Abstract
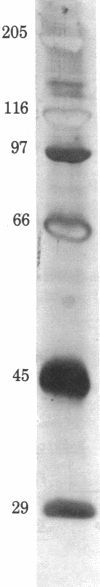
Two monoclonal anti-platelet antibodies, 3B2 and 8G11, have been raised that are specific for normal human platelets. 3B2 is unique in that it has decreased reactivity for platelets from 16 patients with autoimmune thrombocytopenic purpura [mean platelet count, 65,000 +/- 6,000 (SEM)]. With 8G11 in an enzyme-linked immunosorbent assay, the mean of the ratios of patient platelet OD to control platelet OD was 0.95 +/- 0.07, whereas with 3B2, the mean of the ratios of patient platelet OD to control OD was 0.24 +/- 0.04, P less than 0.001. With 3B2 the mean of the OD ratios of five patients with autoimmune thrombocytopenic purpura in remission (greater than 150,000 platelets per mm3) compared to controls was 0.80 +/- 0.14. 3B2 did not react with platelets from a patient with Glanzmann's thrombasthenia, in which membranes lack glycoproteins IIb and IIIa (GPIIb and GPIIIa). Platelet membranes were run on crossed immunoelectrophoresis against a rabbit polyclonal anti-human platelet membrane antibody with 125I-labeled purified 3B2 in an intermediate spacer gel. 3B2 reacted with the GPIIb-GPIIIa-Ca2+ complex in the presence of excess Ca2+ and with GPIIb alone in the presence of excess EGTA. When Triton X-100-solubilized platelet membranes were immunoprecipitated with 3B2 plus rabbit anti-mouse IgG, reduced, and run on NaDodSO4/polyacrylamide gel electrophoresis, a single protein band was obtained with a molecular weight of 120,000 (the molecular weight of GPIIb). Thus, the reactivity of monoclonal antibody 3B2 with GPIIb or the GPIIb-GPIIIa-Ca2+ complex appears to be inhibited by the presence of autoantibody on platelets.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davidson R. L., Gerald P. S. Improved techniques for the induction of mammalian cell hybridization by polyethylene glycol. Somatic Cell Genet. 1976 Mar;2(2):165–176. doi: 10.1007/BF01542629. [DOI] [PubMed] [Google Scholar]
- Hangen I., Bjerrum O. J., Gogstad G., Korsmo R., Solum N. O. Involvement of divalent cations in the complex between the platelet glycoproteins IIb and IIIa. Biochim Biophys Acta. 1982 Feb 4;701(1):1–6. doi: 10.1016/0167-4838(82)90303-x. [DOI] [PubMed] [Google Scholar]
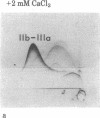
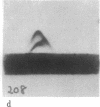
- Howard L., Shulman S., Sadanandan S., Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. The major antigen consists of a complex of glycoproteins, GPIIb and GPIIIa, held together by Ca2+ and missing in Glanzmann's thrombasthenia. J Biol Chem. 1982 Jul 25;257(14):8331–8336. [PubMed] [Google Scholar]
- Jennings L. K., Phillips D. R. Purification of glycoproteins IIb and III from human platelet plasma membranes and characterization of a calcium-dependent glycoprotein IIb-III complex. J Biol Chem. 1982 Sep 10;257(17):10458–10466. [PubMed] [Google Scholar]
- Karpatkin S. Autoimmune thrombocytopenic purpura. Blood. 1980 Sep;56(3):329–343. [PubMed] [Google Scholar]
- Karpatkin S. Heterogeneity of human platelets. VI. Correlation of platelet function with platelet volume. Blood. 1978 Feb;51(2):307–316. [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyman B., Rosenberg L., Karpatkin S. Biochemical and biophysical aspects of human platelet adhesion to collagen fibers. J Clin Invest. 1971 Sep;50(9):1854–1863. doi: 10.1172/JCI106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S., Karpatkin S. Crossed immunoelectrophoresis of human platelet membranes. Diminished major antigen in Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Biol Chem. 1980 May 10;255(9):4320–4327. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- van Leeuwen E. F., van der Ven J. T., Engelfriet C. P., von dem Borne A. E. Specificity of autoantibodies in autoimmune thrombocytopenia. Blood. 1982 Jan;59(1):23–26. [PubMed] [Google Scholar]