Abstract
Tumor cells aberrantly express several T cell inhibitory molecules including members of the B7-H co-regulatory family. Presumably tumor-expressed B7-H1 and B7-H3 confer resistance to elimination by the immune system. In addition, elevated levels of soluble B7-H1 (sB7-H1) has been identified in the sera of cancer patients, including renal carcinoma patients and is associated with increased cancer related death. Here we report that sB7-H1 is produced and released by activated mature dendritic cells (mDC). Immature DC, macrophages, monocytes, or T cells are refractory to releasing sB7-H1. Exposure of CD4+ and CD8+ T cells to mDC-derived sB7-H1 molecules induced apoptosis. These data suggest that the immunobiology of B7-H1 is perhaps more complex than previously thought. sB7-H1 molecules may represent an unanticipated contributing factor to immune homeostasis. That both immune and tumor cells can be sources of sB7-H1 suggests that optimization of co-regulatory blockade immunotherapy for solid malignancies of necessity will require impact of targeting tumor and immune-derived B7-H1 molecules.
Keywords: B7-H1, Soluble, T cell, Dendritic cell, Coregulatory, Tumor cell
1. Introduction
B7-homologues (B7-H) – B7-H1, B7-H2, B7-H3 and B7-H4 and B7-DC – predominantly function as negative costimulatory molecules [1,2]. B7-H molecules were initially discovered as membrane-bound ligands in myeloid-lineage cells and activated T and B cells [3–6]. It was later observed that tumors aberrantly express several types of B7-H molecules, which was associated with increased resistance to immune or pharmacological attack [7]. In particular tumor expressions of B7-H1, B7-H3 or B7-H4 were associated with an increased risk of cancer-related death [8–10].
B7-H ligands have been detected in serum of patients with varying disorders [11,12], including sera from patients with solid malignancies [13–15]. We have detected soluble B7-H1 and B7-H4 molecules in the sera of ccRCC1 patients [13,16]. Tumor-derived sB7-H12 is biologically active and is capable of delivering cell death inducing signals [13].
In addition to tumor cells, immune cells may also release soluble B7-H ligands in the course of immune reactions. For example, sB7-H3 has been detected in cultures of monocytes, DC3 and activated T cells [17]. sB7-H3 has also been detected in serum from healthy individuals [17]. Given the poor prognosis associated with ccRCC expression of B7-H1 and the observation that activated DC and T cells express membrane-bound B7-H1 we investigated whether immune cells have the capacity to release sB7-H1. Understanding the immunobiology of sB7-H1 generation may have applications in clinical settings, notably optimizing B7-H1 blockade immunotherapy for solid malignancies. sB7-H molecules, tumor or immune-derived, may deliver systemic inhibitory messages that could globally adversely impact anti-tumoral immune responses.
2. Materials and methods
2.1. Human cells
Human PBMC4 were isolated from leukoreduction filters (Pall) as previously described [18]. Purified CD3+, CD4+, CD8+ and CD14+ cells were obtained by negative-selection (Miltenyi Biotech). To induce differentiation of DC, CD14+ cells (purity > 90%) were placed in 24-well plates at 2 × 106 cells/well and cultured in complete media supplemented with IL-4 (1000 IU/mL, R&D Systems) and GM-CSF (2800 IU/mL, Bayer). After 3 days of culture, iDC5 were harvested, washed and dispensed at 1 × 106 cells/well in 24-well plates and cultured for 2 days in fresh media containing IL-4, GM-CSF and either TNFα (1100 IU/mL, R&D Systems) and PGE-2 (1 μg/mL, Sigma), poly I:C (20 μg/mL, Sigma), or LPS (100 ng/mL, Sigma) to drive the generation of mDC6. Expression of CD80, CD83, MHC-I, HLA-DR and membrane B7-H1 on DC preparations was determined on days 0, 3 and 5 of culture. Purified CD3+ cells were placed in 24-well plates at 1 × 106 cells/well and cultured in media containing anti-CD3/anti-CD28 microbeads (25 μL/well, Invitrogen), PHA (20 μg/mL, Sigma), CD28 (2 μg/mL BD Biosciences), IFNγ (100 IU/mL, eBioscience), TNFα (100 ng/mL, R&D Systems) or IL-10 (500 ng/mL, BD Biosciences), with or without anti-CD3 (2 μg/mL, BD Biosciences).
2.2. DC: T cell co-cultures
Purified CD4+ or CD8+ T cells were plated at 1 × 106 cells/well at the bottom of 24 well transwell plates pre-coated with azide-free anti-human CD3 (2 μg/mL, BD Biosciences). iDC cells or PBS was added to the upper chamber of the wells along with LPS (100 ng/ml, Sigma) to produce stimulated mDC. Extent of target CD4 and CD8 apoptosis was determined by Annexin-V (BD Biosciences) and propidium iodide (Sigma) staining at day 5 of culture.
2.3. sB7-H1 supernates
Corresponding supernates from intact PBMC (day 3), T cells (day 3), iDC and mDC cultures (days 0, 3 and 5) and transwell cultures (day 5) were clarified twice by centrifugation and stored at −20 °C for later testing.
2.4. Sandwich ELISA for sB7-H1
Soluble B7-H1 ELISA was performed as previously described [13]. High-binding polystyrene plates (Corning Life Sciences) were coated for 2 h at RT with 0.2 μg/well of capture antibody 2.2B. After each step, plates were washed three times (PBS +0.05% Tween-20). Free binding sites were blocked with 200 μL/well of Superblock (Pierce) 1 h at RT. After washing, 50 μL of standard, control or test culture supernate were added in duplicates to 50 μL of assay buffer (PBS +1% BSA) and incubated overnight at 4 °C. Biotinylated 5H1-A3, detection antibody was added (100 μL/well at 1 μg/mL diluted in PBS +0.1% BSA) to the wells and incubated 1 h at RT. Next, 100 μL/well of horseradish peroxidase-conjugated streptavidin (BD Biosciences), diluted in PBS +0.1% BSA, was added and incubated 1 h at RT. Plates were developed with TMB (Pierce), the reaction was stopped using 0.5 N H2SO4 and read at 450 nm using a Benchmark Plus plate reader and associated software (Bio-Rad). For calibration, each plate was loaded with duplicate parallel dilutions of recombinant B7-H1 fusion protein (R&D Systems) ranging in concentration from 0.01 to 20 ng/mL. To normalize the reporting of production of sB7-H1, all results are expressed in picograms of sB7-H1 per day, per mL and million cells (pg/day × mL × 106 cells).
2.5. Statistical analyses
Calibration of the B7-H1 ELISA against standard B7-H1 dilution curves was done as previously described [13]. To compare apoptosis in T cells we used paired t-tests. All p-values were two-sided and considered statistically significant if <0.05.
3. Results and discussion
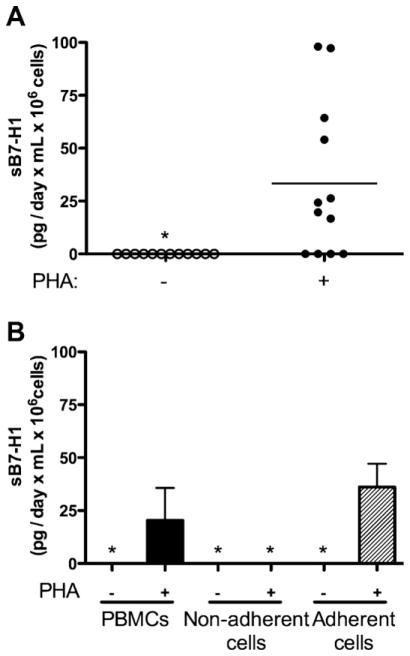
Unfractionated, normal donor PBMC, isolated using Ficoll density gradients and challenged with the mitogen PHA, released sB7-H1 (mean sB7-H1: 33.4 pg/day × mL × 106 cells; 95% CI: 10.2–56.6; Fig. 1A). The release of sB7-H1 was maximal between days 2 and 3, a time point corresponding to both maximal proliferation and induced membrane expression B7-H1 (data not shown). Unstimulated control PBMC cultures did not release detectable sB7-H1 (Fig. 1A), suggesting immune cell activation may be necessary for sB7-H1 production.
Fig. 1.
Immune cells naturally release sB7-H1 upon activation. (A) Human PBMCs release sB7-H1 upon PHA stimulation. Horizontal line represents mean sB7-H1. (B) PHA-stimulated plastic-adherent myeloid PBMCs release sB7-H1. Whole PBMCs were incubated −/+ PHA for 2 days. The non-adherent cells were removed, washed and placed in new wells along with fresh media. Fresh media was also added to the remaining adherent cells. Cells were incubated for 2 more days, and sB7-H1 was measured in the supernatant. Vertical bars represent standard deviation. Star (*) represents undetectable sB7-H1 levels. Data are representative of 6 to 12 individual samples.
As a first approximation in identifying the immune cell source of sB7-H1, PBMC were fractionated into plastic adherent and non-adherent populations and challenged with PHA. Non-adherent PBMCs did not produce detectable sB7H1 whereas PBMC which adhered to plastic produced comparable levels of sB7-H1 (mean sB7-H1: 36.1 pg/day × mL × 106 cells) as unfractionated PBMCs treated similarly (mean sB7-H1: 22.4 pg/day × mL × 106 cells, p = 0.426, Fig. 1B). These observations suggest that cells of myeloid lineage (monocytes, macrophages and DC; plastic adherent) may be a source of sB7-H1.
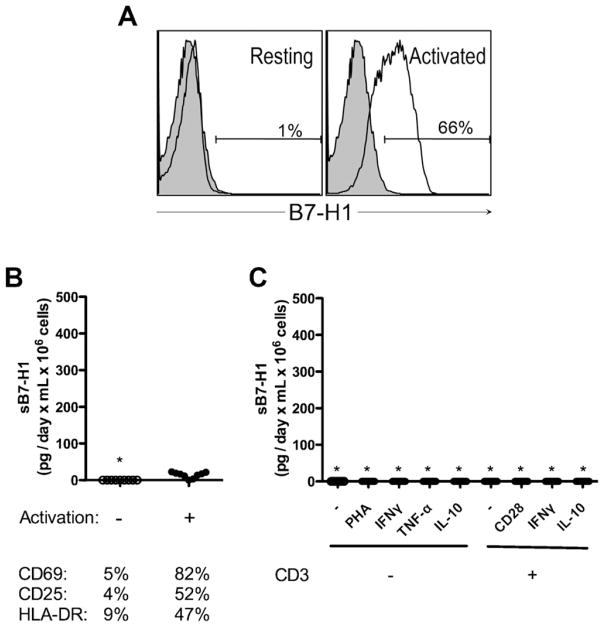
T cell populations were then isolated, challenged and culture fluid levels tested for the presence of sB7-H1. While activation of CD3+ T cells induced expression of membrane-B7-H1 (Fig. 2A) and a panel of commonly accepted membrane markers of activation (Fig. 2B), undetectable or only trace amounts of sB7-H1 (Fig. 2B and C) were released following optimal antigen independent stimulation (<14 pg/day × mL × 106 cells) suggesting that CD3+ T cells are refractory to releasing sB7-H1.
Fig. 2.
T cells do not release measurable amounts of sB7-H1. (A) Expression of membrane-bound B7-H1 (open histogram) in resting (left panel) and activated (right panel) T cells. Shaded histograms represent isotype-matched controls. (B) Measure of sB7-H1 in purified T cells after 3 days of CD3/CD28 bead activation (top panel) and assessment of corresponding activation markers (lower panel). (C) Activation of purified T cells and measure of sB7-H1 after 3 days. Star (*) represents undetectable sB7-H1. Data are representative of 7 to 10 samples analyzed per condition.
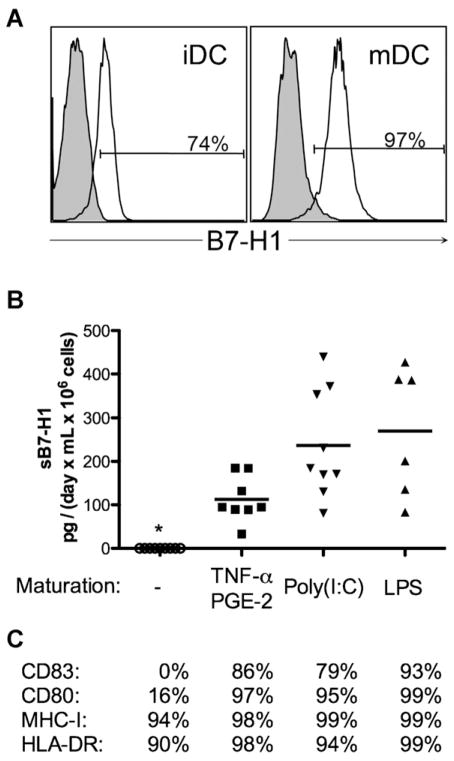
To investigate the capacity of myeloid lineage cells to release sB7-H1, CD14+ cells were isolated by immunomagnetic selection and induced to differentiate into either iDC or mDC. Both iDC and mDC expressed membrane-B7-H1 (Fig. 3A). Although iDC did not release sB7-H1 (Fig. 3B), mDC released sB7-H1 following maturation induced with TNFα/PGE-2, poly(I:C) or LPS (mean sB7-H1: 94.8, 184.3 and 293.4 pg/day × mL × 106 cells respectively, Fig. 3B).
Fig. 3.
DCs release measurable amounts of sB7-H1 after maturation. (A) Expression of membrane-bound B7-H1 (open histograms) in immature (left panel) and mature (right panel) DCs. Shaded histograms represent the isotype-matched controls. (B) sB7-H1 production is independent on which TLR is stimulated. Horizontal lines represent mean sB7-H1. Star (*) represents undetectable levels of sB7-H1. (C) Median % of cells expressing different costimulatory markers representative of DC maturation. Data were obtained using 7 to 10 different samples per experimental condition.
Tumor cell-derived sB7-H1 is biologically active and can induce apoptosis in T cells [13]. To determine whether immune cell-derived sB7-H1 can induce T cell apoptosis, mDC were cocultured with CD4+ or CD8+ T cells. Coincubation of CD4+ or CD8+ T cells with sB7-H1-producing cells mDC induced an increase of T cells undergoing apoptosis (mean: 20.8% for CD4; 26.6% for CD8; Fig. 4). In contrast, co-culture of T cell populations with iDC did not induce detectable apoptosis.
Fig. 4.
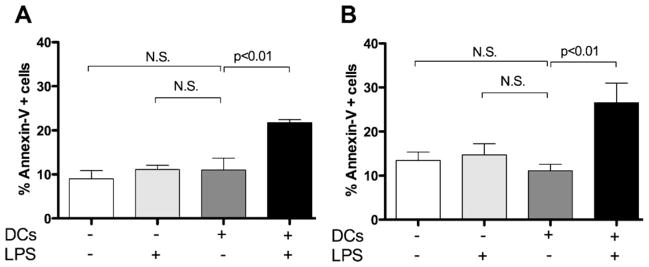
DC-released sB7-H1 produces T cell apoptosis in vitro. Apoptosis of purified (A) CD4+ and (B) CD8+ cells in transwell co-culture experiments without (−) or with (+) LPS and DCs. Bars represent mean sB7-H1 and vertical lines standard deviation. Data are representative of 5 samples analyzed per condition. N.S., statistically not significant.
This explorative investigation revealed, rather surprisingly, that myeloid derived cells have the capacity to release sB7-H1 molecules and may represent a natural source of sB7-H1. In contrast T cells, even when optimally stimulated only contributed trace amounts of sB7-H1. The immunological significance of these findings is not fully understood. That both myeloid and T cell exhibit increased expression of membrane B7-H1 but release of sB7-H1 is a feature of myeloid populations suggests that distinct regulatory mechanisms may be controlling sB7-H1 production. Whether the disparity between mDC and T cells with respect to sB7-H1 release is a reflection of distinct membrane dynamics or intracellular processing is an unresolved question. It has been reported that matrix metalloproteinases can affect release of sB7-H3 from cells [17]. Whether differential membrane enzymatic activity or more sophisticated intracellular regulatory mechanisms account for sB7-H1 release between DC and T cells will require ongoing detailed analyses.
B7-H1 blockade is of intense interest and is undergoing phase I clinical trials. That sB7-H1 can be generated from tumors and subpopulations of immune cells suggest that not all administered anti-B7-H1 immunotherapeutic antibody may reach the surface of tumor cells, with a potentially appreciable proportion being sequestered by sB7-H1 within the circulation. Compensating for potential tie-up of antibody needs to be considered in optimizing B7-H1 blockade therapies.
Acknowledgments
We thank Kim Harris and Noemi Vidal-Folch for technical help. Support for this work was provided by NIH/NCI grant CA134345 as well as generous support from The Richard M. Schulze Family Foundation (EDK), the Mayo Clinic Comprehensive Cancer Center and the Mayo Junior Faculty Development Award (HD).
Abbreviations
- ccRCC
clear cell renal carcinoma
- sB7-H1
soluble B7-H1
- DC
dendritic cells
- PBMC
peripheral blood mononuclear cells
- iDC
immature dendritic cells
- mDC
mature dendritic cells
Footnotes
Conflict of interest statement
EDK, HD, XF, BAI and SMH, have filed for patents of B7-H ligands as prognostic markers and therapeutic targets for cancer.
Author contributions
Xavier Frigola, Brant Inman, Haidong Dong, Al Dietz, and Eugene Kwon: conception and design. Xavier Frigola and Haidong Dong: data analysis; Xin Liu, Susan Harrington and Peggy Bulur: technical support. Xavier Frigola, Brant Inman, Haidong Dong, Al Dietz, Christopher Krco, Eugene Kwon: interpretation of data; Xavier Frigola and Christopher Krco: manuscript writing. Eugene Kwon: final approval of manuscript.
Contributor Information
Xavier Frigola, Department of Urology, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA.
Brant A. Inman, Department of Urology, Duke University Medical Center, Durham, NC, 27710, USA
Christopher J. Krco, Department of Urology, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA
Xin Liu, Department of Urology, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA.
Susan M. Harrington, Department of Urology, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA
Peggy A. Bulur, Department of Transfusion Medicine, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA
Allan B. Dietz, Department of Transfusion Medicine, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA
Haidong Dong, Department of Urology, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA.
Eugene D. Kwon, Department of Urology, College of Medicine, Mayo Clinic, Rochester, MN, 55905, USA.
References
- 1.Flies DB, Chen L. The new B7s: playing a pivotal role in tumor immunity. J Immunother. 2007;30:251–60. doi: 10.1097/CJI.0b013e31802e085a. [DOI] [PubMed] [Google Scholar]
- 2.Inman BA, Frigola X, Dong H, Kwon ED. Costimulation, coinhibition and cancer. Curr Cancer Drug Targets. 2007;7:15–30. doi: 10.2174/156800907780006878. [DOI] [PubMed] [Google Scholar]
- 3.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 4.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2:269–74. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 5.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–61. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 6.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–92. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–43. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103:10391–6. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, et al. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67:7893–900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101:17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Her M, Kim D, Oh M, Jeong H, Choi I. Increased expression of soluble inducible costimulator ligand (ICOSL) in patients with systemic lupus erythematosus. Lupus. 2009;18:501–7. doi: 10.1177/0961203308099176. [DOI] [PubMed] [Google Scholar]
- 12.Wan B, Nie H, Liu A, Feng G, He D, Xu R, et al. Aberrant regulation of synovial T cell activation by soluble costimulatory molecules in rheumatoid arthritis. J Immunol. 2006;177:8844–50. doi: 10.4049/jimmunol.177.12.8844. [DOI] [PubMed] [Google Scholar]
- 13.Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–23. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon I, Liu Y, Krall KL, Urban N, Wolfert RL, Kim NW, et al. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol Oncol. 2007;106:112–8. doi: 10.1016/j.ygyno.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66:1570–5. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 16.Thompson RH, Zang X, Lohse CM, Leibovich BC, Slovin SF, Reuter VE, et al. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68:6054–8. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang G, Hou J, Shi J, Yu G, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123:538–46. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman BA, Frigola X, Harris KJ, Kuntz SM, Lohse CM, Leibovich BC, et al. Questionable relevance of {gamma}{delta} T lymphocytes in renal cell carcinoma. J Immunol. 2008;180:3578–84. doi: 10.4049/jimmunol.180.5.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]