Abstract
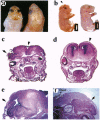
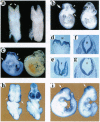
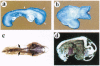
MacMARCKS is a member of the MARCKS family of protein kinase C (PKC) substrates. Biochemical evidence demonstrates that these proteins integrate calcium and PKC-dependent signals to regulate actin structure at the membrane. We report here that deletion of the MacMARCKS gene prevents cranial neural tube closure in the developing brain, resulting in anencephaly. This suggests a central role for MacMARCKS and the PKC signal transduction pathway in the folding of the anterior neural plate during the early phases of brain formation, and supports the hypothesis that actin-based motility directs cranial neural tube closure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Allen L. H., Aderem A. A role for MARCKS, the alpha isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med. 1995 Sep 1;182(3):829–840. doi: 10.1084/jem.182.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J. The MARCKS family of cellular protein kinase C substrates. J Biol Chem. 1993 Jan 25;268(3):1501–1504. [PubMed] [Google Scholar]
- Blackshear P. J., Verghese G. M., Johnson J. D., Haupt D. M., Stumpo D. J. Characteristics of the F52 protein, a MARCKS homologue. J Biol Chem. 1992 Jul 5;267(19):13540–13546. [PubMed] [Google Scholar]
- Chang S., Hemmings H. C., Jr, Aderem A. Stimulus-dependent phosphorylation of MacMARCKS, a protein kinase C substrate, in nerve termini and PC12 cells. J Biol Chem. 1996 Jan 12;271(2):1174–1178. doi: 10.1074/jbc.271.2.1174. [DOI] [PubMed] [Google Scholar]
- Chen Z. F., Behringer R. R. twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995 Mar 15;9(6):686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- Copp A. J., Brook F. A., Estibeiro J. P., Shum A. S., Cockroft D. L. The embryonic development of mammalian neural tube defects. Prog Neurobiol. 1990;35(5):363–403. doi: 10.1016/0301-0082(90)90037-h. [DOI] [PubMed] [Google Scholar]
- Davis C. A., Noble-Topham S. E., Rossant J., Joyner A. L. Expression of the homeo box-containing gene En-2 delineates a specific region of the developing mouse brain. Genes Dev. 1988 Mar;2(3):361–371. doi: 10.1101/gad.2.3.361. [DOI] [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994 Feb;19(2):73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Duncan S. A., Manova K., Chen W. S., Hoodless P., Weinstein D. C., Bachvarova R. F., Darnell J. E., Jr Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7598–7602. doi: 10.1073/pnas.91.16.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein D. J., Vekemans M., Gros P. Splotch (Sp2H), a mutation affecting development of the mouse neural tube, shows a deletion within the paired homeodomain of Pax-3. Cell. 1991 Nov 15;67(4):767–774. doi: 10.1016/0092-8674(91)90071-6. [DOI] [PubMed] [Google Scholar]
- Farese R. V., Jr, Ruland S. L., Flynn L. M., Stokowski R. P., Young S. G. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. B., Pfaff D. W. In situ hybridization detection of trkA mRNA in brain: distribution, colocalization with p75NGFR and up-regulation by nerve growth factor. J Comp Neurol. 1994 Mar 15;341(3):324–339. doi: 10.1002/cne.903410304. [DOI] [PubMed] [Google Scholar]
- Golden J. A., Chernoff G. F. Intermittent pattern of neural tube closure in two strains of mice. Teratology. 1993 Jan;47(1):73–80. doi: 10.1002/tera.1420470112. [DOI] [PubMed] [Google Scholar]
- Golden J. A., Chernoff G. F. Multiple sites of anterior neural tube closure in humans: evidence from anterior neural tube defects (anencephaly). Pediatrics. 1995 Apr;95(4):506–510. [PubMed] [Google Scholar]
- Graff J. M., Stumpo D. J., Blackshear P. J. Molecular cloning, sequence, and expression of a cDNA encoding the chicken myristoylated alanine-rich C kinase substrate (MARCKS). Mol Endocrinol. 1989 Nov;3(11):1903–1906. doi: 10.1210/mend-3-11-1903. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Thelen M., Rosen A., Janmey P. A., Nairn A. C., Aderem A. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-calmodulin. Nature. 1992 Apr 16;356(6370):618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- Hui C. C., Joyner A. L. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat Genet. 1993 Mar;3(3):241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Ang S. L., Shiota K., Nakanishi S., Kageyama R., Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995 Dec 15;9(24):3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Lemire R. J. Neural tube defects. JAMA. 1988 Jan 22;259(4):558–562. [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Li J., Aderem A. MacMARCKS, a novel member of the MARCKS family of protein kinase C substrates. Cell. 1992 Sep 4;70(5):791–801. doi: 10.1016/0092-8674(92)90312-z. [DOI] [PubMed] [Google Scholar]
- Lobach D. F., Rochelle J. M., Watson M. L., Seldin M. F., Blackshear P. J. Nucleotide sequence, expression, and chromosomal mapping of Mrp and mapping of five related sequences. Genomics. 1993 Jul;17(1):194–204. doi: 10.1006/geno.1993.1301. [DOI] [PubMed] [Google Scholar]
- Macdonald K. B., Juriloff D. M., Harris M. J. Developmental study of neural tube closure in a mouse stock with a high incidence of exencephaly. Teratology. 1989 Feb;39(2):195–213. doi: 10.1002/tera.1420390211. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay G., Tuckett F. The role of microfilaments in cranial neurulation in rat embryos: effects of short-term exposure to cytochalasin D. J Embryol Exp Morphol. 1985 Aug;88:333–348. [PubMed] [Google Scholar]
- Parr B. A., Shea M. J., Vassileva G., McMahon A. P. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 1993 Sep;119(1):247–261. doi: 10.1242/dev.119.1.247. [DOI] [PubMed] [Google Scholar]
- Rosen A., Keenan K. F., Thelen M., Nairn A. C., Aderem A. Activation of protein kinase C results in the displacement of its myristoylated, alanine-rich substrate from punctate structures in macrophage filopodia. J Exp Med. 1990 Oct 1;172(4):1211–1215. doi: 10.1084/jem.172.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B., Beddington R. S. Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genet. 1993 May;9(5):162–167. doi: 10.1016/0168-9525(93)90162-b. [DOI] [PubMed] [Google Scholar]
- Sah V. P., Attardi L. D., Mulligan G. J., Williams B. O., Bronson R. T., Jacks T. A subset of p53-deficient embryos exhibit exencephaly. Nat Genet. 1995 Jun;10(2):175–180. doi: 10.1038/ng0695-175. [DOI] [PubMed] [Google Scholar]
- Sakai Y. Neurulation in the mouse: manner and timing of neural tube closure. Anat Rec. 1989 Feb;223(2):194–203. doi: 10.1002/ar.1092230212. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C. Cell movements driving neurulation in avian embryos. Dev Suppl. 1991;Suppl 2:157–168. [PubMed] [Google Scholar]
- Schoenwolf G. C., Folsom D., Moe A. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat Rec. 1988 Jan;220(1):87–102. doi: 10.1002/ar.1092200111. [DOI] [PubMed] [Google Scholar]
- Schoenwolf G. C. On the morphogenesis of the early rudiments of the developing central nervous system. Scan Electron Microsc. 1982;(Pt 1):289–308. [PubMed] [Google Scholar]
- Schoenwolf G. C., Smith J. L. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990 Jun;109(2):243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Gulisano M., Stornaiuolo A., Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992 Aug 20;358(6388):687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Schoenwolf G. C. Further evidence of extrinsic forces in bending of the neural plate. J Comp Neurol. 1991 May 8;307(2):225–236. doi: 10.1002/cne.903070206. [DOI] [PubMed] [Google Scholar]
- Stumpo D. J., Bock C. B., Tuttle J. S., Blackshear P. J. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T., Yamazaki Y., Katoh-Fukui Y., Tsuchiya R., Kondo S., Motoyama J., Higashinakagawa T. Gene trap capture of a novel mouse gene, jumonji, required for neural tube formation. Genes Dev. 1995 May 15;9(10):1211–1222. doi: 10.1101/gad.9.10.1211. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Van Allen M. I., Kalousek D. K., Chernoff G. F., Juriloff D., Harris M., McGillivray B. C., Yong S. L., Langlois S., MacLeod P. M., Chitayat D. Evidence for multi-site closure of the neural tube in humans. Am J Med Genet. 1993 Oct 1;47(5):723–743. doi: 10.1002/ajmg.1320470528. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Bao Z., Li J. MacMARCKS mutation blocks macrophage phagocytosis of zymosan. J Biol Chem. 1995 Jul 28;270(30):17652–17655. doi: 10.1074/jbc.270.30.17652. [DOI] [PubMed] [Google Scholar]
- van Straaten H. W., Hekking J. W., Consten C., Copp A. J. Intrinsic and extrinsic factors in the mechanism of neurulation: effect of curvature of the body axis on closure of the posterior neuropore. Development. 1993 Mar;117(3):1163–1172. doi: 10.1242/dev.117.3.1163. [DOI] [PubMed] [Google Scholar]