Abstract
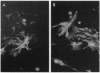
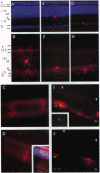
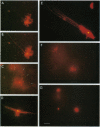
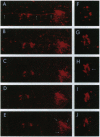
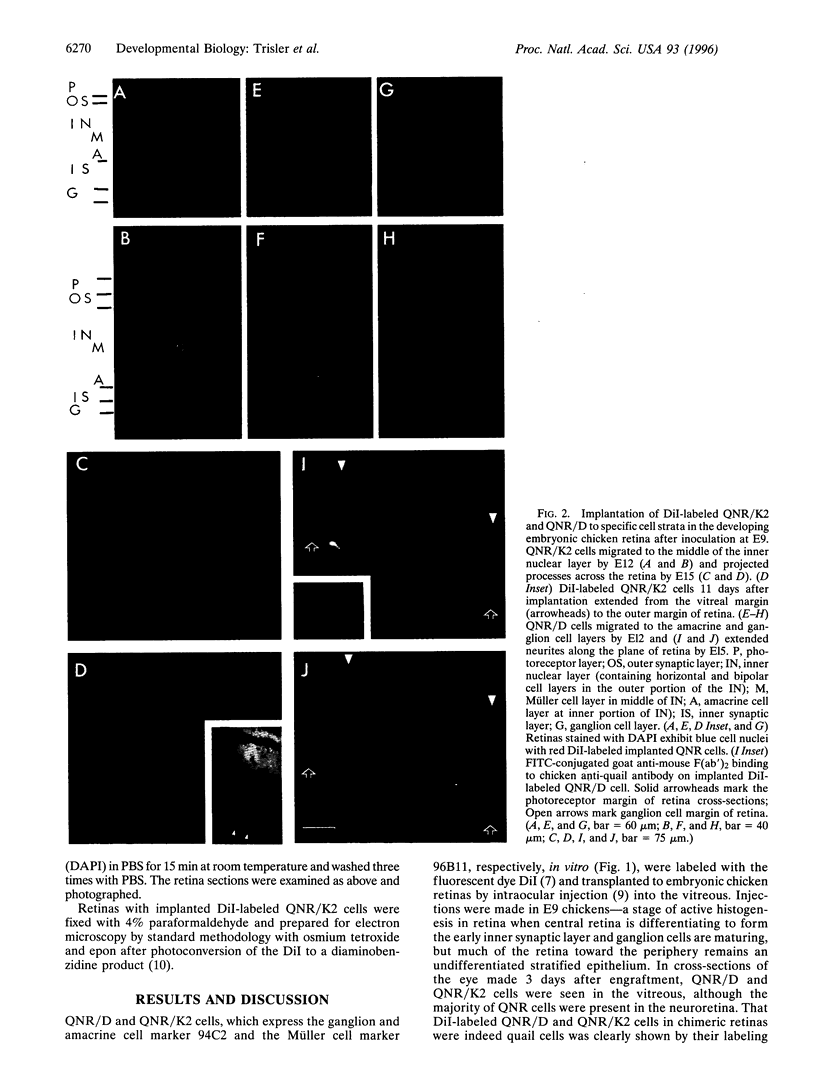
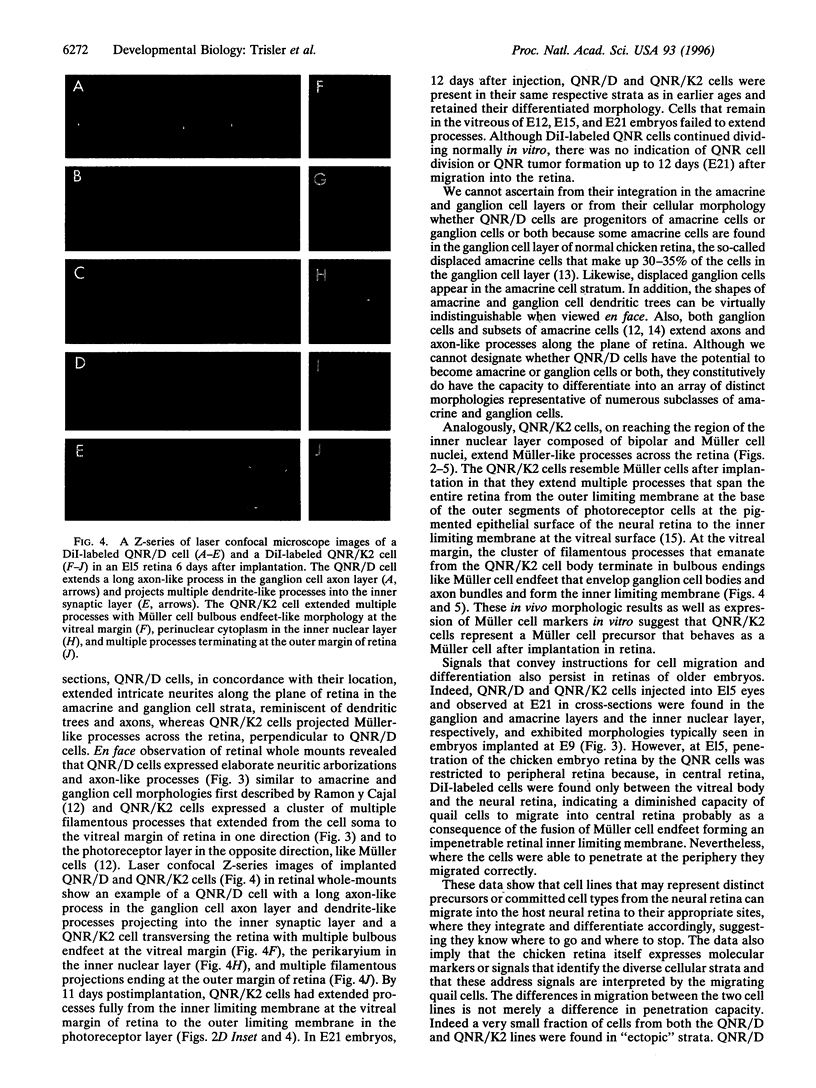
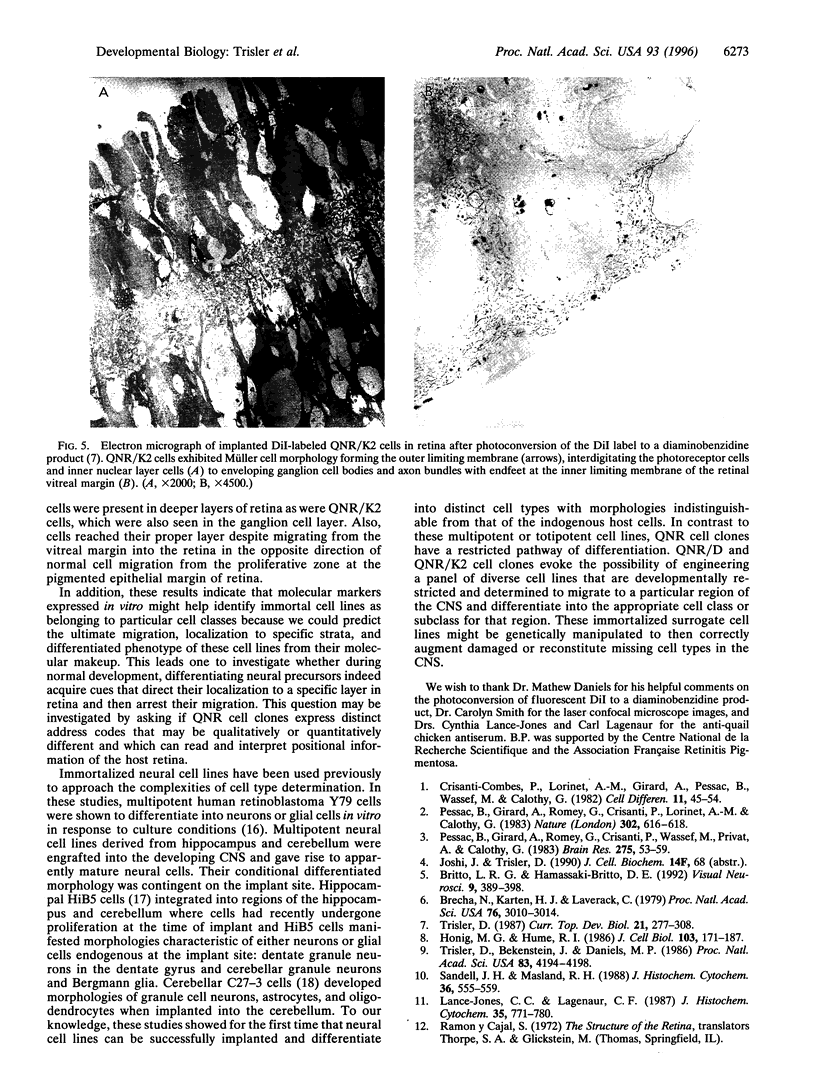
A major question in central nervous system development, including the neuroretina, is whether migrating cells express cues to find their way and settle at specific locations. We have transplanted quail neuroretinal cell lines QNR/D, a putative amacrine or ganglion cell, and QNR/K2, a putative Müller cell into chicken embryo eyes. Implanted QNR/D cells migrate only to the retinal ganglion and amacrine cell layers and project neurites in the plane of retina; in contrast, QNR/K2 cells migrate through the ganglion and amacrine layers, locate in the inner nuclear layer, and project processes across the retina. These data show that QNR/D and QNR/K2 cell lines represent distinct neural cell types, suggesting that migrating neural cells express distinct address cues. Furthermore, our results raise the possibility that immortalized cell lines can be used for replacement of specific cell types and for the transport of genes to given locations in neuroretina.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binggeli R. L., Paule W. J. The pigeon retina: quantitative aspects of the optic nerve and ganglion cell layer. J Comp Neurol. 1969 Sep;137(1):1–18. doi: 10.1002/cne.901370102. [DOI] [PubMed] [Google Scholar]
- Brecha N., Karten H. J., Laverack C. Enkephalin-containing amacrine cells in the avian retina: immunohistochemical localization. Proc Natl Acad Sci U S A. 1979 Jun;76(6):3010–3014. doi: 10.1073/pnas.76.6.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto L. R., Hamassaki-Britto D. E. Enkephalin-immunoreactive ganglion cells in the pigeon retina. Vis Neurosci. 1992 Sep-Oct;9(3-4):389–398. doi: 10.1017/s0952523800010798. [DOI] [PubMed] [Google Scholar]
- Catsicas S., Catsicas M., Clarke P. G. Long-distance intraretinal connections in birds. Nature. 1987 Mar 12;326(6109):186–187. doi: 10.1038/326186a0. [DOI] [PubMed] [Google Scholar]
- Crisanti-Combes P., Lorinet A. M., Girard A., Pessac B., Wasseff M., Calothy G. Expression of neuronal markers in chick and quail embryo neuroretina cultures infected with Rous sarcoma virus. Cell Differ. 1982 Jan;11(1):45–54. doi: 10.1016/0045-6039(82)90016-1. [DOI] [PubMed] [Google Scholar]
- Honig M. G., Hume R. I. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J Cell Biol. 1986 Jul;103(1):171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyritsis A. P., Tsokos M., Triche T. J., Chader G. J. Retinoblastoma--origin from a primitive neuroectodermal cell? Nature. 1984 Feb 2;307(5950):471–473. doi: 10.1038/307471a0. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C. C., Lagenaur C. F. A new marker for identifying quail cells in embryonic avian chimeras: a quail-specific antiserum. J Histochem Cytochem. 1987 Jul;35(7):771–780. doi: 10.1177/35.7.3295031. [DOI] [PubMed] [Google Scholar]
- Pessac B., Girard A., Romey G., Crisanti P., Lorinet A. M., Calothy G. A neuronal clone derived from a Rous sarcoma virus-transformed quail embryo neuroretina established culture. Nature. 1983 Apr 14;302(5909):616–618. doi: 10.1038/302616a0. [DOI] [PubMed] [Google Scholar]
- Pessac B., Girard A., Romey G., Crisanti P., Wassef M., Privat A., Calothy G. Cells with neuronal properties in permanent cultures of quail embryo neuroretinas infected with Rous sarcoma virus. Brain Res. 1983 Sep 19;275(1):53–59. doi: 10.1016/0006-8993(83)90416-x. [DOI] [PubMed] [Google Scholar]
- Renfranz P. J., Cunningham M. G., McKay R. D. Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell. 1991 Aug 23;66(4):713–729. doi: 10.1016/0092-8674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Sandell J. H., Masland R. H. Photoconversion of some fluorescent markers to a diaminobenzidine product. J Histochem Cytochem. 1988 May;36(5):555–559. doi: 10.1177/36.5.3356898. [DOI] [PubMed] [Google Scholar]
- Snyder E. Y., Deitcher D. L., Walsh C., Arnold-Aldea S., Hartwieg E. A., Cepko C. L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992 Jan 10;68(1):33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Trisler D., Bekenstein J., Daniels M. P. Antibody to a molecular marker of cell position inhibits synapse formation in retina. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4194–4198. doi: 10.1073/pnas.83.12.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisler D. Synapse formation in retina is influenced by molecules that identify cell position. Curr Top Dev Biol. 1987;21:277–308. doi: 10.1016/s0070-2153(08)60141-6. [DOI] [PubMed] [Google Scholar]