their airways to respond to MCh (Figure 3 C). As expected, anaphylaxis resulted in a measurable increase in plasma serotonin levels, but this increase was not observed in the PCPA treated mice (Figure 3 D). Thus, the protection of the PCPA treated mice from bronchoconstriction was due to attenuated serotonin release and not to alterations in either smooth muscle function or the responsiveness of mast cells to antigen.
Figure 3. Changes in airway physiology in response to serotonin and after inhibition of serotonergic pathways.
(A) Similar to antigen exposure, exogenous i.v. serotonin induces dose dependent increases in Raw, but no change in G (n=13). (B) Vehicle treated mice (n=10) sensitized and challenged with OVA had a significant increase in Raw. Pretreatment with PCPA (150 mg/kg, n=15) abolished anaphylactic airway constriction in actively sensitized mice challenged with OVA (**P<0.001 compared to all other groups). PCPA treatment had no effect on the ability of mast cells to degranulate; histamine levels were the same in both groups of OVA treated animals (n=10–15 per group). (C) PCPA treatment had no effect on the ability of the airway smooth muscle to constrict to aerosolized MCh administered after antigen challenge (n=3). (D) PCPA treatment significantly attenuated plasma serotonin levels in OVA challenged animals (n=4–6 per group) (*P<0.05 compared to all other groups). (E) Passively sensitized serotonin transporter (5HTT)-deficient (n=4) and hererozygous mice (n=5) challenged with DNP had a significant increase in Raw compared to controls (n=2 per group). This response was similar to that of WT animals (n=5). (*P<0.001; #P<0.001; §P<0.001). (F) Blockade of serotonin receptors with methiothepin (2 mg/kg, n=5) or ketanserin (12 mg/kg, n=5) abolished airway constriction after antigen challenge in passively sensitized animals (**P<0.001 compared to all other groups).
To rule out a possible role for platelet-derived serotonin in this model, we examined the response to IgE and antigen in mice lacking the serotonin transporter. While mast cells express tryptophan hydroxylase and thus can produce serotonin, platelets acquire this mediator via the serotonin transporter as they pass through the intestinal circulation. Thus platelets from mice and rats lacking the transporter also lack serotonin (39, 40). 5HTT-deficient mice demonstrate wild-type levels of constriction after antigen challenge (Figure 3 E) indicating that platelet-derived serotonin is not critical for this response. Serotonin-dependent constriction was also observed in BALB/c mice (Supplemental Figure S3), which are commonly thought of as prototypical Th2 responders.
If bronchoconstriction is mediated by mast cell-derived serotonin, it would follow that agents that block serotonin receptors would also attenuate changes in Raw. Serotonin-induced increases in Raw were abolished by both the non-specific antagonist methiothepin and the 5-HT2A-specific antagonist ketanserin, whereas the 5-HT3-specific antagonist ondansetron failed to attenuate this increase in airway mechanics (data not shown). Both methiothepin and ketanserin abolished allergic airway constriction in passively sensitized mice (Figure 3 F). Together these studies indicate that while mast cells release many mediators that may modulate the inflammatory response, serotonin alone mediates the changes in airway resistance during anaphylaxis through its action on 5-HT2A receptors.
Intact sensory innervation is not required for mast cell mediated bronchoconstriction
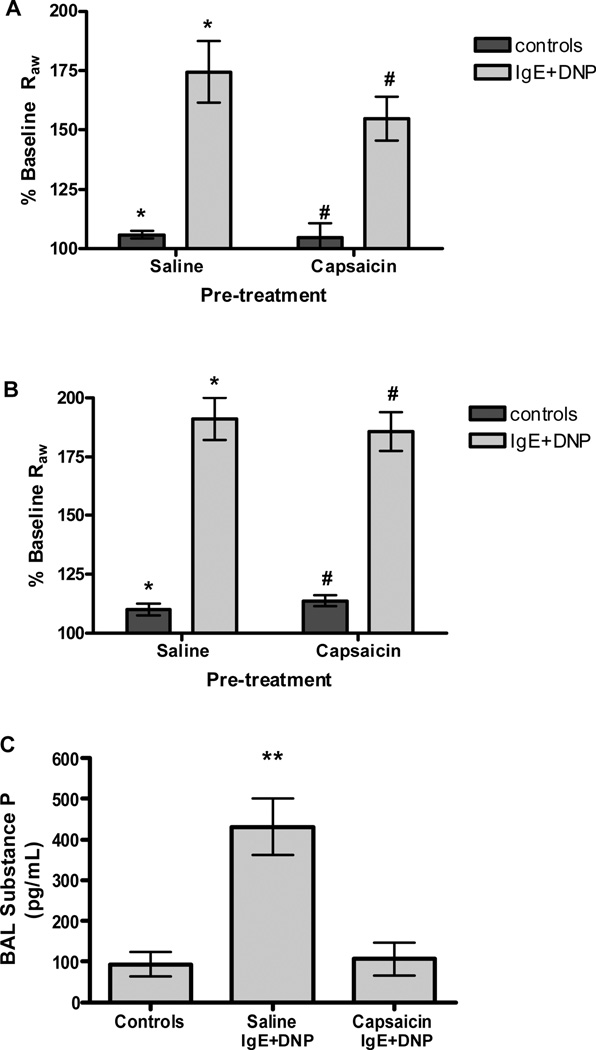
Our observation that the ability of mast cells to mediate bronchoconstriction was sensitive to the location of these cells in the lung, and our observation that serotonin alone mediated this response, suggested to us that the mast cell-mediated constriction may result from indirect stimulation of sensory neurons, triggering a bronchial reflex. To test this hypothesis we examined the ability of IgE and antigen to induce constriction in mice in which sensory neurons had been ablated by treatment with capsaicin. Ablation of sensory C-fibers with capsaicin in both adults and neonates failed to significantly decrease Raw after antigen challenge in passively sensitized mice (Figure 4 A–B), suggesting that capsaicin-sensitive C-fibers do not play a primary role in allergic airway constriction. Substance P, the primary neurotransmitter stored in C-fibers, was measured to evaluate the efficacy of capsaicin treatment. Antigen challenge in passively sensitized mice increased substance P levels in the BAL, but was attenuated in mice pretreated with capsaicin (Figure 4 C), verifying that the capsaicin treatment ablated substance P containing C-fibers in these animals.
Figure 4. Anaphylactic bronchoconstriction following ablation of sensory C-fibers with capsaicin.
(A) Sensory ablation with capsaicin (25–75 mg/kg) in adults (n=6) had no significant effect on airway constriction after antigen challenge in passively sensitized mice (*P<0.001, #P<0.01). (B) Degradation of C-fibers with capsaicin (50 mg/kg) in newborn mice (n=7) had no effect on airway parameters after antigen challenge in passively sensitized mice (*P<0.001, #P<0.001). (C) Antigen challenge in passively sensitized mice (n=10) increased substance P content in the BAL. Neonatal treatment of capsaicin (n=5) reduced BAL substance P after antigen challenge to levels seen in control animals (n=3) (**P<0.05, compared to all other groups).
Vagotomy abolishes anaphylactic airway constriction in both the naïve and allergic airway
The tone of ASM is largely dependent on parasympathetic innervation, which travels in the vagus nerve. Mast cells have been observed close to the vagus nerve and parasympathetic ganglia in other organisms and tissues (7, 13, 41), raising the possibility of cooperation between mast cells and these cholinergic fibers in bronchoconstriction. To test this hypothesis, we examined mast cell-mediated bronchoconstriction in mice in which the cholinergic pathways were disabled using a number of approaches.
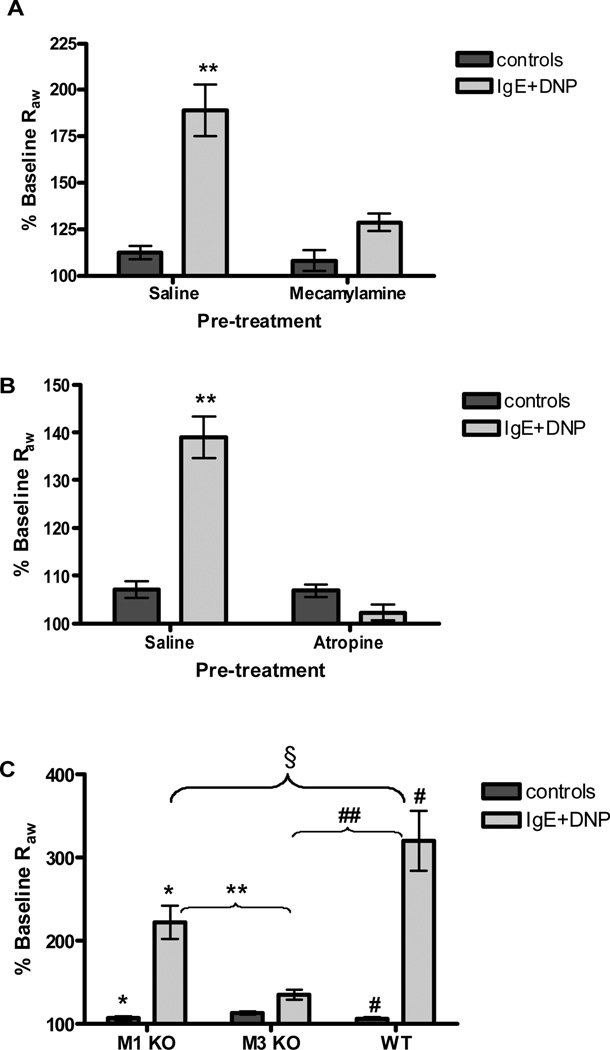
Blockade of nAChRs with the nonspecific nicotinic antagonist mecamylamine, prior to antigen challenge, abolished antigen-induced increases in Raw in passively sensitized mice (Figure 5 A). Post-ganglionic fibers release ACh which binds mAChRs expressed by smooth muscle cells to initiate contraction. Blockade of mAChRs with the nonspecific muscarinic antagonist atropine resulted in complete absence of airway constriction after antigen challenge in both B6 (Figure 5 B) and BALB/c mice (Supplemental Figure S4). To further define the muscarinic receptors required for mast cell mediated bronchoconstriction, we examined a series of mouse lines deficient in M1 or M3 mAChRs. Complete attenuation of Raw was only observed in the mice lacking the M3 mAChR (Figure 5 C), which has been shown to be expressed by airway smooth muscle (3).
Figure 5. Antigen-induced bronchoconstriction following cholinergic inhibition.
(A) Blockade of cholinergic nAChRs with mecamylamine (4 mg/kg, n=8) abolished airway constriction following antigen challenge in passively sensitized wild-type mice (**P<0.001 compared to all other groups). (B) Blockade of mAChRs with atropine (10 µM/kg, n=6) resulted in complete loss of airway constriction after antigen challenge in passively sensitized mice (**P<0.01 compared to all other groups). (C) In passively sensitized animals, loss of the M3 receptor (n=6) resulted in abolishment of anaphylactic airway constriction, whereas M1 receptor deficiency (n=6) resulted in a significant attenuation, but not complete loss of the response to antigen (*P<0.001, **P<0.01, #P<0.001, ##P<0.001, §P<0.01).
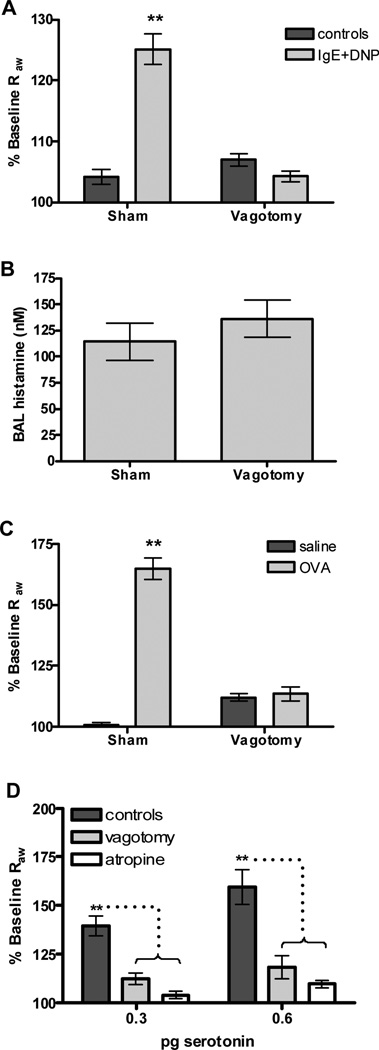
The observation that disruption of both nicotinic and muscarinic neuronal transmission inhibits anaphylactic changes in airway mechanics suggests that an intact parasympathetic pathway is required for this mast cell function. If this is the case, we would expect that severing the incoming neural fibers would eliminate IgE/antigen mediated bronchoconstriction. To test this hypothesis, mice were treated with either IgE or saline and the following day the vagus nerve was severed prior to treatment with antigen. Control groups in which the nerve was isolated but not severed were also prepared. Vagotomy abolished antigen-mediated airway constriction in both B6 (Figure 6 A) and BALB/c mice (Supplemental Figure S4). We next determined whether severing the vagus nerve altered the ability or extent of mast cell degranulation in the airways by measuring histamine levels in the BAL immediately after measurement of airway parameters. No difference in histamine levels was seen following vagotomy (Figure 6 B). To verify that the vagotomy did not alter fundamental properties of the ASM, we confirmed that this surgical procedure did not alter the response of the mice to MCh (Supplemental Figure S5).
Figure 6. Vagotomy attenuates antigen and serotonin-induced changes in airway resistance.
(A) Passively sensitized and challenged wild-type mice after dissection of the vagus nerve (n=18) or a surgical sham (n=28). Antigen challenged mice showed a significant increase in Raw that was abolished in vagotomized animals (**P <0.001 compared to all other groups). (B) Histamine levels in the BAL fluid were measured after induction of passive anaphylaxis. Vagotomy did not affect the levels of histamine in the BAL fluid (n=5 per group). (C) Changes in Raw were abolished after vagotomy in OVA challenged sensitized mice (n=7–14 per group) with allergic lung disease (**P<0.001 compared to all other groups). (D) Serotonin was delivered i.v. following either vagal dissection or pretreatment with atropine. Exogenous serotonin (n=11) resulted in a dose dependent increase in Raw that was sensitive to both vagotomy (n=5) and atropine (n=6) (**P<0.01).
Inflammation results in the recruitment of cells to the lung. This raises the possibility that, in such an environment, mast cells may bring about changes in airway caliber in response to antigen independent of parasympathetic pathways. To test this, we induced allergic lung disease in mice and examined the impact of vagotomy on these animals. Again, vagotomy abolished increases in Raw following antigen challenge (Figure 6 C).
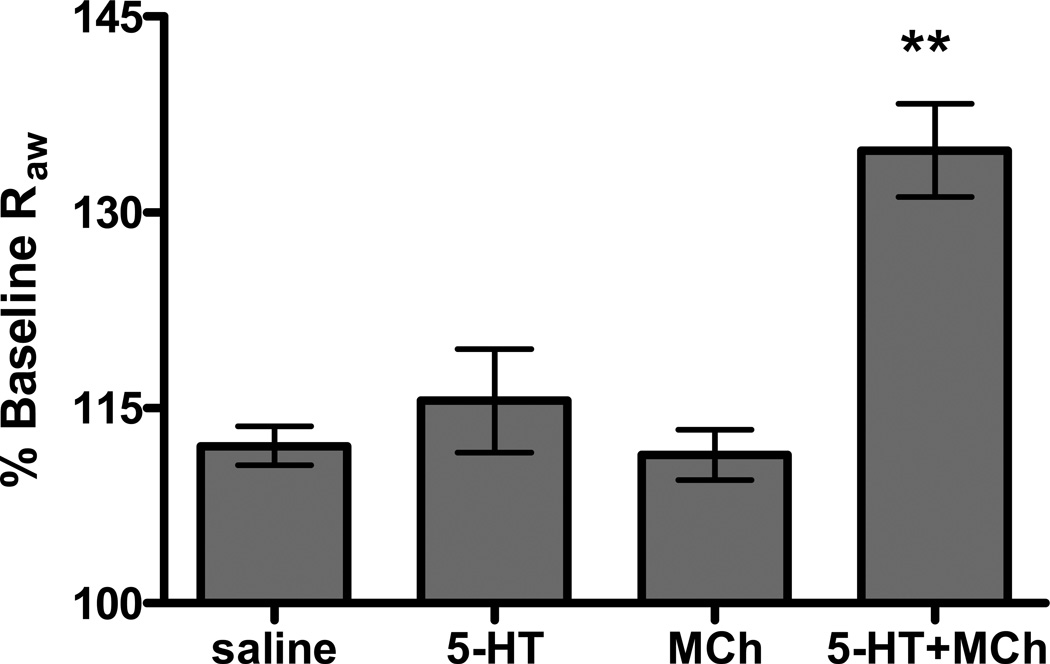
If serotonin released by mast cells collaborates with signals derived from the parasympathetic pathways, one would expect that constriction of the airways to exogenous serotonin to show similar sensitivity to changes in the activity of this system. Supporting the role of serotonin in neural mediated anaphylaxis, administration of exogenous serotonin exhibited dose dependent central airway constriction that was sensitive to both vagotomy and atropine (Figure 6 D). Additionally, pretreatment with a non-provoking dose of serotonin resulted in an additive effect on bronchoconstriction to a non-provoking dose of MCh (Figure 7). Together these studies support a model in which mast cell derived serotonin collaborates with cholinergic pathways of the lung to bring about changes in airway patency during anaphylaxis.
Figure 7. Increased airway resistance in mice treated with non-provoking doses of both serotonin and MCh.
B6 mice exhibited increased sensitivity to serotonin when coupled with exposure to a non-provoking dose of MCh. Low levels of either i.v. serotonin (0.075 pg, n=3) or aerosolized MCh (6 mg/ml, n=3) does not result in a significant increase in Raw compared to saline challenge. A significant increase in Raw was detected after non-provoking doses of serotonin and MCh were delivered together (n=3) (**P<0.001 compared to all other groups).
Discussion
We show here that IgE-mediated degranulation of mast cells leads to an increase in the release of mast cell specific mediators in the mouse lung accompanied by changes in airway mechanics. These changes in airway mechanics are dependent on both mast cell release of serotonin and intact cholinergic innervation of the lung. Similar to previous studies (42) we show that there was a dramatic increase in total RL following antigen challenge, similar in magnitude to that generally achieved with 2 µg i.v. MCh (data not shown). More recently it has become possible to evaluate changes in airway mechanics using FOT. Using the input impedance of prime waves of various amplitudes, fit to the constant phase model of the lung, airflow obstruction following anaphylaxis can be distinguished between the central and peripheral airways (23, 32). Using this method we found that while MCh affects parameters associated with changes in mechanics of both the central and peripheral airways, changes observed after mast cell degranulation were surprisingly limited to the central airways despite the fact that these cells can be found throughout the lung, including within the distal airways and lung parenchyma (31). A number of different explanations are possible for this observation. First while mast cells are found throughout the lung in B6 mice, a larger concentration are localized within close proximity of bronchial smooth muscle while few are found in the alveolar spaces and within the lung parenchyma (31). While antigen exposure may lead to degranulation of cells in the distal lung, the distribution of the receptor(s) sensitive to mast cell mediators may also be biased towards smooth muscle of the central airways. Alternate explanations are also possible. For example, it is possible that cholinergic innervation is more abundant in the central airways, or that the mast cell localization in respect to these neurons differs at different levels of the airways. However, our observation that inhaled serotonin leads to changes in lung mechanics similar to that observed upon mast cell degranulation suggests that the distribution of serotonin receptors plays a role in limiting the response to the larger airways. This finding differs from studies carried out on precision cut lung slices prepared from rats in which constriction of airways was observed in both the distal and proximal airways (43). In this study antigen challenge of passively sensitized lung slices resulted in both stronger and faster constriction of the small airways when compared to the large conducting airways. Similarly, serotonin-provoked responses were also greater in the peripheral airways. In addition, passively sensitized lung slices from humans have also been reported to have an increased response to antigen with decreasing airway size (44). The difference in these findings underscores the continuing importance of verification of ex vivo findings in an animal model. Alternately, this may reflect differences between species in the anatomical distribution of mast cells, ASM, airway receptors, or a combination thereof; and may also indicate that while bronchoconstriction in mice is restricted to central airways this may not be representative of all species.
Mast cells produce a number of mediators that have been shown, both in vitro and in vivo, to mediate airway constriction, including LTC4/LTD4, PGD2, tryptase and histamine. Direct testing using mice lacking 5LO indicated that leukotrienes are not required for IgE/antigen mediated airway constriction, at least in this species. Likewise, inhibition of endogenous prostaglandin production using the NSAID indomethacin failed to inhibit antigen-induced increases in Raw, ruling out the involvement of PGD2 in this response. Furthermore, mice lacking PAR2 responded similarly to wild-type animals, indicating that, in the mouse, activation of PAR2 receptors by tryptase is not essential for airway constriction in this model. Nor did we find evidence supporting a role for mast cell derived histamine in this response, despite the ability of inhaled histamine to induce an increase in airway resistance in humans (45) and to modestly increase Raw in some mouse strains (data not shown). In contrast, both inhibition of serotonin synthesis and blockage of the serotonin 5-HT2A receptor completely abolished the change in airway mechanics after antigen challenge. This finding is consistent with a number of previous studies using mice, in which pharmacological agents were shown to attenuate changes in airway mechanics. For example, Eum et al. reported that pretreatment of antigen challenged mice with the non-specific serotonin receptor antagonist methysergide significantly attenuated anaphylactic bronchoconstriction (42). 5-HT2 receptor specific antagonists have also been shown to attenuate serotonin-induced increases in pulmonary mechanics (46).
Serotonin has been reported to activate human airway smooth muscle ex vivo (47). However, unlike LTC4 and histamine, serotonin inhalation does not lead to increased airway constriction, even in asthmatics (48). Furthermore, until recently, it was believed that human mast cells, unlike the rodent counterpart, could not produce serotonin. Careful study of these cells has revealed both the expression of tryptophan hydroxylase and the localization of serotonin (37); however, these findings are limited to cells derived ex vivo and levels were quite small relative to the amounts stored in rodent mast cell granules. Despite the differing expression between human and mouse mast cells, a re-evaluation of potential roles for this pathway in human mast cells is warranted. For example it is possible that localized release of this mediator in close apposition to nerves or ASM can produce a significant change in the activity of the parasympathetic pathway, and that this activity is not easily mimicked by delivery to the epithelial surface.
The vagus nerve contains both the incoming parasympathetic efferents and the sensory nerves originating from the lung, and thus the loss of response in mice after vagotomy is consistent with a model in which mast cells release mediators that stimulate sensory neurons. Sensory neuron activation can lead to both local release of mediators and a bronchial reflex resulting in increased activity of the parasympathetic pathway, a response lost in the vagotomized mice. The serotonin 5-HT3 receptor is known to be involved in activation and depolarization of sensory neurons in several species including the mouse (15). It is possible that serotonin released by mast cells activates sensory neurons to elicit a bronchial reflex. Past studies in various species have suggested the involvement of sensory neurons in the allergic response. For example, degranulation of purified human lung mast cells enhanced the excitability of rabbit visceral sensory C-fibers in vitro (49). Furthermore, antigenic stimulation of sensitized guinea pig bronchi caused increased sensitivity of sensory nerve endings (50). Consistent with this, Yu et al. showed that mast cell degranulation enhanced the excitability of guinea pig esophageal C-fibers to mechanical and chemical stimulation (51). In the rat, capsaicin-sensitive neurons interact with mast-cells to influence lung solute clearance and chloride ion secretion in the trachea (10–12). Our observation does not support this mechanism, as pretreatment of the mice with capsaicin had little impact on mast cell mediated airway constriction while, as expected, release of substance P by these neurons was all but eliminated. Consistent with this, pretreatment of mice with the 5-HT3 receptor specific antagonist ondansetron did not attenuate serotonin-induced increases in airway mechanics (data not shown). While it is unlikely that substance P is involved in this reaction, as animals were treated both as adults and as neonates, the involvement of this neurotransmitter cannot be completely discounted as effects of specific NK receptor antagonists were not evaluated.
A number of lines of evidence presented here support a critical role for parasympathetic neurons in mast mediated bronchoconstriction. This includes the loss of the antigen-induced contractile response in vagotomized mice, the ability of antagonists of both nAChRs and mAChRs to block the response, and the inability of IgE and antigen to trigger bronchoconstriction in mice lacking the M3 receptor. In recent years, studies have suggested that the cholinergic contractile response to serotonin depends on a non-neuronal source of ACh, specifically, serotonin released from the epithelium (46). This hypothesis was based largely on in vitro studies of mouse isolated trachea challenged with exogenous serotonin. They showed that serotonin induced contractions of tracheal smooth muscle was sensitive to epithelial removal but not to inhibition of neural pathways, and that these contractions were dependent on the release of ACh. Our studies do not support this hypothesis, as it would predict that severing the vagus nerve would have no effect on the ability of serotonin to induce bronchoconstriction. Our data show that in vivo, airway constriction from both exogenous serotonin and mast cell-derived serotonin is sensitive to vagotomy.
A number of models for mast cell interaction with the cholinergic neural pathway are possible. First it is possible that mast cells increase transmission at the preganglionic terminal, either by increasing ACh release or augmenting the activity of nAChRs. Second, mast cell mediators could amplify the release of ACh from postganglionic nerve terminals. Finally, activation of serotonin receptors on ASM may lead to smooth muscle contraction only when ACh, which is released at low levels constitutively by the parasympathetic fibers, occupies M3 receptors present on ASM. A number of studies support the models in which antigen challenge induces an increase in release of ACh.Ex vivo studies have measured an increase in ACh levels after stimulation with antigen in both mouse and canine tracheal rings (52, 53). Drugs which decrease ACh metabolism increased bronchoconstriction after either antigen or 5-HT challenge (42). However, our finding that only the 5-HT2A receptor antagonist attenuates this response, coupled with evidence that supports the localization of this receptor to ASM (54) and not cholinergic fibers, supports the later model in which the impact of engagement of the 5-HT2A receptors is dependent on M3 receptors present on ASM. The need for this “second signal” is not clear. Serotonin alone can shorten smooth muscle in vitro and in ex vivo lung slices (54, 55), and therefore the observation that in the mouse its ability to alter smooth muscle activity in vivo is regulated by parasympathetic pathways is somewhat surprising. However, it is interesting to speculate that this relationship represents one of a number of “checks and balances” designed to safeguard the patency of the airways, limiting the activity of smooth muscle modulators stored by mast cells, while at the same time allowing mast cells to safeguard the airway against infectious agents by rapid deployment of their arsenals of inflammatory mediators.
Supplementary Material
Acknowledgements
The authors would like to thank Kim Burns for assistance with histology and staining, Dr. Bob Bagnell for help with data analysis, Dr. Mauricio Rojas for assistance with jugular catheterization, and Dr. Steve Tilley for helpful discussion.
Footnotes
This work was supported by National Institutes of Health grant NIH/NHLBI RO1 HL080697 (to B.H. Koller).
Disclosures
The authors declare no competing financial interests.
References
- 1.Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 2.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 3.Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- 4.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol. 1989;256:C329–C335. doi: 10.1152/ajpcell.1989.256.2.C329. [DOI] [PubMed] [Google Scholar]
- 5.Yang G, Haczku A, Chen H, Martin V, Galczenski H, Tomer Y, Besien CRVan, Evans JF, Panettieri RA, Funk CD. Transgenic smooth muscle expression of the human CysLT1 receptor induces enhanced responsiveness of murine airways to leukotriene D4. Am J Physiol Lung Cell Mol Physiol. 2004;286:L992–L1001. doi: 10.1152/ajplung.00367.2003. [DOI] [PubMed] [Google Scholar]
- 6.Tolloczko B, Jia YL, Martin JG. Serotonin-evoked calcium transients in airway smooth muscle cells. Am J Physiol. 1995;269:L234–L240. doi: 10.1152/ajplung.1995.269.2.L234. [DOI] [PubMed] [Google Scholar]
- 7.Powers MJ, Peterson BA, Hardwick JC. Regulation of parasympathetic neurons by mast cells and histamine in the guinea pig heart. Auton Neurosci. 2001;87:37–45. doi: 10.1016/S1566-0702(00)00260-5. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W, Kreis ME, Eastwood C, Kirkup AJ, Humphrey PP, Grundy D. 5-HT(3) and histamine H(1) receptors mediate afferent nerve sensitivity to intestinal anaphylaxis in rats. Gastroenterology. 2000;119:1267–1275. doi: 10.1053/gast.2000.19461. [DOI] [PubMed] [Google Scholar]
- 9.Barbara G, Wang B, Stanghellini V, Giorgio Rde, Cremon C, Nardo GDi, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 10.Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH. Mast cell/nerve interactions in vitro and in vivo. Am Rev Respir Dis. 1991;143:S55–S58. doi: 10.1164/ajrccm/143.3_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 11.Bienenstock J, Perdue M, Blennerhassett M, Stead R, Kakuta N, Sestini P, Vancheri C, Marshall J. Inflammatory cells and the epithelium Mast cell/nerve interactions in the lung in vitro and in vivo. Am Rev Respir Dis. 1988;138:S31–S34. doi: 10.1164/ajrccm/138.6_Pt_2.S31. [DOI] [PubMed] [Google Scholar]
- 12.Sestini P, Dolovich M, Vancheri C, Stead RH, Marshall JS, Perdue M, Gauldie J, Bienenstock J. Antigen-induced lung solute clearance in rats is dependent on capsaicin-sensitive nerves. Am Rev Respir Dis. 1989;139:401–406. doi: 10.1164/ajrccm/139.2.401. [DOI] [PubMed] [Google Scholar]
- 13.Myers AC, Undem BJ, Weinreich D. Influence of antigen on membrane properties of guinea pig bronchial ganglion neurons. J Appl Physiol. 1991;71:970–976. doi: 10.1152/jappl.1991.71.3.970. [DOI] [PubMed] [Google Scholar]
- 14.Myers AC, Undem BJ. Antigen depolarizes guinea pig bronchial parasympathetic ganglion neurons by activation of histamine H1 receptors. A m J Physiol. 1995;268:L879–L884. doi: 10.1152/ajplung.1995.268.6.L879. [DOI] [PubMed] [Google Scholar]
- 15.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22:1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frieling T, Rupprecht C, Kroese AB, Schemann M. Effects of the inflammatory mediator prostaglandin D2 on submucosal neurons and secretion in guinea pig colon. Am J Physiol. 1994;266:G132–G139. doi: 10.1152/ajpgi.1994.266.1.G132. [DOI] [PubMed] [Google Scholar]
- 17.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 18.Yamada M, Miyakawa T, Duttaroy A, Yamanaka A, Moriguchi T, Makita R, Ogawa M, Chou CJ, Xia B, Crawley JN, Felder CC, Deng CX, Wess J. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410:207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 19.Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, McBain CJ, Wess J. Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron. 2002;33:615–624. doi: 10.1016/s0896-6273(02)00587-1. [DOI] [PubMed] [Google Scholar]
- 20.Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine ("Ecstasy") in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 21.Allen IC, Pace AJ, Jania LA, Ledford JG, Latour AM, Snouwaert JN, Bernier V, Stocco R, Therien AG, Koller BH. Expression and function of NPSR1/GPRA in the lung before and after induction of asthma-like disease. Am J Physiol Lung Cell Mol Physiol. 2006 doi: 10.1152/ajplung.00174.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hantos Z, Adamicza A, Govaerts E, Daroczy B. Mechanical impedances of lungs and chest wall in the cat. J Appl Physiol. 1992;73:427–433. doi: 10.1152/jappl.1992.73.2.427. [DOI] [PubMed] [Google Scholar]
- 23.Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs forced oscillations. J Appl Physiol. 2002;93:263–270. doi: 10.1152/japplphysiol.01129.2001. [DOI] [PubMed] [Google Scholar]
- 24.Kaczka DW, Ingenito EP, Israel E, Lutchen KR. Airway and lung tissue mechanics in asthma Effects of albuterol. Am J Respir Crit Care Med. 1999;159:169–178. doi: 10.1164/ajrccm.159.1.9709109. [DOI] [PubMed] [Google Scholar]
- 25.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JB, Symanowicz PT, Olsen JE, Thrall RS, Cloutier MM, Hubbard AK. Immediate sensory nerve-mediated respiratory responses to irritants in healthy and allergic airway-diseased mice. J Appl Physiol. 2003;94:1563–1571. doi: 10.1152/japplphysiol.00572.2002. [DOI] [PubMed] [Google Scholar]
- 27.Morris JB, Wilkie WS, Shusterman DJ. Acute respiratory responses of the mouse to chlorine. Toxicol Sci. 2005;83:380–387. doi: 10.1093/toxsci/kfi038. [DOI] [PubMed] [Google Scholar]
- 28.Holzer P, Pabst MA, Lippe IT, Peskar BM, Peskar BA, Livingston EH, Guth PH. Afferent nerve-mediated protection against deep mucosal damage in the rat stomach. Gastroenterology. 1990;98:838–848. doi: 10.1016/0016-5085(90)90005-l. [DOI] [PubMed] [Google Scholar]
- 29.Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 30.Allen IC, Hartney JM, Coffman TM, Penn RB, Wess J, Koller BH. Thromboxane A2 induces airway constriction through an M3 muscarinic acetylcholine receptor-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2006;290:L526–L533. doi: 10.1152/ajplung.00340.2005. [DOI] [PubMed] [Google Scholar]
- 31.Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118:41–49. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- 32.Irvin CG, Bates JH. Measuring the lung function in the mouse: the challenge of size. Respir Res. 2003;4:4. doi: 10.1186/rr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192:455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson WR, Jr., Lewis DB, Albert RK, Zhang Y, Lamm WJ, Chiang GK, Jones F, Eriksen P, Tien YT, Jonas M, Chi EY. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996;184:1483–1494. doi: 10.1084/jem.184.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami M, Bingham CO, 3rd, Matsumoto R, Austen KF, Arm JP. IgE-dependent activation of cytokine-primed mouse cultured mast cells induces a delayed phase of prostaglandin D2 generation via prostaglandin endoperoxide synthase-2. J Immunol. 1995;155:4445–4453. [PubMed] [Google Scholar]
- 36.Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol. 2005;175:2598–2605. doi: 10.4049/jimmunol.175.4.2598. [DOI] [PubMed] [Google Scholar]
- 37.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez VE, McCaskill V, Atkins ND, Wanner A. Variability of airway responses in mice. Lung. 1999;177:355–366. doi: 10.1007/pl00007653. [DOI] [PubMed] [Google Scholar]
- 39.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Homberg J, Mudde J, Braam B, Ellenbroek B, Cuppen E, Joles JA. Blood pressure in mutant rats lacking the 5-hydroxytryptamine transporter. Hypertension. 2006;48:e115–e116. doi: 10.1161/01.HYP.0000246306.61289.d8. author reply e117. [DOI] [PubMed] [Google Scholar]
- 41.Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- 42.Eum SY, Norel X, Lefort J, Labat C, Vargaftig BB, Brink C. Anaphylactic bronchoconstriction in BP2 mice: interactions between serotonin and acetylcholine. Br J Pharmacol. 1999;126:312–316. doi: 10.1038/sj.bjp.0702304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wohlsen A, Uhlig S, Martin C. Immediate allergic response in small airways. Am J Respir Crit Care Med. 2001;163:1462–1469. doi: 10.1164/ajrccm.163.6.2007138. [DOI] [PubMed] [Google Scholar]
- 44.Wohlsen A, Martin C, Vollmer E, Branscheid D, Magnussen H, Becker WM, Lepp U, Uhlig S. The early allergic response in small airways of human precision-cut lung slices. Eur Respir J. 2003;21:1024–1032. doi: 10.1183/09031936.03.00027502. [DOI] [PubMed] [Google Scholar]
- 45.Millman RP, Silage DA, Peterson DD, Pack AI. Effect of aerosolized histamine on occlusion pressure and ventilation in humans. J Appl Physiol. 1982;53:690–697. doi: 10.1152/jappl.1982.53.3.690. [DOI] [PubMed] [Google Scholar]
- 46.Moffatt JD, Cocks TM, Page CP. Role of the epithelium and acetylcholine in mediating the contraction to 5-hydroxytryptamine in the mouse isolated trachea. Br J Pharmacol. 2004;141:1159–1166. doi: 10.1038/sj.bjp.0705720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis C, Kannan MS, Jones TR, Daniel EE. Control of human airway smooth muscle: in vitro studies. J Appl Physiol. 1982;53:1080–1087. doi: 10.1152/jappl.1982.53.5.1080. [DOI] [PubMed] [Google Scholar]
- 48.Cushley MJ, Wee LH, Holgate ST. The effect of inhaled 5-hydroxytryptamine (5-HT, serotonin) on airway calibre in man. Br J Clin Pharmacol. 1986;22:487–490. doi: 10.1111/j.1365-2125.1986.tb02923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greene R, Fowler J, MacGlashan D, Jr., Weinreich D. IgE-challenged human lung mast cells excite vagal sensory neurons in vitro. J Appl Physiol. 1988;64:2249–2253. doi: 10.1152/jappl.1988.64.5.2249. [DOI] [PubMed] [Google Scholar]
- 50.Undem BJ, Riccio MM, Weinreich D, Ellis JL, Myers AC. Neurophysiology of mast cell-nerve interactions in the airways. Int Arch Allergy Immunol. 1995;107:199–201. doi: 10.1159/000236976. [DOI] [PubMed] [Google Scholar]
- 51.Yu S, Kollarik M, Ouyang A, Myers AC, Undem BJ. Mast cell-mediated long-lasting increases in excitability of vagal C fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2007;293:G850–G856. doi: 10.1152/ajpgi.00277.2007. [DOI] [PubMed] [Google Scholar]
- 52.Larsen GL, White CW, Takeda K, Loader JE, Nguyen DD, Joetham A, Groner Y, Gelfand EW. Mice that overexpress Cu/Zn superoxide dismutase are resistant to allergen-induced changes in airway control. Am J Physiol Lung Cell Mol Physiol. 2000;279:L350–L359. doi: 10.1152/ajplung.2000.279.2.L350. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell RW, Ndukuw IM, Ikeda K, Arbetter K, Leff AR. Effect of immune sensitization on stimulated ACh release from trachealis muscle in vitro. Am J Physiol. 1993;265:L13–L18. doi: 10.1152/ajplung.1993.265.1.L13. [DOI] [PubMed] [Google Scholar]
- 54.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol. 2007;36:122–130. doi: 10.1165/rcmb.2006-0036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol. 1999;126:1191–1199. doi: 10.1038/sj.bjp.0702394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.